COVID-19 and Autoimmunity: A Single Center Prospective Follow-up Study
Maria Cristina Sacchi1*, Stefania Tamiazzo1, Ramona Bonometti2, Paolo Stobbione3, Lisa Agatea4, Piera De Gaspari4, Ernesto Cristiano Lauritano2, Antonio Maconi5, Maria Matilde Ciriello1
1Autoimmunology and Analysis Laboratory Unit, “SS Antonio e Biagio e Cesare Arrigo” Hospital, Alessandria, Italy
2Emergency Medicine Unit, SS Antonio e Biagio e Cesare Arrigo” Hospital, Alessandria, Italy
3Rheumatology Unit, “SS Antonio e Biagio e Cesare Arrigo” Hospital, Alessandria, Italy
4Laboratory department, Affiliated to Euroimmun, Padova, Italy
5IRFI (Infrastruttura Ricerca Formazione Innovazione) “SS Antonio e Biagio e Cesare Arrigo” Hospital, Alessandria, Italy
*Corresponding author: Maria Cristina Sacchi, Autoimmunology and Analysis Laboratory Unit, “SS Antonio e Biagio e Cesare Arrigo” Hospital, Alessandria, Italy
Received: 09 December 2021; Accepted: 20 December 2021; Published: 28 December 2021
Article Information
Citation: Sacchi MC, Tamiazzo S, Bonometti R, Stobbione P, Agatea L, De Gaspari P, Lauritano EC, Maconi A, Ciriello MM. COVID-19 and Autoimmunity: A Single Center Prospective Follow-up Study. Archives of Clinical and Biomedical Research 5 (2021): 1018-1026.
View / Download Pdf Share at FacebookAbstract
Background Various factors, such as viral infections, can act as triggers for the development of autoimmune diseases. In our recent study we reported the presence of autoantibodies in patients diagnosed with COVID-19. To verify whether these autoantibodies persisted over time and led to the development of chronic autoimmune diseases, we conducted a follow-up study at 3 and 6 months after admission.
Methods Thirteen of 40 patients enrolled in the previous study gave their consent to the analysis. The same autoimmunity tests performed at the time of diagnosis were carried out in these patients during follow up.
Results We showed, compatibly with an acute inflammatory response, that two patients were negative 6 months after diagnosis. In nine patients, autoantibodies were still present at follow-up. Among them, one patient had only ENA positivity. Another patient developed strong positivity for ANA and M2-β and Ku antigens. Currently, the symptoms do not meet full diagnostic criteria for diagnosis of polymyositis, but the patient is closely monitored to check its possible onset. Three patients developed: transient alopecia, autoimmune thrombocytopenia and hearing loss. Other four patients did not show any clinical symptoms.
Conclusions In conclusion, our data show that after 6 months, the autoantibodies are still present in the majority of patients. Further investigations will be necessary to check whether these patients will become negative over time or may develop clinical symptoms compatible with the onset of longer-term chronic autoimmune diseases.
Keywords
<p>ANA; Autoantibodies; Autoimmunity; COVID-19; Follow-up; Virus infection</p>
Article Details
Abbreviations:
AIED: Autoimmune Inner Ear Disease; ANA: Anti Nucleus Antibodies; ANCA: Anti-Neutrophil Cytoplasmatic Antibodies; ASCA: Anti Saccharomyces Cerevisiae Antibodies; BPCOD: Bronchopulmonary Chronic Obstructive Disease; C3: Complement 3; C4: Complement 4; COVID-19: Corona Virus Disease 2019; CTD: Connective Tissue Disease; ENA: Anti Extractable Nuclear Antigens; HIV: Human Immunodeficiency Virus;
IL6: Interleukin-6; LDH: Lactate Dehydrogenase; MERS: Middle East Respiratory Syndrome; MPO: Myeloperoxidase; PCR: Protein Reactive C; PR3: Proteinase 3; SARS: Severe Acute Respiratory Syndrome; SARS-CoV-2: Severe Acute Respiratory Syndrome Corona Virus 2.
1. Background
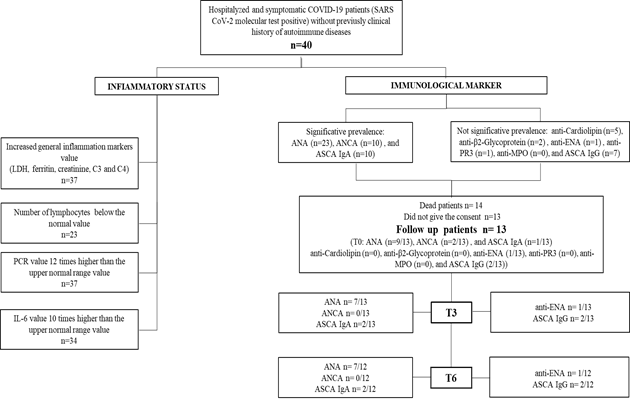
SARS-CoV-2 (Severe Acute Respiratory Syndrome Corona Virus 2) is the agent responsible for the acute respiratory disease named COVID-19 (Corona Virus Disease 2019). The severity of the pathologies deriving from coronavirus infections is very variable, since these viruses are responsible for both some common cold syndromes and severe respiratory syndromes such as SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome) [1-3]. In the case of COVID-19 patients, the main symptomatology is similar to a flu-like illness (e.g. fever, cough, fatigue), but in some cases it can evolve into severe clinical pictures, leading to multiorgan dysfunction syndrome and even leading to death [4-6]. It has been observed that the evolution of SARS-CoV-2 infection seems to have similar characteristics to the cytokine release syndrome [7,8]. These data were confirmed also in a study that we recently published, where we showed how increased inflammatory markers values (i.e. IL6) are related to a worst clinical outcome and could be considered a reliable diagnostic criterion to identify subjects with a worst prognosis [9]. In addition to the inflammatory response, it has been shown that in COVID-19 patients the presence of the virus could affect the autoimmunity too. Although the etiopathogenesis of autoimmune diseases is very complex and the underlying mechanisms have not been fully described yet, it is known how different infectious, bacterial, viral, or fungal agents can represent a trigger for the development of autoimmune diseases [10].
Recent works have described the presence of different types of autoantibodies in patients with COVID-19 [11-14]. Furthermore, different studies have been reported suggesting a possible association between SARS-CoV-2 infection and the development of some autoimmune diseases such as systemic lupus erythematosus [15], Guillain-Barré syndrome, Miller Fisher syndrome, immune thrombocytopenia purpura and Kawasaki disease [16]. However, up to now, the data reported are still controversial. Thus, it is essential to carry out follow-up studies to understand whether the autoantibody presence can be explained as an epiphenomenon, a cross-reaction, a pre-existing undiagnosed pathology, or a new autoimmune disease triggered by COVID-19. During the first wave of the SARS-CoV-2 pandemic, we investigated the autoimmune pattern of hospitalized patients with a severe clinical picture; to check a possible correlation between autoantibody presence and clinical phenotype. We thus enrolled 40 patients between 20 and 97 years (70% male and 30% female) who had been admitted to the sub-intensive medicine ward. None of these patients had a history of autoimmune disease or related symptoms prior to admission. The most common autoantibodies were studied in these patients and a correlation between SARS-CoV-2 infection and the development of some of them was highlighted. In particular, the statistically significant ones were anti-nucleus antibodies (ANA), atypical anti-neutrophil cytoplasmatic antibodies (X-ANCA) and anti-Saccharomyces cerevisiae antibodies (ASCA IgA). Therefore, in our study, we highlighted that there is a correlation between the response to SARS-CoV-2, the presence of autoantibodies and the clinical outcome [9]. To find out whether the autoantibody presence was transient, persisted over time and possibly correlated with the development of autoimmune diseases, we performed a follow-up study on 13 of the 40 patients initially enrolled. Patients were tested at 3 (t3) and 6 (t6) months from hospitalization for the same autoantibodies considered at admission (t0). This work allowed us to observe that most patients maintained an altered autoantibody profile after 6 months.
2. Methods
2.1 Study design
Thirteen convalescent COVID-19 patients already analysed in a previous study [9] were enrolled for a follow-up analysis. The considered time points were 3 (t3) e 6 (t6) months. The patients were aged between 19 and 77 years and were 6 men and 7 women. All patients who participated to the study signed informed consent.
2.2 Blood autoimmunity tests
The autoantibodies considered were analysed as described in the previous study [9]. Briefly, antiphospholipid antibodies were detected using chemiluminescent assay (ACL AcuStar, Instrumentation Laboratory). Anti-Saccharomyces cerevisiae antibody (ASCA IgA and IgG), Proteins Myeloperoxidase (MPO), Proteinase 3 (PR3), Connective Tissue Disease panel were analysed by Fluorescence Enzyme Immunoassay (FEIA) (Phadia 250-Thermoscientific).
Anti-neutrophil Cytoplasmic (ANCA) and Anti-nuclear Antibody (ANA) were detected by indirect Immunofluorescence (IIF) using EUROIMMUN test kits. Confirmatory tests were performed by line-blot technology following the manufacturer's instructions (EUROIMMUN).
2.3 Statistical Analyses
Excel software was used to evaluate the presence of autoantibodies during the follow up.
3. Results
The inflammatory and autoimmune spheres can be influenced by various factors (i.e. virus) and they can change over time [17]. In our recent work, we observed how SARS-CoV-2 infection is also linked to these status [9]. In the study we analysed 40 patients from both an inflammatory and immunological point of view, highlighting modifications of the most common markers of inflammation. Furthermore, as regards the autoimmune profile, a significant prevalence of autoantibodies to ANA, X-ANCA, ASCA IgA was observed (Figure 1). Taking in account that the autoimmune response alterations could be transitory or persist longer and lead to chronic autoimmune disease, we decide to perform a follow-up study. Thirteen patients of the previous study were analysed at 3- and 6-months post hospitalization for the same autoantibodies considered at t0. The 13 patients considered had an age between 19 and 77 years old (average= 55.69 years). None of the 13 patients was affected, in the last five years, by the following diseases: coronary disease, cerebrovascular disease, hepatitis B infection, cancer, immunodeficiency, ischemic heart disease, ictus, dementia, chronic liver disease, HIV infection. The mean hospitalization time was 22.54 days. At the time of discharge, 3 patients (P1, P6, P9) were diagnosed with COVID-19 interstitial pneumonia, 7 patients (P2, P4, P7, P8, P11, P12, P13) were dismissed having severe respiratory failure derived from COVID-19 bilateral interstitial pneumonia. Two of these 7 (P11, P13) presented severe respiratory failure type 1, whereas another (P12) had also thrombocytopenia most probably as consequence of heparin administration. One patient (P5) had immune thrombocytopenic purpura and another one (P10) was defined to have only interstitial anomalies. For one patient we did not have the diagnosis at the discharge (Figure 3).
Concerning the autoimmune sphere, 9/13 patients had ANA antibodies at t0 (Figure 2 table a). A patient (P3), negative at t0, was tested again after 16 days after admission and she resulted positive for ANA, with a cytoplasmatic and granular pattern (1:320). The same patient at t3 showed a combination of granular, cytoplasmic and centriolar pattern, however the granular pattern disappeared at t6. In addition, immunoblot analysis performed at all three time points confirmed the presence of M2-β and Ku autoantibodies. In total, 6 patients (P3, P4, P5, P6, P8 and P13) maintained the positivity during the follow-up, both at t3 and t6. P4 showed a granular pattern at t0 (1:160) and homogeneous pattern (1:80) was also added to t3 and t6 (1:80). P5 showed a centriolar pattern (1:160) which was maintained at follow-up. Furthermore, she was positive at the CTD screen. P6 showed a mid-body and mitotic spindle pattern which was maintained at follow-up. P8 had homogeneous ANA pattern (1:320). P13 showed at t0 a cytoplasmic pattern (1:160) which was maintained at t3 (1:80) and t6 (1:160), furthermore at t6 he also showed a nucleolar pattern (1:160). Three patients (P7, P9, 10), showing at t0 a cytoplasmic pattern, became negative at t3 and t6. A patient (P12) was positive only at t0 and t3, although the pattern changed slightly from cytoplasmic-granular, at t3 it was detected only the cytoplasmic one. Two patients (P1, P2) were negative at t0 and remained negative also during the follow-up. One patient (P11) developed ANA at t3 showing a homogeneous pattern and unfortunately it was not possible to test the patient at t6. Regarding the other autoantibodies (Figure 2 table b), 1 patient (P1) positive for ENA at t0, he was positive also during the follow-up. Two patients (P9 and P10) had also X-ANCA at t0 and one of them (P10) was also positive for ASCA IgG autoantibodies at all the three time points, whereas ASCA IgA were borderline at t0 and then positive at t3 and t6. A patient (P11) was positive for ASCA IgA and IgG only at t0, whereas another patient (P12) developed these autoantibodies during the follow-up.None of the 13 patients showed the presence of the following autoantibodies: anti-β2glicoprotein, anti-cardiolipin, anti PR3, anti MPO (data not shown).
|
a. Antinuclear antibodies (ANA)
b. Other autoantibodies
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Figure 2: Detection of autoantibodies during follow-up in 13 COVID-19 patients.
All the 13 patients were analysed for the following autoantibodies: ANA, ENA, X-ANCA, ASCA IgA, ASCA IgG when admitted to the hospital (t0) and during follow-up after 3 months (t3) and 6 months (t6). a. The table reports the ANA pattern found in each patient at the different time points. b. The table includes the results of the other autoantibodies considered in the study.
(n.d. = not determined; * = the patient developed ANA autoantibodies after 16 days; b.l.= borderline; + = positive; - = negative).
|
Age (years) |
Gender |
Coexisting disorder on admission |
H (days) |
Diagnosis at discharge |
|
|
P1 |
59 |
M |
NO |
10 |
COVID-19 interstitial pneumonia |
|
P2 |
64 |
F |
NO |
22 |
severe respiratory failure derived from COVID-19 bilateral interstitial pneumonia |
|
P3 |
72 |
F |
BPCOD, diabetes, chronic renal disease, atrial fibrillation, obesity, monoclonal gammopathy, tricuspid insufficiency, severe moderate cardiac decompensation |
26 |
n.d. |
|
P4 |
53 |
F |
bronchial asthma, migraine, venous thrombosis |
5 |
severe respiratory failure derived from COVID-19 bilateral interstitial pneumonia |
|
P5 |
19 |
F |
NO |
5 |
immune thrombocytopenic purpura |
|
P6 |
67 |
F |
hypertension |
15 |
COVID-19 interstitial pneumonia |
|
P7 |
36 |
M |
hypertension |
43 |
severe respiratory failure derived from COVID-19 bilateral interstitial pneumonia |
|
P8 |
59 |
F |
hypertension |
17 |
severe respiratory failure derived from COVID-19 bilateral interstitial pneumonia |
|
P9 |
48 |
M |
NO |
42 |
COVID-19 interstitial pneumonia |
|
P10 |
59 |
M |
NO |
52 |
interstitial anomalies |
|
P11 |
77 |
F |
hypertension, previous breast cancer, glaucoma |
16 |
respiratory failure type I derived from COVID-19 bilateral interstitial pneumonia |
|
P12 |
55 |
M |
right renal colic, neuroma C3-C4; lumbar discopathy |
22 |
severe respiratory failure derived from COVID-19 bilateral interstitial pneumonia and thrombocytopenia |
|
P13 |
63 |
M |
asthma |
18 |
respiratory failure type I derived from COVID-19 bilateral interstitial pneumonia |
Figure 3: Clinical picture of the 13 patients observed during follow-up.
The table highlights the age, the gender, the coexisting disorders on admission, the time of hospitalization (days) and the diagnosis at discharge of the patients.
In details, one patient (P3) had clinical record of different pathologies such as: BPCOD, diabetes, chronic renal disease, atrial fibrillation, obesity, monoclonal gammopathy, tricuspid insufficiency, severe moderate cardiac decompensation. Another patient (P12) had right renal colic, neuroma C3-C4 and lumbar discopathy. One patient (P13) had asthma. 4 patients (P6, P7, P8) presented hypertension and one of them (P11) had glaucoma and previous breast cancer history. 5 patients (P1, P2, P5, P9, P10) did not have any coexisting disorders on admission.
At discharge 10 patients (P1, P2, P4, P6, P7, P8, P9, P11, P12, P13) were diagnosed with COVID-19 Interstitial Pneumonia. One patient (P5) had immune thrombocytopenic purpura, one (P10) had only interstitial anomalies and in one case it was not possible to define the diagnosis at the discharge (P3).
4. Discussion
It is known that, in some patients, SARS-CoV-2 infection is able of triggering a strong and harmful immune response [17]; however, the pathogenetic mechanism is not yet fully understood. Some COVID-19 patients may have debilitating symptoms that persist for many months after the initial infections (Long COVID Syndrome) [18].
In this perspective, it is fundamental to monitor COVID-19 patients after viral infection, over long period, to understand the possible clinical outcome. Recently, we reported a case of 85-year-old woman that, after SARS-CoV-2 infection, developed thrombocytopenia, pleural effusion, proteinuria and presented decrease complement level [15]. The patient had ANA (cytoplasmic (1: 160), homogeneous (1: 320) and granular (1: 320) patterns), Ku positivity and X-ANCA. The listed above, are the essential criteria to diagnose systemic lupus erythematosus [19].
These data further support how clinical follow-ups become fundamental tools to observe the possible development of chronic autoimmune diseases in COVID-19 patients having autoimmune alterations.
Therefore, we decided to perform a prospective study on a group of patients enrolled in our previous study, having an altered autoimmune profile on admission (t0). The idea of our follow-up study was to check if the autoimmune changes found resolved at the end of the viral infection or persisted even after its resolution. Our research has shown that only 2 patients (P7 and P9) 6 months after diagnosis were negativized, confirming the fact that it could be a response related to acute infection. On the other hand, a patient who at t0 had a completely negative autoimmune profile, re-evaluated after 16 days, as he continued to be dependent on artificial oxygenation, had ANA antibodies and positivity for M2-β and Ku antigens which also persisted at t3 and t6 (P3). From a clinical point of view, this patient still shows a persistent muscular asthenia at the level of the trunk, but further investigations are currently on going to check the possible onset of polymyositis [20].
Other patients showed symptoms unrelated to the presence of autoantibodies. For example, P8 showed a transient episode of alopecia that can be due to an acute post COVID-19 telogen effluvium and unrelated to alopecia areata generally associated with autoimmune conditions [21]. P4, P6, P13, despite maintaining a positivity to the ANA test, did not show symptoms compatible with connective tissue disease, such as xerostomia, xerophthalmia, oral ulcers, arthritis, synovitis, or skin alterations. P5 presented a positivity to ANA with a centriolar pattern. The young patient developed an initial autoimmune thrombocytopenia that was treated with high dose steroids and immunoglobulins [22]. P12 developed hearing loss during follow-up. Many traditional autoimmune diseases can cause or be associated with autoimmune deafness. The Autoimmune Inner Ear Disease (AIED) has been defined as a condition of a bilateral sensorineural hearing loss, caused by an altered immune system response. AIED is considered primary when the inner ear is the only organ affected; in up to 30% of cases, AIED is secondary to systemic autoimmune disease such as systemic lupus erythematosus, rheumatoid arthritis, Sjögren’s syndrome or Wegener’s granulomatosis [23]. It will therefore be essential to monitor the patient in the long term to determine the cause of hearing loss. In the remaining subjects, despite the persistence of autoantibody positivity, even at high titers, no clinical correlation or development of autoimmune pathologies was found. In accordance with the literature data, the mere presence of autoantibodies has no pathogenic value [24]. However, it is important to follow these patients over time to understand if they will negativize or if in the long term they may develop clinical symptoms.
5. Conclusion
In conclusion, our study is the first to investigate the persistence of autoantibodies in COVID-19 patients over time and highlights how most of the enrolled population maintains an alteration of the autoantibody structure even after 6 months from acute infectious event. Although the number of analysed cases is small, our results suggest the importance of monitoring the presence of autoantibodies over a longer period to evaluate the possible development of autoimmune diseases.
References
- Du Toit A. Outbreak of a novel coronavirus. Nat Rev Microbiol (2020).
- Rothan AH, Byrareddy SN. The Epidemiology and Pathogenesis of Coronavirus diseases (Covid-19) outbreak. J Autoimmun (2020).
- Hu B, Guo H, Zhou P, et al. Characteristics of SARS-COV-2 and CoVID-19. Nat Rev Microbiol 2020.
- Mokhtari T, Hassani F, Ghaffari N, et al. Covid-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol 51 (2020): 613-628.
- Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 26 (2020): 1017-1032.
- Ciaccio M, Agnello L. Biochemical biomarkers alterations in Coronavirus Disease 2019 (COVID-19). Diagnosis 7 (2020): 365-372.
- Chen LYC, Quach TTT. COVID-19 Cytokine storm syndrome: a threshold concept. Lancet 2 (2021): 49-50.
- Chen G, Wu D, Guo W, et al. Clinical and Immunological features of severe and moderate Coronavirus disease. J Clin Invest 130 (2020): 2620-2629.
- Sacchi MC, Tamiazzo S, Stobbione P, et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci 14 (2021): 898-907.
- Pesce G, Saverino D, Bagnasco M. Epidemiologia delle malattie autoimmuni organo-specifiche. In: R. Tozzoli R, Bizzaro N, Villalta D, Tonutti E, editors. Il Laboratorio nelle malattie autoimmuni d’organo (2009): 14-36.
- Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 382 (2020): 38.
- Vlachoyiannopoulos PG, Magira E, Alexoopoulos H, et al. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis 79 (2020): 1661-1663.
- Pascolini S, Vannini A, Deleonardi G, et al. COVID-19 and immunological dysregulation: can autoantibodies be useful?. Clin Transl Sci 14 (2021): 502-508.
- Liu Y, Sawalha A, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol 33 (2021): 155-162.
- Bonometti R, Sacchi MC, Stobbione P, et al. The first case of systemic lupus erythematous (SLE) trigger by COVID19 infection. Eur Rev Med Pharmacol Sci 24 (2020): 9695-9697.
- Ehrenfelda M, Tincanic A, Andreoli L, et al. Covid-19 and autoimmunity. Autoimmun 19 (2020): 102597.
- Catanzaro M, Fagiani F, Racchi M, et al. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Sig Transduct Target Ther 5 (2020): 84.
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet (2021).
- Aringer M. EULAR/ACR classification criteria for SLE. Semin Arthritis Rheum (2019).
- Sacchi MC, Tamiazzo S, Lauritano EC, et al. Case report of COVID-19 in an elderly patient: could SARS-CoV2 trigger myositis?. Eur Rev Med Pharmacol Sci 24 (2020): 11960-11963.
- Forouzan R, Cohen PR. Incipient diabetes mellitus and nascent thyroid disease presenting as beard alopecia areata: case report and treatment review of alopecia areata of the beard. Cureus 12 (2020): 9500.
- Molinaro E, Novara E, Bonometti R, et al. Isolated immune thrombocytopenic purpura in a young adult Covid-19 patient. Eur Rev Med Pharmacol Sc 24 (2020): 10850-10852.
- Ciorba A, Corazzi B, Bianchini C, et al. Autoimmune inner ear disease (AIED): a diagnostic challenge. Int J Immunopathol Pharmacol (2018).
- Kang EH, Ha YJ, Lee YJ. Autoantibody Biomarkers in Rheumatic Disease. Int J Mol Sci 24 (2020): 1382.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 