Biomineralogy of Angiogenesis
Maciej Pawlikowski
AGH University of Science and Technology, Department of Mineralogy, Petrography and Geochemistry, Lab. Biomineralogy, 30-059 Krakow, al. Mickiewicza 30, Poland
*Corresponding Author: Maciej Pawlikowski, AGH University of Science and Technology, Department of Mineralogy, Petrography and Geochemistry, Lab. Biomineralogy, 30-059 Krakow, al. Mickiewicza 30, Poland
Received: 24 May 2017; Accepted: 08 June 2017; Published: 12 June 2017
Article Information
View / Download Pdf Share at FacebookAbstract
Regeneration of blood vessels and their development occur in the damaged spots. This phenomenon suggests that the destruction process itself stimulates development of blood vessels and results in healing and self-repair of tissue. Learning about those self-repair processes is extremely important for restoring tissues and organs to their proper functioning in systems and the whole body. Therefore, recognizing the processes of angiogenesis appears to be the key in treating numerous diseases.
Long-term research on blood vessels, their development and mineralization [1-9] as well as on mineralization and demineralization of bones and the process of bone healing [10-12] and the phenomena of tumor mineralization [9, 12-14] have supplied the author with numerous insights into angiogenesis. This publication is a summary of those insights.
Keywords
Biomineralogy; Angiogenesis; Demineralization; Blood Vessels
Article Details
1. Development of Blood Vessels in Blocked Areas
1.1 Collateral Circulation
In case of blockage of blood vessels, e.g. coronary arteries, by substances crystallizing within [4] increase in levels
of CO2 occurs. It is the effect of hypoxia, i.e. change of proportion of oxygen and CO2 levels in the affected area. Consequently, in the area of the arterial blockage, the level of oxygen is low in comparison to the level of CO2, which is rising due to the dying of the cardiac muscle cells. That phenomenon leads to creation of easily dissociating carbonic acid (H2CO3) formed from local CO2 and water appearing in tissues. The result is a drop in pH below 7.0. Angiogenesis developing in the area of arterial blockage may lead to creation of collateral circulation (Figure 1). Although the described phenomenon doesn’t have direct connection with angiogenesis, one will note that damaged artery wall may undergo the process of self-repair. Newly developing collagen strands “overgrow” the weakened part of arterial wall (Figure 2).
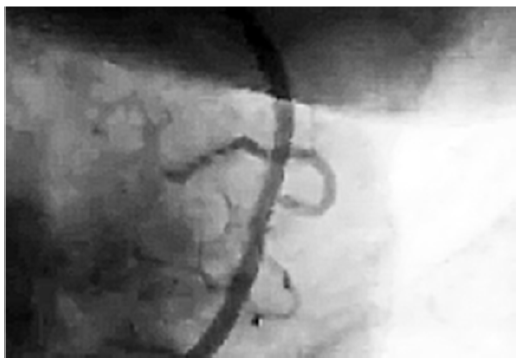
Figure 1: Collateral circulation in a blocked area of coronary artery
1.2 Spontaneous self-repair of damaged arterial walls
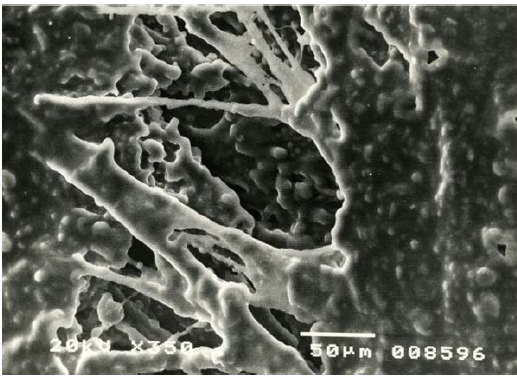
Figure 2: Self-repair of a micro-area of damaged internal wall of an artery. Aneurism of ascending aorta. Male, age 40. SEM [4].
1.3 Development of blood vessels in tumor areas
In cases of tumors, rapid growth in number of cancer cells (Figure 3, 4) changes the ratio of supplied oxygen to produced tumor metabolites [15]. Also in this case locally generated CO2 levels are higher than supplied oxygen levels. That results in acidification of the tumor areas and, in consequence, development of blood vessels (Figure 5).
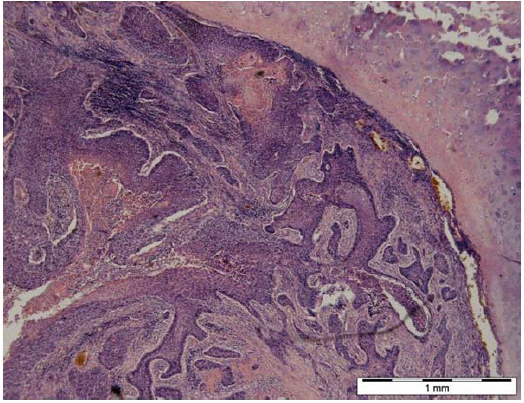
Figure 3: Carcinoma planoepitheliale 1.3.1, source: WHO. Visible epidermoid cancer infiltrating bronchial cartilage. Female, age 60. Biological microscope, magnification according to scale [14].
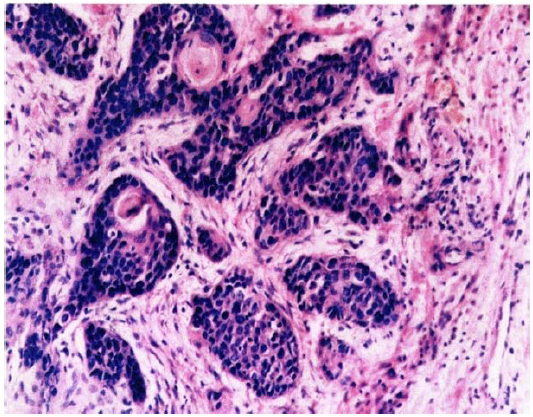
Figure 4: Epidermoid cancer ? group G2. Multiple small centers of cancer infiltrating stroma. Male, age 65. Biological microscope. Material stained with H-E, magnification 220x.
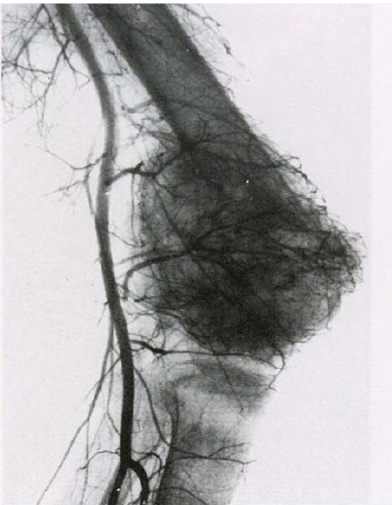
Figure 5: Angiogram of lower limb. Visible net of blood vessels of the knee joint area and multiple pathological vessels connected to the tumor in femur base [12].
1.4 Development of blood vessels in a bone fracture area
In the process of healing a bone fracture, hypoxia of the break area results from destruction of blood vessels and the local concentration of CO2 and other metabolites. Due to destruction of local veins, those substances are not eliminated from the fracture area. Also in this case carbonic acid is created, which acidifies the area, causing drop in the local pH. This phenomenon stimulates angiogenesis. Only after development of new blood vessels, when pH of the environment rises again above 6.6, crystallization of apatite can occur, which is when the mineralization of bone collagen starts (Figure 6A, B).
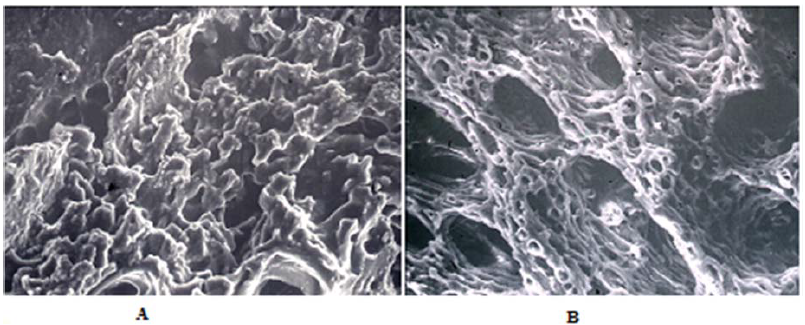
Figure 6: (A) ? Structure of transforming soft callus 10 days after the break. SEM. Magnification 400x. (B) ? Structure of bone developing from soft callus 20 days after the break. SEM. Magnification 400x.
Along with the modification of the soft callus into hard callus, i.e. bone, there are additional processes accompanying development of blood vessels, including modification of bone marrow cells, their evolution into osteoblasts, and creation of collagen strands. It is interesting that collagen strands developing in the callus have crystallization centers located almost identically to those in the “original” bone’s collagen. Owing to that, collagen mineralization by apatite crystallized from alkaline phosphatase results in development of bone whose morphological and physicochemical attributes are the same as those of the original bone.
2. Conclusion
The processes of endothelium development and new blood vessels formation were described by Harper and Shahneh [16-17], among others. It was noted that endothelium cells proliferation and stopping angiogenesis of blood vessels within tumors were essential issues [18].
Common characteristic of all described angiogenesis cases is undoubtedly development of particular physicochemical environment connected with tissue hypoxia, which leads to angiogenesis. Such environment includes local excess of carbon dioxide and cell metabolites in the area, which cannot be eliminated by destroyed blood vessels (or lack of them ? tumors) [18,19].
Conducted research and observations indicate the existence of local acidification of tissue in the areas of angiogenesis, which is a result of the increase in CO2 levels. Such acidification may be the main factor in the local angiogenesis processes.
While debating acidification of tissue environment as the factor stimulating blood vessels development, we need to explain that phenomenon in detail. Since pH is, according to definition, the negative of the logarithm to base 10 of the activity of the hydrogen ion, the potential role of protons (H+) in the blood vessels forming phenomenon needs to be explained.
Free protons are created in acidified tissue environment through dissociation ? from easily dissociating carbonic acid, which develops due to the local excess of CO2. The role of protons appears to be important in the stimulation of intima, and in particular its cells. Its cellular membranes are built of phospholipids with a polar structure. Characteristic of such structure is the presence of charges on the polar opposites of the phospholipids’ particles. It appears that protons present in acidic environment may change or modify polarization of phospholipids. That phenomenon stimulates the endothelium cells to proliferate and develop toward blood vessels formation. Endothelium development is considered to be the main element in blood vessel formation.
That hypothesis should be proved by experiments, which are regrettably impossible to perform in the laboratory which the author has access to at the AGH University of Science and Technology.
If proved, it should allow us to stimulate formation of blood vessels where they are necessary, and stop angiogenesis in places where it is pernicious (tumors). Described process has been confirmed by biomineralogical analysis of bones and mineralization (calcification) of various tissues [11]. Crystallization of apatite and other phosphates in places of calcification occurs in pH over 6.6 (alkalization). Therefore, acidified areas only undergo calcification (mineralization) as the blood vessels develop, tissues are “healed” and their environment returns to slightly alkaline.
Another important issue is stimulation of angiogenesis in areas affected by osteoporosis, as well as stopping its adverse development [20].
On the other hand, creation of collagen, blood vessels, and synthesis of alkaline photosphatase in areas of osteosynthesis in a way similar to that of bone fracture healing should allow for its rebuilding and regeneration.
The potential of these findings, if confirmed, is hard to overestimate in view of the functioning of the whole human body (Pawlikowski 2014, 2015, 2016a, b).
References
- Pawlikowski M. Mineralizacja organizmu cz?owieka ?yj?cego. (Mineralization of human living organism). Prace Mineral 79 (1987): 121.
- Pawlikowski M. Kryszta?y w organizmie cz?owieka. (Crystals of human organism). Secesja. (Atlas), 1993:132.
- Pawlikowski M (2003) Minerals in human blood vessels and their dissolution in vitro. In: Skinner HCW, Berger AW, Geology and health. N.Y. ? Oxford. Oxford University Press, pp.155-158.
- Pawlikowski M. Preliminary results of dissolution of substances mineralizing human arteries. Arch Mineralog 52 (1999): 195-210.
- Pawlikowski M, Pfitzner R. Zastosowanie metod mineralogicznych w badaniach tkanek cz?owieka. I. Sposoby badania mineralizacji. (Mineralogical methods useful for examination of human tissues). Przegl Lekarski 52 (1995): 119-123.
- Pawlikowski M, Pfitzner R Zastosowanie metod mineralogicznych w badaniach tkanek cz?owieka. II. Mineralizacja struktur serca. (Mineralogical methods useful for examination of human tissues. Mineralization of heart structures). Przegl Lekarski 52 (1995): 24-27.
- Pawlikowski M, Pfitzner K., Skinner C. Cholesterol-mineral concentrations of the aneurysmatic wall. Acta Angiologica (1995) Supl. 1 str. 15.
- Pawlikowski M., Pfitzner R. Mineralizacja serca i du?ych naczy?. (Mineralization of heart and big blood vessels). Wyd. IGSMiE PAN Kraków (1999): 142 p.
- Pawlikowski M, Ryskala Z. Charakterystyka mineralogiczno-chemiczna fosforanowej mineralizacji wybranych naczy? t?tniczych cz?owieka. (Mineralogical - chemical characteristic of phosphate mineralization of human arteries). Roczniki Nauk.- Dyd. WSP w Krakowie Prace Fizjologiczne (1991): 81-104.
- Nied?wiedzki T, Pawlikowski M. Zmiany mineralogiczne zachodz?ce w obszarze gojenia z?ama? ko?ci d?ugich. (Mineralogical phenomena observed at healing part of broken bones). Chirurgia Narz. Ruchu i Ortop. Pol. T. LV (1990): 277-281.
- Nied?wiedzki T, D?browski Z, Miszta H, Pawlikowski M. Bone healing after bone marrow stromal cell transplantation to the bone defect. Biomaterials 14 (1993): 115-121.
- Pawlikowski M, Nied?wiedzki T. Mineralogia ko?ci. (Mineralogy of bones). Wyd. PAN Oddzia? w Krakowie (2002): 128.
- Pawlikowski M. Biomineralization of cancer tissues. 20th Int. Symp. Molecular and Physiological Aspects of Regulatory Processes of the Organism. Cracow. Ed. H. Lach. Wyd. Abaton. Kraków (2011):190-191.
- Pawlikowski M. (2013) Mineralizacja guzów nowotworowych p?uc. (Mineralization of lung cancer tumors. Auxiliary sciences in archaeology, preservation of relicts and environmental engineering. CD -no 15, Ed. M Pawlikowski
- Servan-Schreiber D. (2012) Antyrak, Albatros. Warszawa. 304 p.
- Harper J, Moses MA. Molecular regulation of tumor angiogenesis: mechanisms and therapeutic implications. EXS 96 (2006): 223-268.
- Shahneh FZ, Baradaran B, Zamani F, Aghebati-Maleki L. Tumor angiogenesis and anti-angiogenic therapies. Hum Antibodies 22 (2013): 15-19.
- Wietecha MS, Cerny WL, DiPietro LA. Mechanisms of vessel regression: toward an understanding of the resolution of angiogenesis. Curr. Top Microbiol Immunol 367 (2013): 3-32
- Kurzyk A. Angiogenesis - possibilities, problems and perspectives. Postepy biochemii 61 (2014): 25-34.
- Eichholz A, Merchant S, Gaya A. Anti-angiogenesis therapies: their potential in cancer management. Onco. Targets Therapy 3 (2010): 69-82.
- Pawlikowski M. Osteoporosis as a source of tissue mineralization. Research on osteoporosis therapy and dissolution of arterial mineralization. Jour Life Science 8 (2014): 610-625.
- Pawlikowski M. Biomineralogy of osteoporosis. Academia Journal of Biotechnology 4 (2016): 138-144.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 