Pilot Study on Non-Invasive Diagnostics of Volatile Organic Compounds over Urine from COVID-19 Patients
T Boeselt1*, P Terhorst1, J Kroenig1, C Nell1, M Spielmanns2,3, H Heers5, U Boas1, M Veith1, C Vogelmeier1, T Greulich1, AR Koczulla1,4, B Beutel1
1Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Center Giessen and Marburg, Philipps-University Marburg, Germany, Member of the German Center for Lung Research (DZL).
2Pulmonary Rehabilitation, Zuercher Reha Zentren Klinik Wald, Switzerland
3Faculty of Health, Department of Pneumology, University of Witten/Herdecke, Germany
4Department of Pulmonology, Schoen-Kliniken Berchtesgaden, Philipps-University Marburg, Germany. PMU Salzburg
5Department of Urology and Paediatric Urology, University Medical Center Giessen and Marburg, Philipps-University Marburg, Germany
*Corresponding author: Tobias Boeselt, Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Center Giessen and Marburg, Philipps-University Marburg, Germany.
Received: 30 November 2021; Accepted: 07 December 2021; Published: 12 January 2022
Article Information
Citation: T Boeselt, P Terhorst, J Kroenig, C Nell, M Spielmanns, H Heers, U Boas, M Veith, C Vogelmeier, T Greulich, AR Koczulla, B Beutel. Pilot Study On Non-Invasive Diagnostics Of Volatile Organic Compounds Over Urine From COVID-19 Patients. Archives of Clinical and Biomedical Research 6 (2022): 65-73.
View / Download Pdf Share at FacebookAbstract
Introduction: The new beta - coronavirus SARS-CoV 2, which causes the disease COVID-19, can be detected by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) from a nasopharyngeal and/or oropharyngeal swab or Bronchoalveolar Lavage (BAL). The diagnosis of COVID-19 infection is based on the detection of the virus in addition to the typical symptoms. Pre-analytics play a crucial role in this process, as a meaningful result can only be obtained if a sufficient sample quantity is available. This pilot study investigated the possibility of detecting volatile organic compounds (VOCs) from the urine of positive COVID-19 patients using an electronic nose. A SARS-CoV 2-negative control group was additionally studied.
Methods: Between June 2020 and February 2021, the urine of 65 symptomatic, SARS-CoV -2 PCR positive, patients was analyzed. 65 asymptomatic and PCR negative subjects served as control group. VOCs in the headspace of the samples were analyzed using an electronic nose (Cyranose 320) and signals were analyzed in a linear discriminant assay.
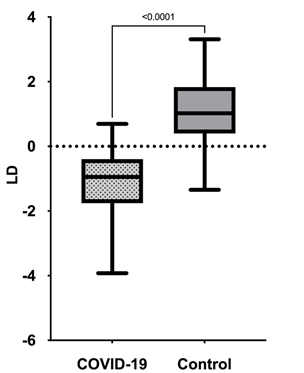
Results: Discriminant analysis of a total of 130 urine samples, 65 of which were SARS-CoV-2 positive and 65 negative, showed good overall separation. A sensitivity of 92% and a specificity of 89 % could be determined. The Mahalanobis distance was 1.5. Overall, 92 % of COVID-19 positive urine samples could be correctly matched. This resulted in a positive predictive value of 90 %.
Discussion: The results show for the first time that Cyranose can differentiate between air over urine (Headspace) of SARS-CoV-2 positive patients versus negative subjects. Thus, urine could become a promising, non-invasive and cost-effective diagnostic medium. Further urine-based studies on SARS-CoV-2 using other VOC detection methods need to follow to confirm validity.
Keywords
<p>Covid-19; Diagnostics; Electronic nose; Smell prints; Urine; VOC</p>
Article Details
1. Introduction
The COVID 19 pandemic claimed a high number of lives worldwide. This was compounded by social upheaval and considerable economic damage. Currently, the crisis continues in many parts of the world. Infection with coronavirus (SARS-CoV-2) often leads to illness with pneumonia and even Acute Respiratory Distress Syndrome (ARDS) after an incubation period of approximately 5.2 days [1]. However, between 80-90% of affected individuals have only mild to no symptoms [2]. The most common symptoms represent fever, cough, dyspnea, fatigue, and sometimes gastrointestinal symptoms [3]. Complications such as pulmonary artery embolism, microthrombi, myocardial damage, acute renal failure, and secondary bacterial and mycotic infections often occur during the course of the disease [4, 5]. Currently, nearly 4 million people have died worldwide because of the viral infection despite intensified measures by governments. WHO recommendations included physical distancing and improved hygiene measures [6]. As another strategy, widespread testing stations were established in many countries to further contain the pandemic [7]. The current gold standard is reverse transcription polymerase chain reaction (RT-PCR) based on a nasopharyngeal and/or oropharyngeal swab. Despite high sensitivity and specificity of this test, false positive or negative results occur from time to time [2, 8]. Specific findings from computed tomography scans of the thorax (CT thorax) provided the first insights into false-negative results [9, 10]. A native CT chest is now standard in addition to RT-PCR and often shows basal to global milk glass infiltration in COVID-19 infections [11, 12]. For asymptomatic patients who nevertheless pose a relative risk of infection, the accuracy of current methods needs to be reevaluated. In practice, there is the challenge of discharging a patient based solely on a nasal or throat swab. The measures represent a significant time and financial issue, require trained personnel, and expose the patient to x-ray radiation in the case of a CT chest scan. Diagnosis and screening of COVID-19 remain central to pandemic containment.
The primary entry receptor for SARS-CoV-2 in many experimental models is angiotensin-converting enzyme 2 (ACE2), which is highly expressed in renal proximal tubular epithelial cells [13-15]. Increasing numbers of autopsy reports that have been able to isolate the virus from patient urine also suggest infection of the kidney with SARS-CoV-2. However, it seems unclear whether direct infection of the kidney is responsible for the severity of COVID-19 disease [16-18].
Volatile organic compounds (VOCs) are opening up a promising non-invasive diagnostic approach. VOCs are gaseous molecules that are often released as a degradation product of various metabolic processes in the body which may be altered in pathological processes or infections [19]. In acute SARS-CoV 2 infections, a virus is usually detectable in blood, pharynx, feces, and in some cases in cerebrospinal fluid, exhaled air [20-22] and also in urine [23]. First data could show that SARS-CoV 2 pos. patients can be differentiated by VOCs [24]. Especially in the case of viral infections (particularly adenoviruses) of the urogenital system, it is possible to detect the agent in the urine (viruria). Viruses are usually detected in urine in three ways. First, as detection of inclusion bodies in the cells of the urinary sediment, further as specific immunofluorescence of the cells and isolated from tissue cultures. To date, viruria has been demonstrated in measles, human cytomegalovirus, human adenovirus, polyomavirus-associated nephropathy (PAN), and enterovirus (neonates: hepatitis and myocarditis) [23]. Recent case reports now also support possible detectability of corona viruses (MERS-CoV, e.g. SARS-CoV-19,) in urine and stool [25]. Even though SARS-CoV-2 spreads particularly strongly in the lower respiratory tract, small amounts of virus could nevertheless also be detected in urine. Currently, there are very few data regarding such diagnostics. The present pilot study investigates the possibility of non-invasive diagnostics via the urine of COVID-19 positive and negative patients and volunteers.
2. Material and Methods
2.1 Study participants
This pilot study was conducted at the University Hospital of Philipps University Marburg, Germany, from July 2020 to February 2021. Patients with positive RT-PCR test from nasopharyngeal swab with symptoms requiring hospital admission for surveillance were included. Participants were excluded if they were on the intensive care unit due to an excessively severe course and were not able to give informed consent. A control group was formed from subjects who presented with appropriate symptoms and suspected COVID-19 but had a negative RT-PCR result from a nasopharyngeal swab at the time of study inclusion and had no contact with COVID-19 positive patients. The study protocol was approved by the Medical Ethics Committee of the University Hospital of Philipps University of Marburg (AZ 72/20) and was conducted in accordance with the Declaration of Helsinki. Verbal and written informed consent was obtained from all eligible participants before providing a spot urine sample.
2.2 VOC analysis
In the underlying feasibility study, Cyranose 320 from Sensigent (USA) was used (See Figure 1). The device contains 32 different conductive biopolymer sensors (thin film carbon polymer chemiresistors) that can detect complex gas mixtures of volatile organic compounds (VOCs) at concentrations ranging from 100 ppb to 100 ppm [26, 27]. When the sensors are exposed to a gas, they respond by changing their electrical resistance. Subsequently, the chemical signal is converted into a digital signal. At the beginning of each measurement day and for the duration of all measurements, the measurement room is sealed airtight and an equilibration/calibration measurement is performed under ambient air. Before and after each sample measurement, a zero measurement is performed for 60 seconds each under ambient air. The sample measurement itself takes place for 60 seconds during which a signal in the sense of a "steady state" is determined. Each sensor reacts to the gas mixture with an individual sensor response due to different fabrication. In Cyranose, the total VOCs in the gas are measured and the respective sample receives a unique response from all 32 sensors in terms of pattern recognition. These profiles are called "smellprints" or "breathprints" as described earlier. With the help of statistical methods such as linear discriminant analysis, it is possible with a model setup to assign subjects to, for example, a "sick" and a "healthy" group based on their "breathprints" [26]. The measurement procedure was analogous to previous studies of our research group on the diagnosis of urinary bladder tumors from the headspace of urine samples [28].
2.3 Statistics and data analysis
All analyses were calculated with SPSS 22 (IBM SPSS Statistics, Version 22.0. Armonk, New York, US) and Prism 5.03 (GraphPad Software, Inc., La Jolla, US). For comparing the two groups for ordinal scaled parameters the Mann-Whitney-U-Test for unpaired samples was used. The Fisher’s exact test were performed for categorical variables. All tests are two-sided (p <0.05 was considered to be significant). The analysis method of the eNose is described elsewhere [29].
3. Results
Table 1 gives an overview over relevant patient characteristics in both groups.
|
Variables |
COVID-19 (n=65) |
controls (n=65) |
p-Wert |
|
Male gender, n (%) |
38 |
41 |
n.s. |
|
Age [years] |
73.0 ± 14.02 |
59,1 ± 13.1 |
<0.001 |
|
BMI (kg/m2), mean ± SD |
35.25 + 5.8 |
32.41 + 6.2 |
n.s. |
|
Smoking status “Never“, n (%) |
52 |
45 |
n.s. |
|
Comorbidities |
|||
|
Hypertension, n (%) |
12 |
15 |
n.s. |
|
Diabetes mellitus, n (%) |
18 |
12 |
n.s. |
|
Coronary disease, n (%) |
5 |
8 |
n.s. |
|
COPD/asthma, n (%) |
13 |
8 |
n.s. |
|
Malignancy, n (%) |
8 |
6 |
n.s. |
|
Kidney disorders, n (%) |
12 |
7 |
n.s. |
|
Medication use |
|||
|
PPI, n (%) |
16 |
7 |
n.s. |
|
NSAID, n (%) |
6 |
8 |
n.s. |
|
Corticosteroid, n (%) |
14 |
6 |
n.s. |
|
Angiotensin receptor blocker, n (%) |
5 |
7 |
n.s. |
|
n.s. = not significant |
|||
Table 1: Patient characteristics.
Each sample of the total 130 samples was exposed to three headspace measurements. After calculating the arithmetic mean from the 3 measurements of each subject, a positive predictive value of 90% was achieved in the linear discriminant analysis (LDA). More than 92% of the COVID-19 positive urine samples were also assigned to the correct group (see table 1-2). The sensitivity was 92 % and the specificity 89 % with a significant p value < 0.001 after Mann-Whitney U-test.
|
Covid-19 |
Control |
P |
|
|
Covid-19 |
60 (92,31%) |
7 (10.77%) |
<0,001 |
|
Control |
5 (7,69%) |
58 (89,23%) |
Table 2: CVV table.
A boxplot of the data comparing Covid-19 positive and negative urine samples is shown in Figure 1.
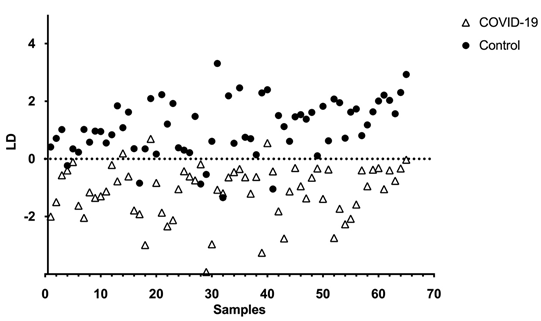
Figure 2 shows the linear discriminant analysis of the two groups. The Mahalanobis distance between the two centroids of the groups is 1.5.
4. Discussion
This pilot trial was able to show that differentiation between SARS-CoV-2 positive patients and negative subjects using analysis of VOCs from urine samples is feasible. The study was blinded to the analyzer. Comorbidities were equally distributed in both groups. In 2020, the Hanover group was able to show that VOCs in exhaled air differed between SARS-CoV-2 positive and negative individuals. These data are important for a first diagnostic approach and could be used for future diagnostic tests. Using accessory data (urine and metabolites), the urine headspace signal was shown to indicate possible systemic SARS-CoV-2 infection or renal infection [30]. Our study results suggest that VOC-based urine diagnostics have the potential to become a rapid, inexpensive, and non-invasive triage test for COVID-19. With a positive predictive value of 90%, Cyranose was able to differentiate between COVID-19 positive and negative VOC patterns. The Mahalanobis distance is a dimension of the distance between two points (centroids) in a space defined by two correlated variables. The distance in our study between the two groups is more than one (1.5) standard deviations away from the centroid. Thus, we can conclude that the centroids of the two groups have no correlations. The use of VOC analysis by an electronic nose in SARS-CoV-2 has been described in previous studies. For example, the research group led by Wintjes et al. (2020) demonstrated the use of an eNose prior to surgery [31]. Using analysis of VOCs from patients' exhaled air, a negative predictive value of 0.92 and a sensitivity of 0.86 were shown. Thus, a comparable eNose technology has also demonstrated good diagnostic accuracy in detecting COVID-19 positive patients from exhaled air.
The present study demonstrates several strengths over conventional methods. This is the first study of an eNose to demonstrate separation of COVID-19 positive patients from negative subjects based on urine. The procedure does not require dedicated personnel or use costly consumables. A clear advantage in the use of urine specimens is the much lower cost and also less uncomfortable examination compared to nasopharyngeal swabs or BAL. Obtaining the samples is painless for the patients, which is also why the dropout rate was 0%. This was also confirmed by the results of other eNose studies [32]. The data are quickly available due to a real-time analysis and can provide a significant time advantage in case of doubt in a triage situation. However, the present study also has some limitations. Furthermore, one of the major limitations is the need for RT-PCR testing, as this is the standard for diagnosis of COVID-19 at the current time of the study. Also limiting this study is that only symptomatic patients requiring hospitalization were included in the test group. It remains unclear to what extent the procedure is also informative in mildly symptomatic or asymptomatic patients. It is possible that the individual components of urine appear altered by Covid-19 infection. In a study by the Helms et al. study group, proteomic upregulation of a total of nine proteins was detected in the urine of COVID-19+ patients [30]. Whether these are purely from renal infection or this is a combination of systemic and renal response to infection remains open. However, it seems possible that SARS-CoV-2 can directly infect and damage renal tubular epithelial cells [30]. From this viewpoint, the damaged epithelium of the kidney could model features of the lung epithelium in acute respiratory distress syndrome that appear relevant to COVID-19 pneumonia. Further analysis of the interrelationships of affected organs appears to play a major role in SARS-CoV-2 infection. In light of this, urine samples can play a part in studying the pathophysiology of COVID-19 kidney and contribute to the development of therapies and predictive models [33-35]. At the current time, we only know that Cyranose can detect a difference between sample groups. The specific molecules relevant to the group separation need to be identified in further studies using more elaborate methods.
5. Conclusion
In summary, to our knowledge, this is the first diagnostic study on SARS-CoV-2 based on urine. It could be clearly shown that the VOC pattern over the urine samples of SARS-Cov-2 positive patients is different compared to SARS-CoV-2 negative subjects. We could draw evidence for a systemic infection which might involve the kidneys. If confirmed in larger studies, VOC analysis over urine samples might become a helpful and rapid diagnostic method for COVID 19.
Conflict of interest statement
None of the authors have a conflict of interest in this paper.
Funding statement
The preparation of this work was not supported by any financial means.
References
- Rothan HA, Byrareddy SN, The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109 (2020): 102433.
- Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med 288 (2020): 192-206.
- Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75 (2020): 1730-1741.
- Huang C, Wang Y, Li X, et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (2020): 497-506.
- Siordia JA. Epidemiology and clinical features of COVID-19: A review of current literature. J Clin Virol 127 (2020): 104357.
- Organization WH, Coronavirus disease (COVID- 19) advice for the public 2020, W.H. Organization, Editor. 2020: Geneva (2020).
- Cheng MP, Papenburg J, Desjardins J, et al., Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review. Ann Intern Med 172 (2020): 726-734.
- Long C, Xu H, Shen Q, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol 126 (2020): 108961.
- Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 296 (2020): 32-40.
- Xie X, Zhong Z, Zhao W, et al. Chest CT for Typical Coronavirus Disease 2019 (COVID-19) Pneumonia: Relationship to Negative RT-PCR Testing. Radiology 296 (2020): 41-45.
- Iyer M, Jayaramayya K, Subramaniam MD, et al. COVID-19: an update on diagnostic and therapeutic approaches. BMB Rep 53 (2020): 191-205.
- Xie J, Ding C, Li J,et al. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol 92 (2020): 2004-2010.
- Wysocki J, Lores E, Ye M, et al. Kidney and Lung ACE2 Expression after an ACE Inhibitor or an Ang II Receptor Blocker: Implications for COVID-19. J Am Soc Nephrol 31 (2020): 1941-1943.
- Ye M, Wysock J, William J, et al., Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 17 (2006): 3067-3075.
- Shang J, Wan Y, Luo C, et al., Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 117 (2020): 11727-11734.
- Braun F, Wong MN, Carsten A, et al., SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396 (2020): 597-598.
- Sun J, Zhu A, Li H, et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes Infect 9 (2020): 991-993.
- Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe 1 (2020): 245-253.
- Haick H, Broza YY, Mochalski P, et al. Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev 43 (2014): 1423-1449.
- Fabian P, et al. Influenza virus in human exhaled breath: an observational study. PLoS One 3 (2008): 2691.
- Kharitonov SA, Yates D, Barnes PJ. Increased nitric oxide in exhaled air of normal human subjects with upper respiratory tract infections. Eur Respir J 8 (1995): 295-297.
- Milton DK, Fabiana MP, Cowling BJ, et al., Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog 9 (2013): 1003205.
- Gourinat AC, Connor O, Calvez E, et al. Detection of Zika virus in urine. Emerg Infect Dis 21(2015): 84-86.
- Jendrny P, Schulz C, Twele F, et al. Scent dog identification of samples from COVID-19 patients - a pilot study. BMC Infect Dis 20 (2020): 536.
- Li Y, Wang Y, Liu H, et al. Urine proteome of COVID-19 patients. Urine (Amst) 2 (2020): 1-8.
- Bikov A, Lázár Z, Horvath I. Established methodological issues in electronic nose research: how far are we from using these instruments in clinical settings of breath analysis? Journal of breath research 9 (2015): 034001.
- Koczulla R, Hattesohl A, Biller H, et al. Vergleich von vier baugleichen elektronischen Nasen und drei Messaufbauten. Pneumologie 65 (2011): 465-470.
- Heers H, Gut JM, Hegele A, et al., Non-invasive Detection of Bladder Tumors Through Volatile Organic Compounds: A Pilot Study with an Electronic Nose. Anticancer Res 38 (2018): 833-837.
- Bach JP, Gold M, Mengele D, et al. Measuring Compounds in Exhaled Air to Detect Alzheimer's Disease and Parkinson's Disease. PLoS One 10 (2015): 0132227.
- Helms L, Marchiano S, Stanaway IB, et al. Cross-validation of SARS-CoV-2 responses in kidney organoids and clinical populations. JCI Insight 2021.
- Wintjens A, Hintzen KFH, Engelen SME, et al. Applying the electronic nose for pre-operative SARS-CoV-2 screening. Surg Endosc 2020.
- van Geffen WH, Bruins M, Kerstjens HA. Diagnosing viral and bacterial respiratory infections in acute COPD exacerbations by an electronic nose: a pilot study. J Breath Res 10 (2016): 036001.
- Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 181 (2020): 905-913.
- Wysocki J, Ye M, Hassler L, et al. A Novel Soluble ACE2 Variant with Prolonged Duration of Action Neutralizes SARS-CoV-2 Infection in Human Kidney Organoids. J Am Soc Nephrol 2021.
- Hunt AC, Case JB, Park YJ, et al. Multivalent designed proteins protect against SARS-CoV-2 variants of concern. bioRxiv 2021.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 