Can Pre-Treatment Assessment of Aqueous Vascular and Inflammatory Biomarkers In Patients with Macular Oedema Secondary to Retinal Vein Occlusion Guide Treatment Options?
Jai Shankar1*, Stephen Fôn Hughes2, Peter Ella-Tongwiis2
1Department of Ophthalmology, Wrexham Maelor Hospital, Betsi Cadwaladr University Health Board,
Croesnewydd Road, Wrexham LL13 7TD, UK
2Maelor Academic Unit of Medical & Surgical Sciences (MAUMSS) Wrexham Maelor Hospital, Betsi Cadwaladr University Health Board, Croesnewydd Road, Wrexham LL13 7TD, UK
*Corresponding author:
Jai Shankar, Department of Ophthalmology, Wrexham Maelor Hospital, Croesnewydd Road, Wrexham LL13 7TD, UK
Received: January 16, 2024;Accepted: January 19, 2024;Published: January 31, 2024
Article Information
Citation: Jai Shankar, Stephen Fôn Hughes, Peter Ella-Tongwiis. Can Pre-Treatment Assessment of Aqueous Vascular and Inflammatory Biomarkers In Patients with Macular Oedema Secondary to Retinal Vein Occlusion Guide Treatment Options?. Archives of Clinical and Biomedical Research. 8 (2024): 20-26.
View / Download Pdf Share at FacebookAbstract
Background: Macular oedema following Retinal Vein occlusion is the second most common cause of visual loss due to retinal vascular disease. The Royal College of Ophthalmologists recommends intra-vitreal anti- Vascular Endothelial Growth Factor (anti-VEGF) for patients with glaucoma and in young patients & steroid injections in those with recent cardiovascular events. Treatment is, both, expensive and demanding on secondary care. There is no recommendation regarding pre-treatment biomarker testing to determine which would be a better treatment option.
Aims: The study aimed to determine whether vascular and inflammatory biomarkers in BRVO and CRVO differ from normal levels, and whether one can formulate an individualised treatment plan to predict whether anti-VEGF or steroids are more likely to be beneficial.
Methods: Eight patients each with newly diagnosed BRVO and CRVO with macular oedema without any previous intra-vitreal injections were recruited (n=16). 100-200μl of aqueous sample was taken from the anterior chamber just prior to intra-vitreal injection of anti-VEGF agent or steroid. Aqueous samples were also collected from eight patients undergoing routine cataract surgery (n=8). ELISA was employed to assess the samples for VEGF and IL-8 levels.
Results: VEGF and IL-8 levels were significantly higher in RVO as compared to controls and higher in CRVO as compared to BRVO. There were some samples with predominance of VEGF and others with IL-8.
Conclusion: This study concludes that pre-treatment assessment of biomarkers may help determine whether anti-VEGF or steroid would be a better treatment option and thereby result in a significant cost reduction for the healthcare provider.
Keywords
<p>Retinal vein occlusion; Aqueous biomarkers; Macular oedema</p>
Article Details
Introduction
Retinal vein occlusion (RVO) is the second most common cause of visual loss due to retinal vascular disease after diabetic retinopathy [1,2]. Macular oedema is caused by breakdown of the blood retinal barrier from increased venous hydrostatic pressure. However, it is now known that oedema is not merely as a result of a mechanical effect but is mediated by complex underlying ischaemic and inflammatory pathologies [3]. Vascular Endothelial Growth Factor (VEGF) is expressed by retinal pigment epithelial cells, retinal microglia and retinal vascular endothelial cells. There is significant upregulation of VEGF expression in Central Retinal Vein Occlusion (CRVO) [4]. As some patients of CRVO develop macular oedema in the absence of ischaemia, VEGF could not have been the only pathogenic agent. Cytokines such as Interleukin-6 (IL-6) and Interleukin-8 (IL-8) and growth factors such as Placental Growth Factor and Platelet Derived Growth Factor have been implicated [5]. Branch Retinal Vein Occlusion (BRVO) is caused by compression at an arterio-venous crossing [6]. BRVO leads to increased intra- vascular pressure, inflammation and leakage of fluid into the surrounding retina, with resultant macular oedema. Similar to CRVO, macular oedema in BRVO is not merely a mechanical effect. Ischemia produced by impaired venous outflow results in the release of VEGF and increased vascular permeability. VEGF expression in BRVO, unlike CRVO is variable [7]. Until 2010, there was effectively no specific treatment for macular oedema secondary to CRVO. Argon laser was useful for the management of neovascular complications following CRVO but it had no role in the management of the resultant macular oedema [8]. The BRVO Study [1] showed that Argon grid laser for macular oedema resulted in a 1.3- line (8 letter) improvement in vision but only 60% of patients retained 6/12 or better vision. The study group recognised the need for better treatment modalities. Pharmacological therapy for RVO related macular oedema is either by neutralisation or entrapment of VEGF, or by down regulation of expression of pro-inflammatory chemicals with steroids. Miller [9], Kotake [10] and Liang [11] have demonstrated a reduction in VEGF levels and cytokine markers post-treatment when compared to the levels before anti-VEGF treatment. Dexamethasone (Ozurdex® implant) was the first drug to be approved by the National Institute for Health and Clinical Excellence (NICE) [12]. This was followed by the recommendation of anti-VEGF agents Ranibizumab in 2013 [13] and Aflibercept for CRVO in 2014 [14] and for BRVO in 2016 [15]. It was first hypothesised in 1948 that there may be a vasoactive substance produced by ischaemic retinal tissue [16]. Animal models of RVO have shown upregulation of a bio-marker that was named vascular endothelial growth factor (VEGF). VEGF is a potent stimulator for vasculogenesis. Expression is potentiated by hypoxia and various cytokines [18]. The first report of intra-retinal expression of VEGF mRNA in humans was by Pe’er et al [19]. Increased expression of VEGF was noted in the inner retina. Cytokines, first described in 1957, are small peptides and include chemokines, interferons, interleukins, lymphokines and tumour necrosis factor. They are responsible for maintaining inflammatory balance. Some of them facilitate inflammatory cascades whereas others supress inflammation. Increased levels of VEGF in aqueous humour samples obtained from human subjects with macular oedema secondary to BRVO was first identified by Noma [20] who reported increased aqueous levels of two biomarkers, VEGF and IL-6, in patients with BRVO. They also measured their levels in serum samples and found no correlation between the levels measured in serum and that from the aqueous. They concluded that increased vascular permeability was a local phenomenon and not a systemic disease. Measurement of serum levels could therefore not be used as a surrogate marker for intra-ocular levels of bio-markers. Noma [21] conducted a similar study to determine the levels of VEGF and IL-6 in aqueous and vitreous samples from patients with macular oedema secondary to BRVO. They found that there was significant correlation between aqueous levels and vitreous levels and concluded that measurement of aqueous levels may be clinically useful. Aqueous sampling is far less invasive than vitreous sampling. This justifies the design of other studies wherein only aqueous sampling is performed to determine biomarker levels. Increased levels of VEGF in aqueous samples were also identified in patients with BRVO by Park [22]. In a prospective observational study conducted on a sample of 102 patients with BRVO, aqueous was collected just prior to intra-vitreal therapy. Samples from 10 cataract patients were used as controls. Mean VEGF levels in BRVO was significantly higher than in controls. Direct comparison between biomarker levels in controls and patients with macular oedema secondary to BRVO and CRVO was reported by Shchuko et al [23]. This group reported significantly higher levels of VEGF and pro-inflammatory cytokines and chemotaxins – IL-1β, IL-6, IL-8, IL-12, IL15, IFN-γ TNF-α, MCP-1 and RANTES in RVO patients as compared to control. They concluded that RVO is associated with overexpression of VEGF and other pro-inflammatory cytokines and chemokines. Surprisingly, they also reported statistically significant higher levels of anti-inflammatory markers like IL-4, IL-10, IL-13, IL-17 and RAIL-1 (Receptor antagonist interleukin-1). Therefore retinal activity was concluded to be a balance between pro-inflammatory and anti- inflammatory mechanisms [24]. They also noted that there was a significant reduction in VEGF levels following treatment with the anti-VEGF agent, Ranibizumab, in those patients who had a good clinical response. Those patients who did not show a good clinical response also showed significantly lower levels of VEGF post-treatment, but the reduction of inflammatory cytokines was only modest. This indicates that whilst VEGF is an important mediator of macular oedema, other factors may also play a significant role, particularly in those patients who do not respond well to anti-VEGF agents alone. The literature review has shown that a majority of the publications in peer-reviewed journals pertain to studies done on South-East Asians, either Japanese or Korean [6, 21, 22, 24, 25, 26, 27, 28, 29]. None of the studies have been UK based, let alone in a Welsh cohort of patients. Furthermore, no study has explored whether pre-treatment quantification of aqueous levels could help in making an informed choice as to which NICE approved drug is likely to prove more beneficial- steroid or anti-VEGF agents. Intra-vitreal therapy places a heavy burden on ophthalmic services, both in terms of drug cost and available clinic capacity [30]. Ranibizumab is injected monthly whilst Aflibercept is injected every 2 months after the first three monthly loading injections. Some patients are put on a treat and extend regime. Faricimab needs four loading injections. On an average, patients need monitoring and treatment about 8-9 times a year in the first year and 4-5 injections in the second year. The NICE estimate of 2-year drug cost is £10,700. There is often an indefinite need for monitoring of patients and with no indication of levelling off or decline in demand [31].
Aims
The management of macular oedema secondary to retinal vein occlusion is by administering multiple intra-vitreal injections of anti-VEGF agents or long acting steroids. There is no NICE or Royal College guidance as to which of these two group of agents is to be used as first line treatment in the absence of contraindications.
This pilot study aimed:
To measure the VEGF and inflammatory marker IL-8 levels in normal human controls and patients with retinal vein occlusion before treatment in a Welsh population, and also compare the levels of these biomarkers in CRVO versus BRVO. Ultimately, information provided from this study may determine whether pre-treatment measurement of these biomarkers might guide the clinician as to which agent may be used first-line thereby saving vision, cost and maximising available clinic capacity.
Methods
Ethics
The pilot project was designed as a prospective case controlled observational study. The study was conducted in accordance with the Declaration of Helsinki. Approval for the project was obtained from the local Research Ethics Committee (GREC), the NHS Ethics Committee (IRAS reference 269524) and the Health Research Authority (HRA) and Health and Care Research Wales (HCRW) through IRAS. This was a pilot feasibility study and as such, no formal sample size calculation was performed.
Patient recruitment
Eight control patients were recruited from a pool of adults undergoing routine cataract surgery at Wrexham Maelor Hospital in North Wales (n=8). Subjects with any previous or current ocular inflammatory conditions, those with any previous or current vascular retinopathy or any participant with traumatic, steroid induced or post-inflammatory cataracts were excluded. 16 patients with RVO who were undergoing intra-vitreal injection of anti-VEGF agents or steroids for treatment of macular oedema secondary were recruited. These study patients were offered treatment as per The Royal College of Ophthalmologists and NICE guidelines. Those patients who had previously received an anti-VEGF or steroid injection were excluded.
Measurement of VEGF and IL-8 levels
100µL to 200µL of aqueous was obtained from the anterior chamber using a Rycroft cannula during cataract surgery. In those undergoing intra-vitreal injections for RVO, samples were collected using a 30G needle on 1 ml syringe. Samples were placed in a suitably sized capped Eppendorf micro- centrifuge tube and frozen immediately at -80°C and stored for later laboratory biomarker analysis. Aqueous samples were stored allowing for analysis to be conducted together in one batch. Following thawing, samples were analysed using the ELISA technique on a Bioplex Luminex® 200 Analyser.
Statistical Analysis
Test for normality was performed using the Shapiro- Wilk Test using SPSS (version 27.0). Where the data did not follow a normal distribution, non-parametric testing using the Friedman test was used performed on SPSS27 software. Wilcoxon Rank Test was employed to make comparisons were between the two biomarkers tested i.e. VEGF and IL-8, between controls and RVO patients. Comparisons of these biomarkers were also made between BRVO and CRVO. A p<0.05 was considered statistically significant.
Data was presented as Mean + Standard Deviation (SD) and as Median with inter-quartile ranges (IQR).
Results
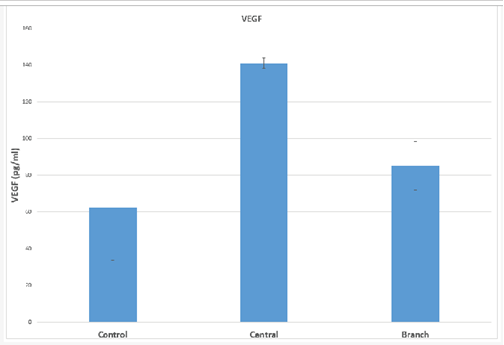
Mean VEGF value in the control sample was 67.04+10.94 pg/ml. Mean aqueous humour levels in patients with CRVO was 187.88+77.56 pg/ml, while that in patients with BRVO was 82.94+5.89 pg/ml. Median values were 62.41 pg/ml in controls, 141.08 pg/ml in CRVO and 85.17 pg/ml in BRVO. Table 1 and Figure 1 show the results of testing for VEGF. Statistical analysis using the Friedman test was employed on median values. The levels of VEGF in CRVO were more than twice the level in controls and this difference was statistically significant (p<0.05). The difference between controls and BRVO was not statistically significant (p=0.072). There was also a statistical difference between the VEGF levels in CRVO versus BRVO with VEGF values being significantly higher in CRVO (p<0.05)
Table 1: VEGF values
|
VEGF (pg/mL) |
|||||
|
Mean |
SD |
Median |
IQR |
P value |
|
|
Control |
67.04 |
10.94 |
62.41 |
28.81 |
|
|
CRVO |
187.88 |
77.56 |
141.08 |
2.83 |
p<0.05 |
|
BRVO |
82.94 |
5.89 |
85.17 |
13.31 |
p=0.072 |
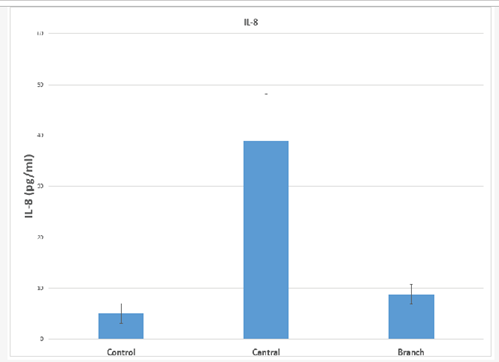
Mean IL-8 value in the control sample was 10.60+25.24 pg/ml. Mean aqueous humour levels in patients with CRVO was 33.58+27.34 pg/ml, while that in patients with BRVO was 10.35+18.38 pg/ml. Median values were 5.05 pg/ml in controls, 38.98 pg/ml in CRVO and 8.85 pg/ml in BRVO. Table 2 and Figure 2 show the results for IL-8 levels.
Statistical analysis using the Friedman test was employed on median values. The levels of IL-8 in CRVO and BRVO were higher than controls and this difference was statistically significant (p=0.03 and p=0.017 respectively). Levels were higher in CRVO as compared to BRVO and this difference, too, was statistically significant (p<0.05).
In Central Retinal Vein Occlusion, there were some samples that predominantly showed that VEGF is the dominant biomarker (CR3 & CR7). There were other samples where IL-8 was a more dominant biomarker. Figure 3 shows a bar chart demonstrating this.
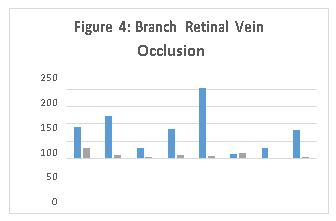
Similarly in Branch Retinal Vein Occlusion, there were some samples that predominantly showed that VEGF is the dominant biomarker (CR6). There were other samples where IL-6 (BR6) or IL-8 (BR2) was a more dominant biomarker as demonstrated in Figure 4.
Table 2: IL-8 values in pg/mL
|
IL-8 (pg/mL) |
|||||
|
Mean |
SD |
Median |
IQR |
P value |
|
|
Control |
10.6 |
25.24 |
5.05 |
1.97 |
|
|
CRVO |
33.58 |
27.34 |
38.98 |
9.13 |
P<0.05 |
|
BRVO |
10.35 |
18.38 |
8.85 |
1.97 |
P<0.05 |
Discussion
Mean VEGF values in the control samples was 67.04+10.94 pg/ml with a median value of 62.41 pg/ml. These values are very similar to the control values obtained by Noma et al [25] where they reported mean control values of 62.4 pg/ml. These values were higher than the 40.4 pg/ml reported by Funk et al [32] in the same year. Control values of VEGF seem very variable, ranging from as low as 15.6 pg/ml reported by Noma et al [27], 112 pg/ml by Park et al [22], 119 pg/ml by Noma el al [20] to as high as 126.61 pg/ ml by Shchuko et al [23]. It was interesting to note that the same research team, Noma et al [20, 21, 27] have reported varying control values in different published articles (Table 3). One possible explanation is that each laboratory may have slightly different processes in place for analysis of samples and different standard reagents. Therefore comparison of aqueous level of biomarkers in each published paper needs to be measured against their own control values. Mean aqueous humour VEGF levels in our patients with BRVO was 82.94+5.89 pg/ml while that in patients with CRVO was 187.88+77.56 pg/ml. Median values were 85.17 pg/ml in BRVO and 141.08 pg/ml in CRVO. Our results are in keeping with this upward trend of increased levels of VEGF in BRVO and still higher levels which have consistently been reported by many authors (Table 3). The levels of VEGF in CRVO were more than twice the level of controls and this difference was statistically significant (p<0.05). Although the median levels of VEGF in BRVO (85.17 pg/mL) were higher than controls (62.41 pg/mL), this difference in the levels of VEGF in controls versus was not statistically significant (p=0.072). However on comparing the difference between the VEGF levels in CRVO versus BRVO, the levels were statistically higher in CRVO as compared to BRVO (p<0.05). This trend of levels of VEGF being higher in CRVO as compared to BRVO has been noted by several previous researchers [23, 32].
RVO is associated with breakdown of blood retinal barrier and increased vascular permeability. Damaged retinal vascular endothelial cells leads to intra-luminal thrombus formation and areas of retinal non-perfusion [11]. This leads to upregulation of VEGF. The area of retinal ischemia is larger in CRVO. This fits in well with the trend noted in our study where VEGF levels were higher in CRVO as compared to BRVO.
Table 3: Trend in aqueous levels of VEGF
|
VEGF levels in pg/ml |
|||
|
Control |
BRVO |
CRVO |
|
|
Funk et al (2009) |
40.4 |
106.9 |
351.8 |
|
Noma et al (2005) |
119 |
351 |
|
|
Noma et al (2009) |
62.4 |
435 |
|
|
Noma et al (2013) |
15.6 |
83.3 |
|
|
Park et al (2010) |
112 |
328 |
|
|
Shchuko et al (2015) |
126.61 |
919 |
1725 |
|
Current study (2022) |
67.04 |
82.94 |
187.88 |
Table 4: Trend in aqueous levels of IL-8
|
IL-8 levels in pg/ml |
|||
|
Control |
BRVO |
CRVO |
|
|
Funk et al (2009) |
1.8 |
12.9 |
114.9 |
|
Shchuko et al (2015) |
30.1 |
55.41 |
181.27 |
|
Current study (2022) |
5.05 |
8.85 |
38.98 |
Mean IL-8 value in the control samples was 10.60+25.24 pg/ml and a median value of 5.05 pg/ml. These levels are much higher than values reported by Funk et al [32] of 1.8 pg/ ml, but only about a third of the reported 30.1 pg/ml published by Shchuko et al [23]. Mean aqueous humour levels of IL-8 in our patients with BRVO was 10.35+18.38 pg/ml and those with CRVO was 33.58+27.34 pg/ml. Median values were 8.85 pg/ml in BRVO and 38.98 pg/ml in CRVO. This difference was statistically significant (p<0.05). In general, a similar increasing trend from controls to BRVO, increasing further in CRVO, was reported by Funk et al [32] Shchuko et al [23]. (Table 4). IL-8 is a pro-inflammatory cytokine. Peripheral retinal ischaemia leads to the release of pro-inflammatory cytokines. Larger the areas of the retina involved in the retinal vein occlusion, higher the aqueous and vitreous levels of IL-8 [3, 21]. This would explain the trend of higher levels of IL-8 in CRVO as compared to BRVO. Clinically too, patients with CRVO have greater visual impairment in CRVO as compared to BRVO and in general tend to have a poorer prognosis [6].
The difference in the levels of VEGF between controls and CRVO was statistically significant (p=0.017). Comparison of VEGF levels in BRVO with that of controls showed that although the levels of VEGF in BRVO were slightly higher than controls, this was not statistically significant (p=0.072). Concentration of VEGF in CRVO was significantly higher than in BRVO (p<0.05). This indicates that VEGF is an important biomarker in RVO and that larger the area of retina involved in the vascular occlusion, higher the levels of VEGF [18, 19]. Previous studies are in keeping with this observation [23, 32]. Studies have also shown a significant decrease in the aqueous levels of VEGF following treatment with intra- vitreal injections of anti-VEGF agents like Bevacizumab [32], Ranibizumab or Aflibercept [10]. The difference in IL-8 concentration between BRVO and CRVO was also found to be statistically significant (p=0.017) with levels in CRVO much higher. This indicates that, apart for retinal ischaemia, inflammation plays an important role [3]. It is on this basis that intra-vitreal implantation of long-acting Dexamethasone is an NICE approved treatment for macular oedema secondary to RVO [12]. The literature search has shown that biomarker levels reported in controls, BRVO and CRVO are all widely variable. For example, VEGF levels ranged from 15.6 pg/ml to as high as 126.61 pg/ml. Therefore no single normative value can be used as a yardstick for comparing with values in RVO. One can surmise that the variation in values is probably related to the technique of testing, specific commercially available assay kits or perhaps the brand of analyser used. However, all reported studies, including our own, clearly show a consistent trend where VEGF levels and those of pro-inflammatory cytokine IL-8 are higher in retinal vein occlusion. Levels in CRVO and consistently higher than levels in BRVO. The study has shown the feasibility of performing biomarker analysis on small aqueous samples locally. The future direction of the research would be to increase sample size and perhaps make biomarker analysis part of routine management of patients with RVO. Modi et al [34] have recently shown in a case report that an individualised treatment plan based on biomarker testing may be particularly useful in patients where first line management fails. Our analysis included only 24 samples. Due to the small sample size, the results perhaps did not follow a normal distribution necessitating the use of non-parametric statistical methods. There is clear evidence that while some patients may present with predominantly increased aqueous VEGF levels, others may present with raised inflammatory markers. This indicates that some patients who might respond better to an anti-VEGF agent whereas others may respond better to a steroid. Pre- treatment aqueous sampling and testing for biomarkers can guide clinicians to choose one of the above treatment options rather than merely trying one agent and switching to the other if the first agent does not work. Both anti-VEGF agents and long-acting steroids are expensive drugs, which need to be administered regularly with patients needing monitoring and treatment 6-8 times in the first year and 4-6 times in the second year. Formulating an individualised treatment plan and choosing what is likely to be the most effective treatment will not only help from patient perspective by preserving vision, but from a health economics point of view, would be a cost saving measure. This cost saving will, of course, be offset by the laboratory cost of biomarker assay. Since several samples can be assayed simultaneously, a cost effective model would be to centralise the laboratory services so that aqueous samples from several hospitals can be pooled and analysed in a single run. Future direction of the study could be to have a larger sample size and expand the range of biomarkers. It is possible that each individual case may present with alteration of concentration of some biomarkers and not others, both pro-inflammatory and anti-inflammatory biomarkers. Some studies have analysed up to 27 biomarkers including IL- 1β, IL-6, IL-8, IL-12, IL15, IFN-γ, TNF-α, MCP-1, and RANTES. Commercially available test kits are capable to assaying several biomarkers simultaneously without the need to increase sample volume.
Conclusion
We identified that aqueous levels of VEGF and inflammatory bio-markers are statistically significantly higher in patients with RVO as compared to controls. Furthermore, our study also demonstrated that the levels were significantly higher in CRVO as compared to BRVO. Ultimately, pre- treatment assessment of biomarkers may help clinicians chose between anti-VEGF and steroids as first line treatment.
Funding
Funding for the research was through a Pathway to Portfolio grant by the Academic Unit of Betsi Cadwaladr University Health Board.
Conflicts of Interest
The authors declare no conflict of interest
References
- Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous haemorrhage in branch vein occlusion. A randomized clinical trial. Branch Vein Occlusion Study Arch Ophthalmol 104 (1986): 34-41.
- The Royal College of Ophthalmologists: Retinal Vein Occlusion Guidelines (2015).
- Noma H, Yasuda K, Shimura Cytokines and pathogenesis of central retinal vein occlusion. J Clin Med 9 (2020): 3457.
- Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 113 (1995): 1538–1544.
- Yong H, Qi H, Yan H, Wu Q, Zuo L. The correlation between cytokine levels in the aqueous humor and the prognostic value of anti-vascular endothelial growth factor therapy for treating macular edema resulting from retinal vein occlusion. Graefe's Arch Clin Experimental Ophthalmol 259 (2021): 3243-3250.
- Ho M, Liu DT, Lam DS, & Jonas Retinal vein occlusions, from basics to the latest treatment. Retina 36 (2016): 432-448.
- Noma H, Yasuda K, Shimura Cytokines and the pathogenesis of macular edema in branch retinal vein occlusion. J Ophthalmol (2019).
- Central Vein Occlusion Study A Randomized Clinical Trial of Early Pan-retinal Photocoagulation for Ischaemic Central Vein Occlusion: The Central Vein Occlusion Study Group N Report. Ophthalmol 102 (1995): 1434-1444.
- Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor in intraocular vascular disease. Ophthalmol 120 (2013): 106-114.
- Kotake O, Noma H, Yasuda K, Motohashi R, Goto H. Comparing cytokine kinetics between Ranibizumab and Aflibercept in central retinal vein occlusion with macular edema. Ophthalmic Res 61 (2019): 210-217.
- Liang Correlation analysis of IL-6 and MCP-1 concentration in aqueous humor with retinal vein occlusion-macular edema. Int Eye Sci (2020): 1206-1210.
- TA229: Dexamethasone intravitreal implant for the treatment of macular oedema secondary to retinal vein occlusion: NICE (2011).
- TA283: Ranibizumab for treating visual impairment caused by macular oedema secondary to retinal vein NICE (2013).
- TA305: Aflibercept for treating visual impairment caused by macular oedema secondary to central retinal vein NICE (2014).
- TA409: Aflibercept for treating visual impairment caused by macular oedema after branch retinal vein occlusion. NICE (2016).
- Michaelson The mode of development of the vascular system in the retina: With some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK 68 (1948): 137-180.
- Mirshahi A, Hoehn R, Lorenz K, Kramann C, & Baatz
- Anti-tumor necrosis factor alpha for retinal diseases: current knowledge and future concepts. J Ophthalmic & Vision Res 7 (2012): 39.
- Stone J, Itin A, Alon T, Pe'er J, Gnessin H, et Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 15 (1995): 4738-4747.
- Pe’er J, Folberg R, Itin A, Gnessin H, Hemo I, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmol 105 (1998): 412-416.
- Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol 140 (2005): 256-261.
- Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, et Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye 22 (2008): 42-48.
- Park S P, Ahn J K, & Mun G Aqueous vascular endothelial growth factor levels are associated with serous macular detachment secondary to branch retinal vein occlusion. Retina 30 (2010): 281-286.
- Shchuko AG, Zlobin IV, Iureva TN, Ostanin AA, Chernykh ER, et al. Intraocular cytokines in retinal vein occlusion and its relation to the efficiency of anti-vascular endothelial growth factor therapy. Indian J Ophthalmol 63 (2015): 905.
- Hayasaka S, Nagaki Y, Matsumoto M, Sato S. Interferon associated retinopathy. British J Ophthalmol 82 (1998): 323-325.
- Noma H, Funatsu H, Mimura T, Harino S, Hori Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmol 116 (2009): 87-93.
- Jiang WG, Sanders AJ, Ruge F, Harding Influence of interleukin-8 (IL-8) and IL-8 receptors on the migration of human keratinocytes, the role of PLC-γ and potential clinical implications. Experimental Therapeutic Med 3 (2012): 231-236.
- Noma H, Mimura T, Tatsugawa M, Shimada K. Aqueous flare and inflammatory factors in macular edema with central retinal vein occlusion: a case BMC Ophthalmol 13 (2013): 78.
- Suzuki Y, Nakazawa M, Suzuki K, Yamazaki H, Miyagawa Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Japanese J Ophthalmol 55 (2011): 256-263.
- Ip M, Hendrick Retinal vein occlusion review. The Asia-Pacific J Ophthalmol 7 (2018): 40-45.
- Jackson TL, Kirkpatrick Cost comparison of Ranibizumab and bevacizumab. BMJ 343 (2011).
- Hollingworth W, Jones T, Reeves BC, Peto A longitudinal study to assess the frequency and cost of antivascular endothelial therapy, and inequalities in access, in England between 2005 and 2015. BMJ open 7 (2017): 018289.
- Funk M, Kriechbaum K, Prager F, Benesch T, Georgopoulos M, et Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Investigative Ophthalmol & Visual Sci 50 (2009): 1025-1032.
- Kaneda S, Miyazaki D, Sasaki SI, Yakura K, Terasaka Y, et al. Multivariate analyses of inflammatory cytokines in eyes with branch retinal vein occlusion: relationships to bevacizumab treatment. Investigative Ophthalmol & Visual Sci 52 (2011): 2982-2988.
- Modi A, Sharma K, Sudhakar NP, Yadav NK. Aqueous Humor Cytokines and Therapeutic Customization in Nonresponding Macular Edema Secondary to Retinal Vein Occlusion. Retinal Cases and Brief Rep 15 (2021): 127-130.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 