Renal Cystic Nephroma: Histologic and Immunohistochemical Analyses and Review of Literature
Darouichi M1*, Till JP2, Constanthin PE2
1Institute medical and Chirurgical Champel, Geneva, Switzerland
2University Hospital of Geneva, Switzerland
*Corresponding Author: Darouichi M, Institute medical and Chirurgical Champel, Geneva, Switzerland
Received: 03 March 2019; Accepted: 18 March 2019; Published: 21 March 2019
Article Information
Citation: Darouichi M, Till JP, Constanthin PE. Renal Cystic Nephroma: Histologic and Immunohistochemical Analyses and Review of Literature. Archives of Clinical and Biomedical Research 3 (2019): 003-011.
View / Download Pdf Share at FacebookAbstract
Background: A case of renal Cystic Nephroma (CN) was reported in 32 years-old women presenting with an incidental renal mass on radiological imaging.
Methods: On CT-scan examination, a renal cell carcinoma was suspected and right nephrectomy was performed. The resected tumor measured 17 × 10 × 7 cm. Macroscopically, the tumor was a multilocular mass with cysts ranging from 0.5 cm to 4.5 cm. The tumor was well delineated relative to non-tumoral renal parenchyma, which was physiological in appearance with a cortex at 1 cm without evidence of infiltration of the tumor. At the hilum, the arterial, venous and ureter were normal. No lymph node was identified. Microscopically, the lesion was well defined by a fibrous capsule, consisting of multiple cystic formations of variable sizes bordered by a mono-stratified epithelium, which ranged from flat to cubic. The lining epithelial cells had a hobnail appearance, abundant cytoplasm and an oval nucleus associated with a nucleolus. No mitosis were founded. The cysts were subtended by cellular fibrous septa of ovarian type. On immunohistochemical examination, the epithelial cells were positive for cytokeratins 7 and 19; stromal cells were positive for estrogen receptors, progesterone receptor and CD10. In the septa, there were some bundles of smooth muscle cells positive for desmin and smooth muscle actin.
Conclusion: The patient was alive 8 years after treatment, without metastasis or recurrence. Histologic and immunohistochemical analyses may be helpful in differential diagnosis between benign cystic renal neoplasms, CN, mixed epithelial and stromal tumor (MEST) and malignant tumors such as clear cell renal carcinoma (CCRC) in adults.
Keywords
<p>Histologic; Immunohistochemically; Renal cystic nephroma</p>
Article Details
1. Introduction
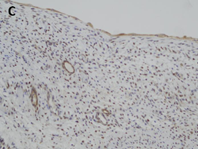
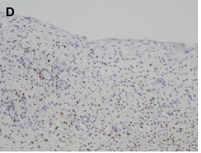
Renal cystic nephroma (CN) is a rare benign renal tumor, which is recognized as a specific entity characterized by the association of epithelial and stromal neoplasms [1]. The histologic features of CN are comparable to that epithelial type of the Wilm’s tumor in pediatric forms or to the mixed epithelial and stromal tumor of the kidney in adulthood [2]. In the present study, we describe histological findings of CN and its differential diagnosis using immunohistochemical staging and histology analysis (Figure 1).
2. Materials and Methods
2.1 Macroscopically
CN is an encapsulated, well-demarcated tumor and is typically composed entirely of multiples cysts and cyst septa without solid areas. The cysts contain sero-sanguineous fluid and the tumor may be focal or replace the entire kidney (varying in size ranging from 5 to 20 cm) (Figure 2).
2.2 Microscopically
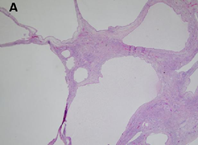
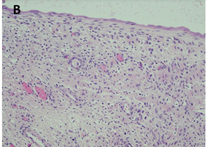
There is well-visible demarcation from the surrounding parenchyma kidney by a thick fibrous capsule. The cysts have a variable size ranging from microscopic to up to 5 cm and are lined by flattened cuboidal or hobnail epithelium. Occasionally, the lining cells have clear cytoplasm. Interestingly, many tumors have areas of cystic surface that lack epithelium. The septa are thin (under 5 mm of width), translucent and uniform and correspond to the outlines of the cysts. The septal stroma consists of fibrous tissues, which vary from myxoid to collagenous; occasionally it is cellular and has a wavy appearance resembling ovarian-like stroma [3]. The septa contain small cysts lined by bland cuboidal epithelial cells resembling renal tubules and may become calcified (Figure 3).
2.3 Immunohistochemistry profile
CN demonstrate reactivity of the epithelial component to antibodies directed against cytokeratins (Figure 3a, b, c and d). The stromal component expresses vimentin, smooth muscle actin, caldesmon and desmin. In general, CD10, calretinin, inhibin, estrogen receptor (RE) and progesterone receptor (PR) expression are seen.
The predominantly cystic renal tumors are still the source of confusion and diagnostic controversy. All imaging techniques (US, CT scans and MRI) show a multilocular cystic tumor with septa but do not differentiate benign CN from other benign or malignant complex cystic kidney masses [4]. In front of a renal tumor with a cystic component, the differential diagnosis is essential with four entities having a systematically different growth and prognosis profile. However, these entities are very similar in their macroscopic appearances and cannot be differentiated by modern preoperative imaging [5]. Thus, in young children, partially differentiated cystic nephroblastomas and highly cystic Wilms tumors with little or no local or distant invasion are diagnosed as partially differentiated nephroblastoma [6]. Similarly, in adults, mixed epithelial and stromal tumors (MEST), CCRC, and multiple cell renal cell carcinoma (MLCRC) and sometimes cystic renal hamartoma are entities that, even if they each have a different immunohistochemical identity, have common clinical, epidemiological and radiologic aspects [7]. Cystic nephroma, which is a benign tumor composed of a stroma and an epithelium of unknown histogenesis, should always be considered in this group of heterogeneous neoplasms. Another rare, potential diagnosis that should not be forgotten is sarcoma.
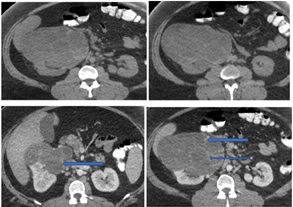
Figure 1: Radiographic features (a and b: enhanced axial), (c and d: contrast enhanced axial). CT imaging of the upper abdomen showed an exophytic complex renal mass in the middle third and the anterior inferior portion of the right kidney. The mass had multiple thick septa and showed IV-contrast enhancement without infiltration of the adjacent structures (arrows).
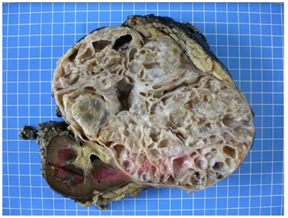
Figure 2: Macroscopical findings-the kidney measured 20 × 16 × 13 cm, and weighted 1682 g. At the cut, a voluminous multilocular mass, measuring 17 × 12 × 12 cm, with cysts ranging from 0.5 cm to 4.5 cm was observed. The tumor was well-delineated relatively to the non-tumoral renal parenchyma, which was physiological in appearance with a cortex at 1 cm. The mass arrived at 0.2 cm from the renal capsule, which presented focal fat tissue without evidence of infiltration of the tumor. At the hilum, the arterial, venous and ureteral section slices were identified with a ureter that measured 4 cm in length and 0.3 cm in diameter. No lymph nodes were identified.
2.4 Mest
Mest is a benign surgically-treatable cystic tumor like CN with clinical, epidemiological, morphological and pathological attributes very similar to those of CN and mainly affecting women. It is defined by an epithelial and stromal component mixture composed of a mixture of cystic and solid areas. Its first description was made in 1993 by Pawade et al. [8] under the name of cystic hamartoma of the kidney, but the term of mixed epithelial and stromal tumor was proposed by Michel and Syrucenk [9]. Its histogenesis is still unknown, and the most probable theory states that it is characterized by cystic structures aligned by epithelium mixed with ovarian stroma. In the World Health Organization 2004 renal neoplasms classification [10], MEST and CN were considered separate entities. Of note, recent studies suggest that MEST and CN might represent two different parts of the morphological spectrum of the same disease, as incorporated in the WHO 2016 classification, and both entities cannot be distinguished radiologically. Macroscopically, it appears as a complex cystic lesion with thin septa, with or without herniation in the renal pelvis. The difference lies in the tumor composition, CN is a multilocular cyst without macroscopic solid area with a cystic septum less than 5 mm thick in microscopy, while MEST is a cystic or partially cystic mass with solid areas and partitions greater than or equal to 5 mm. However, it is difficult to differentiate between MEST and CN classified as Category III or IV in Bosnian based on preoperative imaging results or macroscopy and to differentiate them from malignant lesions such as MLCRC [11].
2.5 Multilocular cystic renal cell carcinoma or MLCRC
Multilocular cystic renal cell carcinoma or MLCRC (or multilocular cystic renal neoplasm of low malignant potential in the new WHO classification) occurs in men above 30 years old, like the CN. Microscopically, it contains micro-clusters of epithelial cells with clear cytoplasm. Immunohistochemistry for epithelial membrane antigen, cytokeratin’s, vimentin or CD68 can confirm the nature of the cells. MLCRC is a rare entity. Its exact incidence and biological behavior are not well known. It is a rare subtype of renal cell carcinoma (RCC), occurring in 2.5% of cases and has a good prognosis after tumor surgical removal. Its differential diagnosis also contains cystic nephroma and papillary RCC with clear cells. Cystic renal cell carcinoma is a predominantly cystic lesion with a small solid component, whereas renal cell carcinoma is generally in the form of a solid mass, but may occur as a unilocular or multilocular cystic mass [12]. Under the microscope, the cysts of a multilocular cystic RCC are lined with epithelial cells with clear cytoplasm, which represent the clear malignant cell coat, whereas the septa are made up of fibrous tissue. A similar appearance can be seen in cystic nephroma; the subtle difference between the two is mainly in the cell type of the epithelial wall. Cystic nephroma consists of a circumscribed mass of cysts with intermediate fibrous septa, sometimes areas of calcification and regions of ovarian-type cell stroma. Cysts are covered with a single layer of benign, flattened, cuboid or clavicular epithelium [13]. The stromal cells are immunoreactive for the estrogen and progesterone receptors and focally for CD10. Unlike multilocular cystic RCC, the cyst wall and septa do not show signs of clear cell proliferation [14]. Multilocular cystic RCC sometimes has a high percentage of fibrosis, which on pathological examination is correlated with a high density of internal vessels compatible with vascularized fibrosis [15].
2.5.1 CPDN: Partially differentiated cystic nephroblastoma (CPDN) is a rare multicystic renal tumor in adults [16]. It is part of the spectrum of benign cystic nephroma and is considered a highly cystic WT without an expandable solid nodule, with immature nephroblastomatous epithelial and stromal elements and a characteristic blastema [17]. This entity is an intermediate between CN (benign) and WT (malignant). Only rare cases of CPDN are reported in the English literature, and its diagnosis should be made only after formally excluding both RCC and NC. CN and CDPN are almost identical radiologically and macroscopically: both types lack solid and expansive nodules that are characteristic of cystic WT [18]. Histopathological examination reveals a blastema and/or nephroblastomatous elements presenting the diagnosis of CDPN. However, CN and CPDN may not be in the same spectrum, as genetic analyzers have shown that the DICER1 mutation is the major genetic element in CN tumorigenesis but not in the CPDN. Moreover, CN has a potential for malignant transformation [19], which is not the case in the CPDN.
2.5.2 Wilm’s Tumor (WT): also called partially cystic nephroblastoma, it occurs in children under 2 years of age. It is well circumscribed from the remaining kidney by a fibrous pseudo-capsule and composed of cysts of variable size. The septa are thin and present with no nodules [20]. Cysts are coated with flattened cuboidal epithelium or naked epithelium. The septa are of variable cellular nature and contain undifferentiated and differentiated mesenchymal epithelial elements, blastemas and nephroblates. Skeletal muscle and myxoid mesenchyme are present in the septa. The epithelial component consists mainly of mature and immature microscopic cysts resembling the tubule cross-section. The Wilm’s solid tumor with a multifocal cystic change presents mainly with solid areas containing cystic focal areas and exhibits malignant behavior [21]. In contrast, partially differentiated cystic nephroblastoma is predominantly cystic with blastema or other embryonic cells in the septa of cysts; it lacks solid nodular regions. Both are WT1 blastemic cells [22]. Finally, CPDN is a predominantly cystic lesion without solid nodular regions, in which blastema cells or other embryonic cells are present in the septa of the cysts, whereas WT is a solid tumor with a multifocal cystic change and must be distinguished from INDC [23].
2.6 Cystic kidney hamartoma
Cystic kidney hamartoma is a rare benign tumor categorized in the same group with mixed epithelial and stromal tumors. It is composed of a stromal and complex, smooth muscle association and of a variety of epithelial elements. Histologically, there is a presence of both benign mesenchymal and epithelial elements [24]. The histological appearance is distinctive and characterized by disordered biphasic proliferation of epithelial and mesenchymal elements. The epithelial component consists of tubules and cysts coated with cuboidal and cylindrical epithelium showing focal changes of oncocytes. The tubular epithelium is positive for CAM 5.2, epithelial membrane antigen, carcinoembryonic staining and immunostaining against vimentin. The stroma is cellular and mainly fibroblastic with scattered bundles of smooth muscle cells [25]. Similarly, it contains fusiform cells with monomorphic nuclei and abundant eosinophilic cytoplasm that resembles smooth muscle and reacts positively with antibodies to smooth alpha-muscular actin, desmin, and vimentin [26].
3. Discussion
CN is a rare unilateral, solitary renal tumor. It is recognized as a separate entity thought to have benign biological behavior [27]. This neoplasm has a bimodal distribution, in children under 4 years and in adults ranged from 40 to 65 years, much more frequent in women with a ratio of 8/1 [28]. Before the operation, CN doesn’t have any specific clinical presentation or any distinctive imaging features from other renal cystic lesions, in particular MEST and CCRC [29]. Pathogeny of this entity is unknown and it has been proposed that the epithelial stromal components of the tumor are neoplastic. It has also been suggested that CN is characterized by cystic structures lined by epithelium mixed with ovarian-like stroma [30]. The term renal epithelial and stromal tumor has been recently proposed in cystic renal classification [31]. These diagnostic criteria of CN were initially established by Joshi and Beckwith [32] for cystic, partially differentiated nephroblastoma and subsequently by Eble and Bonsib for adult [33].
4. Conclusion
In conclusion, cystic kidney tumors are still prone to confusion and dilemma in preoperative diagnosis because they have a diffuse cystic growth development and are similar in their macroscopic appearances. Cystic nephroma is a benign stromal tumor and is a composite of epithelium of an unknown origin with the potential for secondary development of sarcoma. Wilms tumor, with metastatic capacity, might sometimes be misdiagnosed as partially differentiated nephroblastoma. The multilocular cystic renal cell carcinoma has a potential for cystic as well as malignant development, is an expansive mass surrounded by a fibrous wall, and its interior is entirely cystic, separated by septa containing clusters of epithelial cells with clear cytoplasm. Cystic hamartoma is rare, composed of a stroma with a large, smooth muscle component and a variety of epithelial elements. Until today, only histological and immunohistochemical analyzes are available for a positive diagnosis of CN, MEST and of the other entities with malignant potential.
Acknowledgment
We thank Aida Darouichi for professional advice and support during this study.
References
- Jevremovic D, Lager DJ, Lewin M. Cystic nephroma (multilocular cyst) and mixed epithelial and stromal tumor of the kidney: a spectrum of the same entity? Annals of diagnostic pathology 10 (2006): 77-82.
- Michal M, Hes O, Bisceglia M, et al. Mixed epithelial and stromal tumors of the kidney: a report of 22 cases. Virchows Arch 445 (2004): 359-367.
- Wilkinson C, Palit V, Bardapure M, et al. Adult multilocular cystic nephroma: Report of six cases with clinical, radio-pathologic correlation and review of literature.Urol Ann 5 (2013): 13-7.
- Hirai T, Ohishi H, Yamada R, et al. Usefulness of colour Doppler flow imaging in differential diagnosis of multilocular cystic lesions of the kidney. J Ultrasound Med 14 (1995): 771-776.
- Silver IM, Boag AH, Soboleski DA. Best cases from the AFIP: Multilocular cystic renal tumor: Cystic nephroma. Radiographics 28 (2008): 1221-1225.
- Kim SH, Choi BI, Han MC, et al. Multilocular cystic nephroma: MR findings. AJR Am J Roentgenol 153 (1989): 1317.
- Turbiner J, Amin M, Humphrey PA, et al. Cystic nephroma and mixed epithelial and stromal tumor of kidney: a detailed clinicopathologic analysis of 34 cases and proposal for renal epithelial and stromal tumor (REST) as a unifying term. Am J Surg Pathol 31 (2007): 489-500.
- Pawade JGN Soosay, Delprado W, Parkinson MC, et al. Cystic hamartoma of the renal pelvis. Am J Surg Pathol 17 (1993): 1169-1175.
- Michal M, Syrucek M. Benign mixed epithelial and stromal tumor of the kidney. Pathol Res Pract 194 (1998): 445-558.
- Eble JN. Mixed epithelial and stromal tumour. In Eds.: Eble JN, Sauter G, Epstein II, et al. Tumours of the Urinary System and Male Genital Organs. Lyons, France: IARC Press, World Heath Organization Classification of Tumours (2004): 77-78.
- Bosniak M. Diagnosis and management of patients with complicated cystic lesions of the kidney. AJR 169 (1997): 819-821.
- Han KR, Janzen NK, McWhorter VC, et al. Cystic renal cell carcinoma: biology and clinical behaviour. Urol Oncol 22 (2004): 410-414.
- Hora M, Hes O, Michal M, et al. Extensively cystic Renal Neoplasms in Adults (Bosniak II or III)-possible “Common” Histological Diagnosis: Multilocular cystic renal carcinoma, Cystic Nephroma, and Mixed epithelial and stromal tumour of the kidney. Int J Urol Nephrol 37 (2005): 743-750 .
- Murad T, Komaiko W, Oyasu R, et al, Multilocular cystic renal cell carcinoma. Am J Clin Pathol 95 (1991): 633-637.
- Bielsa O, Lloreta J, Gelabert MA. Cystic renal cell carcinoma (pathological features, survival and implications for treatment). Br J Urol 82 (1998): 16-20.
- van den Hoek J, de Krijger R, van de Ven K, et al. Cystic nephroma, cystic partially differentiated nephroblastoma and cystic Wilms’ tumor in children: A spectrum with therapeutic dilemmas. Urol Int 82 (2009): 65-70.
- Jevremovic D, Lager DJ, Lewin M. Cystic nephroma (multilocular cyst) and mixed epithelial and stromal tumor of the kidney: a spectrum of the same entity? Ann Diagn Pathol 10 (2006): 77-82.
- Mazzucchelli R, Lopez-Beltran A, Martignoni G, et al. Cystic nephroma and mixed epithelial and stromal tumour of the kidney: opposite ends of the spectrum of the same entity? Eur Urol 54 (2008): 1237-1246.
- Jujju Jacob Kurian, Susan Jehangir. Multiloculated Cystic Renal Tumors of Childhood: Has the Final Word Been Spoken. Indian Assoc Pediatr Surg 23 (2018): 22-26.
- Kurian JJ, Jehangir S, Korula A. Multiloculated Cystic Renal Tumors of Childhood: Has the Final Word Been Spoken. J Indian Assoc Pediatr Surg 23 (2018): 22-26.
- Walford N, Delemarre JF. Wilms' tumour associated with deep cystic nephroma-like changes: three cases of a putative Wilms' tumour precursor. Histopathology 18 (1991): 123-131.
- Tajima S, Waki M. Cystic partially differentiated nephroblastoma in an adult: a case imitating the process of normal nephrogenesis along with corresponding WT1 expression. Int J Clin Exp Pathol 8 (2015): 989-994.
- Sangkhathat S, Kanngurn S, Chaiyapan W, et al. Wilms’ tumor 1 gene (WT1) is overexpressed and provides an oncogenic function in pediatric nephroblastomas harboring the wild-type WT1. Oncol Lett 1 (2010): 615-619.
- Pawade J, Soosay GN, Delprado W, et al. Cystic hamartoma of the renal pelvis. Am J Surg Pathol 17 (1993): 1169-1175.
- van den Hoek J, de Krijger R, van de Ven K, et al. Cystic nephroma, cystic partially differentiated nephroblastoma and cystic Wilms’ tumor in children: a spectrum with therapeutic dilemmas. Urol Int 82 (2009): 65-70.
- Doros LA, Rossi CT, Yang J, et al. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol 27 (2014): 1267-1280.
- Adsay V, Eble JN, Srigley JR, et al. Mixed epithelial and stromal tumor of the kidney. Am J Surg Pathol 24 (2000): 958-970.
- Xiang H, Ding W, Liu F, et al. Clinicopathologic analysis of mixed epithelial and stromal tumor of kidney and adult cystic nephroma]. Chinese article 8 (2009): 436-440.
- Picken MM, Bova D, Pins MR, et al. Mixed Epithelial and Stromal Tumor of the Kidney with Extension into Inferior Vena Cava: Case Report and Discussion of Adult Biphasic Cystic Renal Lesions and the Significance of Vascular Involvement. Case Rep Pathol (2018): 8234295.
- Antic T, Huang M, Picken MM. Evolving concepts of cystic renal lesions: The controversy over cystic nephroma and mixed epithelial and stromal tumor of the kidney. Pathology Case Reviews 11 (2006): 173-177.
- Pierson CR, Schober MS, Wallis T, et al. Mixed epithelial and stromal tumor of the kidney lacks the genetic alterations of cellular congenital mesoblastic nephroma. Hum Pathol 32 (2001): 513-520.
- Joshi VV, Beckwith JB. Multilocular cyst of the kidney (cystic nephroma) and cystic, partially differentiated nephroblastoma. Terminology and criteria for diagnosis. Cancer 64 (1989): 466-479.
- Eble JN, Bonsib SM. Extensively cystic renal neoplasms: Cystic nephroma, cystic partially differentiated nephroblastoma, multilocular cystic renal cell carcinoma, and cystic hamartoma of renal pelvis. Semin Diagn Pathol 15 (1998): 2-20.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 