Influence of NUDT15 Genotyping on Dose Intensity of Thiopurine Administration and Long-Term Clinical Outcomes (Hospitalization and Surgery)
Shotaro Tsunoda1, Yuichiro Kojima1*, Yosuke Hirotsu2, Hiroyuki Amano1, Yuko Miura1, Hiroshi Ashizawa1, Hiroshi Ohyama1, Kenji Hosoda1, Yoji Suzuki1, Hitoshi Mochizuki1,2, Shin Maeda3, Masao Omata1,2,4
1Department of Gastroenterology , Yamanashi Prefectural Central Hospital, Yamanashi, Japan
2Genome Analysis Center, Yamanashi Prefectural Central Hospital, Yamanashi, Japan
3, Department of Gastroenterology, Yokohama City University, Graduate School of Medicine, Yokohama, Japan
4 University of Tokyo, Tokyo, Japan
*Corresponding author: Yuichiro Kojima, Department of Gastroenterology, Yamanashi Prefectural Central Hospital, 1-1-1 Fujimi, Kofu, Yamanashi 400-8506, Japan.
Received: 17 December 2023; Accepted: 22 December 2023; Published: 31 January 2024
Article Information
Citation: Shotaro Tsunoda, Yuichiro Kojima, Yosuke Hirotsu, Hiroyuki Amano, Yuko Miura, Hiroshi Ashizawa, Hiroshi Ohyama, Kenji Hosoda, Yoji Suzuki, Hitoshi Mochizuki, Shin Maeda, Masao Omata. Influence of NUDT15 Genotyping on Dose Intensity of Thiopurine Administration and Long-Term Clinical Outcomes (Hospitalization and Surgery). Archives of Clinical and Biomedical Research. 8 (2024): 10-19.
View / Download Pdf Share at FacebookAbstract
Background: Thiopurines are one of the major drugs for treatment of inflammatory bowel disease. It is well known that SNPs in TPMT are the main cause of leucopenia and hair loss in European descent. In Asian individuals, the SNP p.Arg139Cys in exon 3 of NUDT15 is associated with leucopenia and hair loss. Previously, we demonstrated that thiopurineinduced leucopenia is related not only to exon 3 but also exon 1 SNPs in a cohort followed for a short term. The aim of this study was to evaluate the long-term effects of NUDT15 on clinical outcomes.
Methods: Patients (ulcerative colitis130 cases, Crohn’s disease 55 cases) were divided into mutation and wild-type NUDT15 groups, and the daily dosage of thiopurines and the effect of mutation on hospitalization and surgery were retrospectively investigated over a long period of up to 10 years (median, 7.8 years).
Results: Regarding TPMT SNPs, p. Pro80Ala, p. Thy154Ala and p. Tyr240Cys were not detected, and all genes were wild-type. Compared to the NUDT15 mutation group (n = 48), the daily thiopurine dosage was increased in the wild-type group (n = 137) (p = 0.024). The time to dose reduction and discontinuation of thiopurines was significantly shorter in the NUDT15 mutation group (p < 0.001, p = 0.039). The NUDT15 mutation group tended to have more hospitalizations (p = 0.067), and surgeries were significantly more frequent (p = 0.028). In ulcerative colitis patients, thiopurine discontinuation was associated with hospitalization and surgery (p = 0.003, HR 2.87, 95% CI 1.44-5.71, p = 0.036, HR 5.45, 95% CI 1.12-26.5). In Crohn's disease patients, the presence of SNPs was associated with hospitalization (p = 0.019, HR 3.68, 95% CI 1.24-10.97) and surgery (p = 0.036, HR 6.81, 95% CI 1.14-40.86).
Conclusions: The presence of the NUDT15 mutation affects maintenance of thiopurine dosage in the long term. In ulcerative colitis, it is important to continue thiopurines with fine-tuning of the dosage to avoid hospitalization and surgery. In Crohn's disease, a direct association between NUDT15 SNPs and hospitalization and surgery was found.
Keywords
<p>Inflammatory Bowel Disease; Thiopurines; NUDT15; Clinical outcome</p>
Article Details
Introduction
Thiopurines such as 6-mercaptopurine (6-MP) and azathioprine are widely used for treatment of Ulcerative Colitis (UC) and Crohn's disease (CD) [1,2]. In particular, thiopurines are recommended for treatment of steroid-dependent or steroid-resistant UC [3]. When combined with infliximab, thiopurine use lowers the relapse rate of CD [2]. Leucopenia, hair loss, pancreatitis and liver dysfunction are major adverse events associated with thiopurine use [4] . Leucopenia and hair loss occur in Asian patients with NUDT15 variants in exon 3 [5]. In particular, for patients homozygous for the p. 139 Arg<Cys (T/T) SNP, thiopurines are contraindicated because all patients develop severe leucopenia and hair loss [6] . In individuals of European descent, it is reported that TPMT is mainly related to adverse events, including leucopenia. In contrast, the incidence of NUDT15 variants is reported to be very low in individuals of European descent [7]. Genetic variants of NUDT15 in exon 3 are mainly associated with the adverse event leucopenia [5] . Previously we reported that thiopurine-induced leucopenia is observed not only with exon 3 variants but also exon 1 variants in the short term after starting thiopurines [6] . In addition, it was revealed that compound heterogous SNPs in exon 1 and exon 3 of NUDT15 may result in loss of function and are related to side effects. However, the majority of studies to date have focused on short effects of less than two years [8], and no long-term data for the influence of NUDT15 are available. Therefore, we evaluated the long-term outcome of patients, especially with respect to the influence of SNPs on hospitalization and surgery.
Methods
Patients
We enrolled 185 patients with inflammatory bowel disease treated with thiopurines at our hospital between October 2015 and December 2019 (Table 1). These 185 patients comprised 68 females and 117 males. Of the 185 patients, 96 were included in our previous short-term study of SNPs and incidence of adverse effects [6]. All 185 patients were treated with 6-MP, which was started at a dose of 30 mg daily. Written informed consent to conduct genetic analysis of NUDT15 and TPMT was obtained from all 185 patients. The study protocol was approved by the Institutional Review Board of our hospital (No. Genome2018-1, approved on November 15, 2011).
|
Wild (%) |
Mutant (%) |
p-value |
|
|
n |
137 |
48 |
0.921 |
|
Sex (M:F) |
87:50 |
30:18 |
0.901 |
|
Age at diagnosis Median, (range) |
26.5 (3-86) |
33 (12-79) |
0.839 |
|
Family history,yes |
9 (6.6) |
5 (10.4) |
0.799 |
|
Smoking status |
0.993 |
||
|
never |
54 (39.4) |
19 (39.6) |
|
|
current |
9 (6.6) |
3 (6.3) |
|
|
past |
28 (20.4) |
9 (18.8) |
|
|
unknown |
46 (33.6) |
17 (35.4) |
|
|
Appendectomy , yes |
6 (4.4) |
1 (2.1) |
0.914 |
|
Disease location (UC) |
0.095 |
||
|
total |
54 (56.3) |
25 (74.3) |
|
|
left-sided |
33 (34.3) |
5 (14.3) |
|
|
proctitis |
9 (9.4) |
4 (11.4) |
|
|
Disease location (CD) |
0.12 |
||
|
small intestinal |
5 (12.2) |
2(14.3) |
|
|
small intestinal and colonic |
29 (70.7) |
6 (42.9) |
|
|
colonic |
7 (17.1) |
6 (42.9) |
|
|
Disease severity (UC) |
0.073 |
||
|
mild |
32 (33 .3) |
15 (44.1) |
|
|
moderate |
46 (47 .9) |
18 (52.9) |
|
|
severe |
18 (18.8) |
1 (2 .9) |
|
|
Disease activity (CD:CDAI, median,SD) |
210±99.3 |
311±103.3 |
0.12 |
|
Medication |
|||
|
5ASA |
123 (89.8) |
43 (89.6) |
0.969 |
|
steroid |
76 (55.5) |
21 (43.8) |
0.162 |
|
ED≧900Kcal/day (CD only) |
12/41 (29.3) |
4/14 (28.6) |
0.96 |
|
GMA |
4 (2.9) |
2 (4.2) |
0.675 |
|
tacrolimus (UC only) |
21/96 (1.5) |
1/34 (2.9) |
0.769 |
|
cyclosporine (UC only) |
21/96 (1.5) |
1/34 (2.9) |
0.769 |
|
infliximab |
30 (21.9) |
13 (27.1) |
0.464 |
|
adalimumab |
9 (6.6) |
3 (6.3) |
0.938 |
|
golimumab (UC only) |
0 (0.0) |
0 (0.0) |
|
|
vedolizumab |
0 (0.0) |
0 (0.0) |
|
|
ustekinumab |
0 (0.0) |
0 (0.0) |
|
|
tofacitinib (UC only) |
0 (0.0) |
0 (0.0) |
Table 1: Demographic data of the patients
Patient follow-up
Patients visited the hospital weekly for the first month, and then once every 1-2 months thereafter. The patient’s general condition was evaluated at each visit, and blood count and biological tests were performed. The 185 patients were followed for up to 10 years (median 7.8 years, 6.8 ± 3.08 years, mean ± SD) after starting 6-MP.
For UC, severity according to Montreal criteria, i.e., frequency of defecation, degree of hematochezia, ESR, and pulse rate, were evaluated [9,10]. For CD, the Crohn's disease activity index (CDAI), i.e., the number of diarrhea and soft stools, abdominal pain, subjective general condition, fever over 37.8°C, arthritis and joint pain, iritis and uveitis, erythema nodosum, pyoderma gangrenosum, aphtha fissures, hemorrhoids, perianal abscesses, other fistulas, use of loperamide and opiates, abdominal mass, hematocrit, and body weight, were investigated [11].
Clinical outcome
Studies on short-term outcomes included the presence or absence of hair loss, liver dysfunction, pancreatitis, and leucopenia; long-term outcomes included thiopurine dose intensity, frequency of use of biologic agents, cumulative hospitalization rate, and surgery.
Dose adjustment of 6-MP
To carefully evaluate the side effects of 6-MP, we set the criteria as follows: when WBC counts were below 3,000/mm3, we reduced the dose of 6-MP by 5 mg; when it stayed above the level, we increased the dose by 5 mg [6]. There for the WBC count was adjusted between 3,000 and 4000/mm3.
NUDT15 and TPMT genotyping
Peripheral blood samples were obtained from the 185 patients. Buffy coats were isolated by centrifugation of the blood samples at 820 × g at 25°C for 10 min and stored at -80 °C until required for DNA extraction. Buffy-coat DNA was extracted using a QIAamp® DNA Blood Mini QIAcube Kit (Qiagen, Hilden, Germany) with a QIAcube (Qiagen). The total genomic DNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States), as described previously [12,13].
PCR was performed using genomic DNA as a template and primer pairs flanking SNP sites in exon 1 (rs869320766, p. Val18_Val19insGlyVal; rs186364861, Val18Ile) and exon 3 (rs116855232, Arg139Cys; rs147390019, Arg139His) of the NUDT15 gene . The PCR products were cleaned using ExoSAP-IT™ Reagent (Affymetrix, Santa Clara, CA, United States) and sequencing was performed with a BigDye Terminator v3.1 (Thermo Fisher Scientific) using forward or reverse primers. The PCR products were purified and subsequently analyzed by a 3500 Genetic Analyzer (Thermo Fisher Scientific) [14,15].
Real-time PCR was conducted using a ViiA7 system (Thermo Fisher Scientific) and TaqMan Genotyping Master Mix (1 ×) (Life Technologies Corp.), forward and SNP genotyping was conducted by the allelic discrimination method. NUDT15 (rs186364861, Val18Ile; rs116855232, Arg139Cys) and TPMT (rs1800462, rs1800460, and rs1142345) genotyping primers and probes were purchased. NUDT15 SNP typing results were validated by Sanger sequencing.
The GenBank sequences of human NUDT15 (accession number: NP_060753.1) and TPMT (accession number: NP_000358.1) were accessed at the NCBI Reference Sequence Database.
Statistical analysis
All statistical analyses were performed using IBM SPSS software ver. 26. The statistical significance of differences in mean values between two cohorts was assessed by Student's t-test if variances were equal in an F test, or by the nonparametric Mann-Whitney test if the variances were not equal. Demographic data and drug introduction were evaluated by the χ2 test, and hospitalization and surgery were evaluated by the log-rank test and the Cox proportion hazard test.
Results
Genotyping TPMT and NUDT15
Genetic analysis of three SNPs of TPMT, including c.283G>C, c. 460G>A and c.719A>G, were not detected in 185 Japanese patients (Table 2). NUDT15 genotyping of exon1 and exon 3 showed five genetic variations (Groups A-E) (Table 2). Wild-type sequences of both exon1 and 3 were observed in 137 patients (74.1%, Group A). Heterozygous p.Val18Ile in two cases (1.1%, Group B), heterozygous p.Val18_Val19insGlyVal in exon 1 and p.Arg139Cys in exon 3 in eight cases (4.3%, Group C), heterozygous p.Arg139Cys in 36 cases (19.5%, Group D), and homozygous p.Arg139Cys in exon 3 in two cases (1.1%, Group E) were detected (Table 2). There were no cases of p. Gly17_Val18del (c.37_42delGGGAGTC), p. Arg34Thr (c.101G>C), p. Lys35Glu (c.103A>G), p.Arg139His (c.416G> A).
Altogether, 137 cases were wild-type NUDT15 and 48 mutant NUDT15.
|
Patients |
Exon1 |
Exon 3 |
TPMT |
|
Group A (n=137) |
|||
|
#1-#137 |
Wild |
Wild |
Wild |
|
Group B (n=2) |
|||
|
#138-#139 |
c .52G>A (G/A) (p.Val1811e) |
Wild |
Wild |
|
Group C (n=8) |
|||
|
#140-#147 |
c.50_55dupGAGTCG (G/GGAGTCG) (p.Val18_Val19insGlyVal) |
c.415C>T (C/ T) (p.Arg139Cys) |
Wild |
|
Group D (n=36) |
|||
|
#148-#183 |
Wild |
c.415C>T (C/T) (p.Arg139Cys) |
Wild |
|
Group E(n=2) |
|||
|
#184-#185 |
Wild |
c.415C>T (T/T) (p.Arg139Cys) |
Wild |
Table 2: Genotypes of NUDT15 and TPMT.
Comparison of wild and mutant types of NUDT15
Then, the patients with wild-type (Group A, n=137) and mutant (Group B + Group C + Group D + Group E, n=48) NUDT15 were compared regarding clinical features and dose intensity of 6-MP (Table 1). Baseline demographic, sex, age, UC vs. CD, family history, smoking status, appendectomy, disease location, disease severity (UC), disease activity (CD), medication data between the wild-type and mutant groups showed no significant differences (Table 1). There were no cases vedolizumab, ustekinumab or tofacitinib use at the time of thiopurine administration (Table 1).
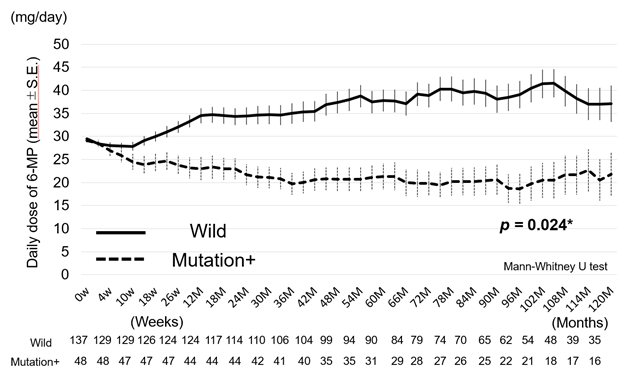
We studied the dose of 6-MP (dose intensity) during treatment up to 10 years (median 7.8 years, 6.8 ± 3.08 years, mean ± SD) after starting 6-MP. 6-MP was usually started at 30 mg daily and the subsequent dose of 6-MP was adjusted based on white blood cell counts. The WBC count was adjusted between 3,000 and 4000/mm3. We compared the dose intensity of 6-MP between the patients with wild-type (Group A) and mutant (Group B + Group C + Group D + Group E) NUDT15 (Figure 1).
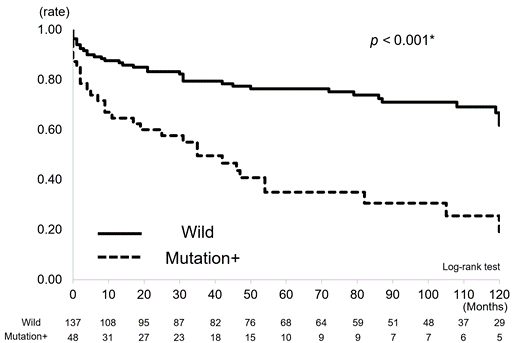
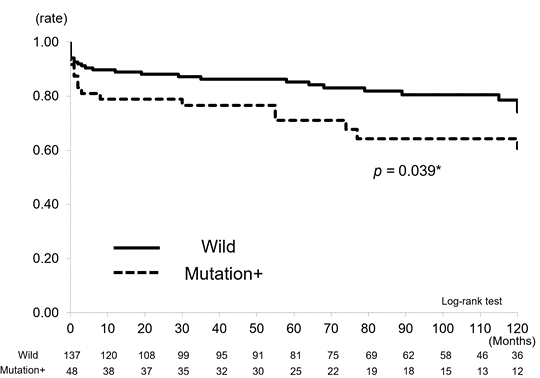
The dose of 6-MP in the patients with wild-type NUDT15 was significantly higher than that in the patients with mutant NUDT15 (p = 0.024) (Figure1). We also evaluated the time to dose reduction and discontinuation of 6-MP between the cohorts (Figure2a, 2b). The time to the 6-MP dose reduction in the mutation cohort was significantly shorter than that in the wild-type cohort (p < 0.001) (Figure 2a). Similarly, the time to discontinuation of 6-MP was significantly shorter and the rate higher in the mutation cohort than in the wild-type cohort (p = 0.039) (Figure 2b).
The time periods of dose reduction of 6-MP were significantly shorter in the mutation cohort than in the wild-type cohort (p < 0.001).
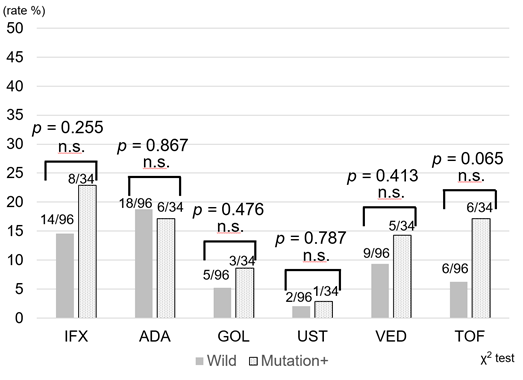
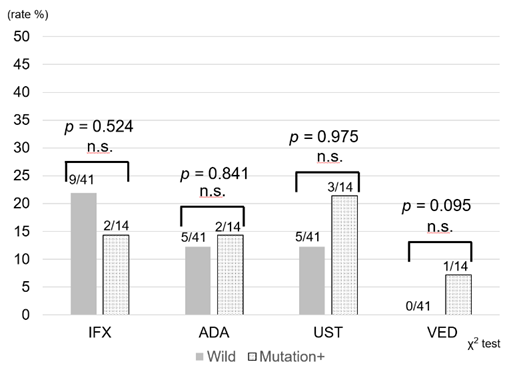
Use of treatment regimens other than 6-MP was compared between the two groups. In the UC patients, there was no significant difference in usage of infliximab, adalimumab, golimumab, ustekinumab, vedolizumab, and tofacitinib (Figure 3a). Similarly, there was no difference in the frequency of infliximab, adalimumab, ustekinumab, and vedolizumab use in the CD patients (Figure 3b). Therefore, despite dose reduction or discontinuation of 6-MP in the mutation cohort, no obvious difference in the introduction of other types of new drugs was revealed.
Figure 3a: Frequency of introduction of biologics and JAK inhibitors after initiation of thiopurines for UC. In UC, there was no significant difference between infliximab, adalimumab, golimumab, ustekinumab, vedolizumab, and tofacitinib use. IFX: Infliximab, ADA: Adalimumab, GOL: Golimumab. UST: Ustekinumab, VED: Vedolizumab, TOF: Tofacitinib
Long term outcome (hospitalization and surgery)
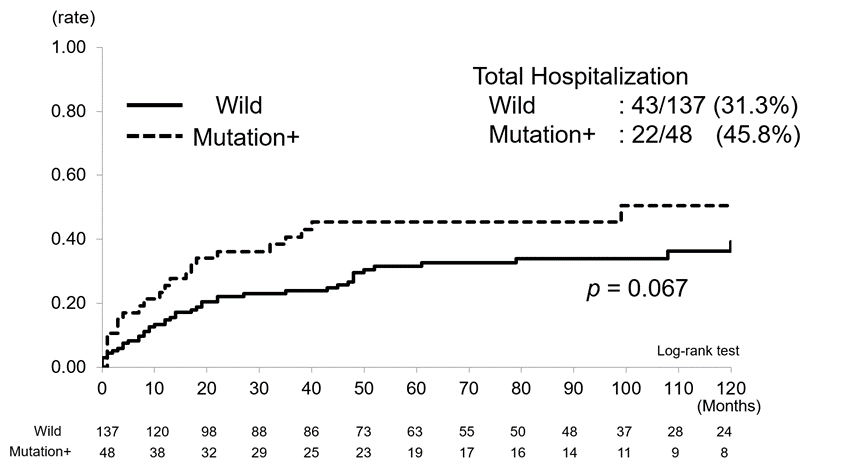
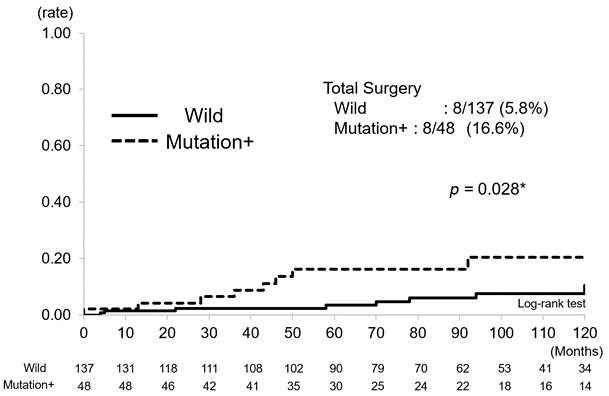
Finally, we studied the long-term possible influence of NUDT15 on the cumulative hospitalization rate and surgery. Cumulative hospitalization rates for combined UC and CD were 31.3% (43/137) in the wild-type group and 45.8% (22/48) in the mutation group and tended to be higher in the latter (p = 0.067) (Figure4a). Cumulative surgery rates were 5.8% (8/137) in the wild-type group and 16.6% (8/48) in the mutation group and were significantly higher in the latter (p = 0.028) (Figure4b).
Factors involved in hospitalization and surgery
Factors involved in hospitalization and surgery were then examined among patients with UC and CD (Table 3), including age, sex, NUDT15 mutation, disease location, disease severity and thiopurine continuation in UC and age, sex, NUDT15 SNP, disease location, CDAI at diagnosis, elemental diet and thiopurine continuation in CD.
In UC, discontinuation of thiopurine was associated with both hospitalization (p = 0.003, HR 2.87, 95% CI 1.44-5.71) and surgery (p = 0.036, HR 5.45, 95% CI 1.12-26.5); disease severity was also associated with hospitalization (moderate, p = 0.015, HR 2.71, 95% CI 1.21 - 6.04, severe, p = 0.032, HR3.36, 95% CI 1.11 – 10.16) (Table 3). In CD, NUDT15 mutation alone was associated with hospitalization and surgery (p = 0.019, HR 3.68, 95% CI 1.24 -10.97, p = 0.036, HR 6.81, 95% CI 1.14 - 40.86) (Table 3)
Discussion
It is widely recognized that leucopenia and hair loss are major thiopurine-induced adverse events and closely related to variants in NUDT15 exon 3 in Asian patients. In European patients, the adverse events are linked to TPMT variants [16] . The protein encoded by NUDT15 hydrolyzes the thiopurine active metabolites 6-thio-GTP and 6-thio-dGTP to 6-thio-GMP and 6-thiodGMP, which results in their reduced incorporation into DNA and RNA. In patients with NUDT15 variants, these pathways are disturbed, resulting in higher levels of the active metabolites 6-thio-GTP and 6-thio d GTP [17]. NUDT15 has nucleotide diphosphate activity, and variations result in loss of function of enzymatic activity to different extents [17]. Myelosuppression is induced by functional loss, and patients homozygous for p.139Arg<Cys (T/T) inevitably develop severe leucopenia and hair loss with thiopurine use; thus, thiopurines are now contraindicated [18]. Therefore, it is necessary to asses p.139Arg<Cys in Asian patients being treated with thiopurines for IBD. Previously, we demonstrated that thiopurine-induced leucopenia is related to not only variants in exon 3 but also in exon 1 of NUDT15 in the early stage after starting thiopurines [6] . In this study, we expanded on analysis of exon1 and exon 3.
Studies of SNPs in NUDT15 and thiopurine administration have mainly been concerned with short-term follow-up, especially in relation to side effects [8,19]. On the other hand, the association between NUDT15 SNPs and clinical features of IBD has not been reported. In the present study, patients with IBD were divided into mutation and wild-type groups. The influence of daily thiopurine dosage, introduction of new drugs, and other clinical features on long-term outcomes such as hospitalization and surgery was examined over a long period of up to 10 years (median, 7.8 years). The results showed that the daily dose of 6-MP tended to increase with time in the wild-type group, but that it tended to decrease in the mutation group (p = 0.024). Duration of thiopurine reduction and discontinuation were clearly shorter in the mutation group than in the wild-type group (p < 0.01, p = 0.039). This indicates that the patients with mutation not only experienced the initial acute side effect leucopenia, but that the patient’s condition was also directly or indirectly affected tor a longer period of time.
|
Hospitalization |
Surgery |
|||||
|
p-value |
HR |
95%CI |
p-value |
HR |
95%CI |
|
|
UC |
||||||
|
Age at diagnosis |
0.997 |
1.00 |
0.16-5.51 |
0.308 |
0.12 |
0.00-10.17 |
|
Sex |
||||||
|
Female |
0.36 |
1.34 |
0.71-2.53 |
0.826 |
0.83 |
0.13-5.01 |
|
Male |
1 |
1 |
||||
|
NUDT15 Mutation |
||||||
|
Mutation+ |
0.236 |
1.56 |
0.75-3.23 |
0.606 |
1.61 |
0.26-9.84 |
|
Wild |
1 |
1 |
||||
|
Disease location |
||||||
|
Total |
1 |
1 |
||||
|
Left sided |
0.741 |
0.62 |
0.38 -1.99 |
0.522 |
0.47 |
0.05 - 4.71 |
|
Proctitis |
0.617 |
1.28 |
0.48 -3.40 |
0.856 |
1.22 |
0.13 - 11.16 |
|
Disease Severity |
||||||
|
Mild |
1 |
1 |
||||
|
Moderate |
0.015* |
2.71 |
1.21-6.04 |
0.324 |
2.46 |
0.41-14.79 |
|
Severe |
0.032* |
3.36 |
1.11-10.16 |
no data |
||
|
Continuous administration of thiopurines |
||||||
|
No |
0.003* |
2.87 |
1.44-5.71 |
0.036* |
5.45 |
1.12-26.5 |
|
Yes |
1 |
1 |
||||
|
Crohn's disease |
||||||
|
Age at diagnosis |
0.052 |
1.04 |
1.00-1.07 |
0.064 |
1.05 |
1.00-1.14 |
|
Sex |
||||||
|
Female |
0.963 |
0.97 |
0.29-3.23 |
0.951 |
0.94 |
0.14-6.44 |
|
Male |
1 |
1 |
||||
|
NUDT15 Mutation |
||||||
|
Mutation+ |
0.019* |
3.68 |
1.24-10.97 |
0.036* |
6.81 |
1.14-40.86 |
|
Wild |
1 |
1 |
||||
|
Disease location |
||||||
|
small intestine |
1 |
1 |
||||
|
small intestine and colon |
0.261 |
2.89 |
0.45-18.32 |
0.449 |
2.59 |
0.22-30.40 |
|
colon |
0.524 |
1.90 |
0.26-13.88 |
0.550 |
2.29 |
0.15-34.78 |
|
CDAI at diagnosis |
0.429 |
1.00 |
0.99-1.00 |
0.589 |
1.00 |
0.99-1.01 |
|
ED ( 900 kcal /day≦) |
||||||
|
No |
0.964 |
0.98 |
0.35-2.74 |
0.32 |
0.42 |
0.07-2.33 |
|
Yes |
1 |
1 |
||||
|
Continuous administration of thiopurines |
||||||
|
No |
0.684 |
1.29 |
0.39-4.35 |
0.768 |
1.36 |
0.17-10.69 |
|
Yes |
1 |
|||||
|
Cox proportional hazard test. • significantly different |
||||||
Table 3: Factors affecting hospitalization and surgery.
When the two groups of UC and CD patients were combined, the mutation group tended to have more hospitalizations than the wild-type group (p = 0.067) (Figure 4a) as well as significantly more surgeries (p = 0.028) (Figure 4b). The association between various factors and outcomes was analyzed for UC and CD. As we selected the Montreal classification for UC and CDAI for CD, we did not examine inflammatory markers such as CRP and ESR. In UC, thiopurine discontinuation was associated with both hospitalization (p = 0.003, HR 2.87, 95% CI 1.44-5.71) and surgery (p = 0.036, HR 5.45, 95% CI 1.12-26.5), and disease severity was associated with hospitalization (moderate, p = 0.015, HR 2.71, 95% CI 1.21-6.04, severe, p = 0.032, HR 3.36, 95% CI 1.11–10.16) (Table 3). In CD, NUDT15 SNPs were significantly associated with both hospitalization (p = 0.019, HR 3.68, 95% CI 1.24-10.97) and surgery (p = 0.036, HR 6.81, 95% CI 1.14-40.86) (Table 3). These findings demonstrate not only the short-term influence of the SNPs, but also that the genetic diversity of NUDT15 is associated with long-term outcomes of IBD, namely, hospitalization and surgery. Limitations of this study include that it was conducted at a single institution, with accordingly small number of patients. Furthermore, criteria for admission and surgery may vary from case to case. Further investigation of these issues using a greater number of cases is warranted. Despite these limitations, the importance of thiopurine use in UC is now recognized in terms of long-term prognosis, and direct association between NUDT15 SNPs and hospitalization and surgery in CD was demonstrated. Further clarification of the mechanism is needed.
Declarations
Ethical approval and consent to participate
All procedures in this study were performed in accordance with the ethical standards of the Helsinki declaration and its later amendments or compatible ethical standards. This study was approved by the Institutional Review Boards of Yamanashi Prefectural Central Hospital (No. Genome2018-1, approved on November 15, 2011) and all patients were informed about the study and signed a consent form for participation.
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analyzed for this study are included in the published article.
Competing interests
The authors have no conflict of interest to declare. All coauthors have seen and agree with the contents of the manuscript, and there is no financial interest to declare.
Funding
Not applicable.
Author contributions
ST: Collecting patient data and statistical analysis and genomic analysis.
YK: Writing the paper, acquisition of data, and analysis and interpretation of data.
YH: Genomic analysis.
HA, Y. M, H. A., H. O., Y. S., H. M.: Acquisition of data.
SM: Conception and design of the study.
MO: Conception and design of the study and final approval of the submitted version.
Acknowledgements
Not applicable
Reference
- Timmer A, Patton PH, Chande N, et al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev (2016): Cd000478.
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 362 (2010): 1383-1395.
- Singh S, Feuerstein JD, Binion DG, et al. AGA Technical Review on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology 156 (2019): 769-808.
- Present DH, Meltzer SJ, Krumholz MP, et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med 111 (1989): 641-649.
- Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet 46 (2014): 1017-1020.
- Kojima Y, Hirotsu Y, Omata W, et al. Influence of NUDT15 variants on hematological pictures of patients with inflammatory bowel disease treated with thiopurines. World J Gastroenterol 24 (2018): 511-518.
- Coenen MJH, de Jong DJ, van Marrewijk CJ, et al. Identification of Patients With Variants in TPMT and Dose Reduction Reduces Hematologic Events During Thiopurine Treatment of Inflammatory Bowel Disease. Gastroenterology 149 (2015): 907-917.
- Akiyama S, Matsuoka K, Fukuda K, et al. Long-term effect of NUDT15 R139C on hematologic indices in inflammatory bowel disease patients treated with thiopurine. J Gastroenterol Hepatol 34 (2019): 1751-1757.
- Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 55 (2006): 749-753.
- Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 380 (2012): 1606-1619.
- Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 70 (1976): 439-444.
- Hirotsu Y, Nakagomi H, Sakamoto I, et al. Detection of BRCA1 and BRCA2 germline mutations in Japanese population using next-generation sequencing. Mol Genet Genomic Med 3 (2015): 121-129.
- Sakamoto I, Hirotsu Y, Nakagomi H, et al. BRCA1 and BRCA2 mutations in Japanese patients with ovarian, fallopian tube, and primary peritoneal cancer. Cancer 122 (2016): 84-90.
- Hirotsu Y, Nakagomi H, Amemiya K, et al. Intrinsic HER2 V777L mutation mediates resistance to trastuzumab in a breast cancer patient. Med Oncol 34 (2017): 3.
- Hirotsu Y, Nakagomi H, Sakamoto Iet al. Multigene panel analysis identified germline mutations of DNA repair genes in breast and ovarian cancer. Mol Genet Genomic Med 3 (2015): 459-466.
- Wusk B, Kullak-Ublick GA, Rammert C, et al. Thiopurine S-methyltransferase polymorphisms: efficient screening method for patients considering taking thiopurine drugs. Eur J Clin Pharmacol 60 (2004): 5-10.
- Moriyama T, Nishii R, Perez-Andreu Vet al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 48 (2016): 367-373.
- Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J 16 (2016): 280-285.
- Maeda T, Sakuraba H, Hiraga H, et al. Long-term efficacy and tolerability of dose-adjusted thiopurine treatment in maintaining remission in inflammatory bowel disease patients with NUDT15 heterozygosity. Intest Res 20 (2021): 90-100.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 