Rheumatic Valve Disease or Cardiotoxicity. Key Points for Differential Diagnosis
Ricardo Alvarez-Santana MD1,2, Mara Escudero-Salamanca MD1,3, Jesus A Garcia-Diaz MD1,4, Alberto Aranda-Fraustro5, Erick Alexanderson-Rosas1,6, Nilda Espinola-Zavaleta MD, PhD1,7*
1Department of Nuclear cardiology, National Institute of Cardiology Ignacio Chavez, Mexico City, Mexico
2Institute of Medical Sciences. Autonomous University of Ciudad Juarez, Ciudad Juarez-Chihuahua, Mexico
3Mexican Faculty of Medicine La Salle University, Mexico City, Mexico
4 Academic Unit of Medicine. Autonomous University of Nayarit, Tepic-Nayarit, Mexico
5Department of Pathology, National Institute of Cardiology Ignacio Chavez, Mexico City, Mexico
6Department of Physiology, Faculty of Medicine. Autonomous National University of Mexico, Mexico City, Mexico
7 Department of Echocardiography, ABC Medical Center, I.A.P, Mexico City, Mexico
*Corresponding author: Nilda Espinola-Zavaleta, Department of Nuclear cardiology, National Institute of Cardiology Ignacio Chavez, Mexico City, Mexico
Received: 30 June 2020; Accepted: 09 July 2020; Published: 13 July 2020
Article Information
Citation: Ricardo Alvarez-Santana, Mara Escudero-Salamanca, Jesus A Garcia-Diaz, Alberto Aranda-Fraustro, Erick Alexanderson-Rosas, Nilda Espinola-Zavaleta. Rheumatic Valve Disease or Cardiotoxicity. Key Points for Differential Diagnosis. Archives of Clinical and Biomedical Research 4 (2020): 268-273.
View / Download Pdf Share at FacebookKeywords
<p>Cardiotoxicity; Diagnosis</p>
Article Details
Introduction
The etiology of valvular heart disease is rheumatic, congenital or degenerative, but secondary to chemotherapy only in isolated cases. Rheumatic heart disease remains the leading cause of valvular disease in developing countries. In this pathology, echocardiography provides anatomical details of both the mitral and aortic valves [1].
There are some authors who claim that chemotherapy agents do not directly affect the heart valves, and that the most common valve abnormalities caused by radiation are fibrosis and calcification of the aortic and mitral valves. International guidelines state that an annual physical examination and echocardiography are recommended in patients, who received chemotherapy [2]. It is well known that the prevention of cardiotoxicity by cardio-protectors is the main recommendation for patients who started treatment, but what about the survivors?
Are patients with multivalvular heart disease a new group of cardiotoxicity survivors that should be differentiated from rheumatic heart disease?
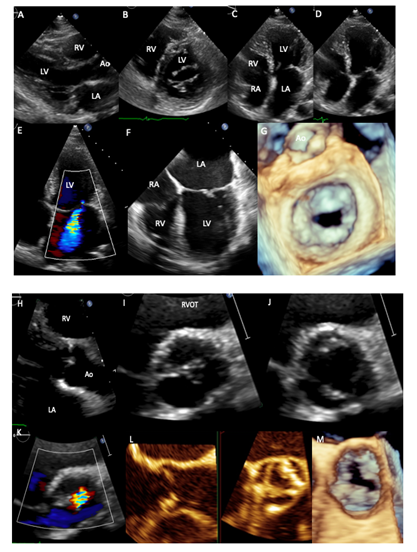
A 65-year-old female with history of cervical cancer in 1996 that underwent hysterectomy and intestinal Hodgkin lymphoma in 2012 treated with hemicolectomy and 6 cycles of anthracyclines and Rituximab, with remission since 2015. On cardiac examination, palpable apex in 6th intercostal space, mid-systolic aortic murmur II/IV soft diastolic murmur in Erb´s point II/IV, diastolic and systolic mitral murmur IV/IV and systolic tricuspid murmur I/IV were detected. Transthoracic echocardiogram (2018) showed left atrial enlargement (42 ml/m2), thickening and calcification of the mitral leaflets with valvular area of 1.46 cm2, without commissural fusion and thickening of the subvalvular apparatus (A-D), moderate-to-severe mitral regurgitation (E), these data were corroborated by transesophageal echocardiography, where thickening and calcifications of the mitral leaflets are clearly observed (F, G). Aortic valve had thickening and focal calcifications of the leaflets and double aortic lesion with valve area of 1.2 cm/m2 and mean systolic gradient of 37 mmHg (H-M). Abbreviations: RA-right atrium, RV-right ventricle, LA-Left atrium, LV-Left ventricle, Ao-aorta, RVOT-Right ventricular outflow tract.
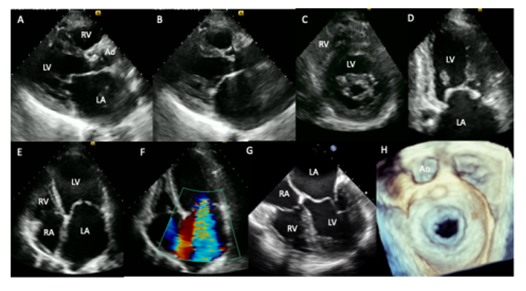
A 34-year-old female patient with a history of rheumatic heart disease in childhood. She came to our institution for fatigue, progressive dyspnea from moderate to small efforts, paroxysmal nocturnal dyspnea and stabbing chest pain of 5-months evolution. On cardiac auscultation, arrhythmic heart sounds, regurgitant murmur in mitral focus III / IV and holosystolic murmur in accessory aortic focus III / IV were found. Transthoracic and two-and three-dimensional transesophageal echocardiogram (2017) showed severe left atrial enlargement (128 ml/m2), mitral valve with leaflets thickening, the anterior has the appearance of a “hockey stick” and the posterior is fixed, with commissural fusion and thickening of sub-valvular apparatus, mitral area of 0.8 cm2, mean gradient of 12 mmHg and severe mitral regurgitation (A,B,C,D,E,F). Aortic valve with leaflets thickening and double aortic lesion with moderate aortic stenosis (mean gradient: 30 mmHg), severe aortic insufficiency (pressure half time: 258 ms) and normal systolic biventricular function (I-N).
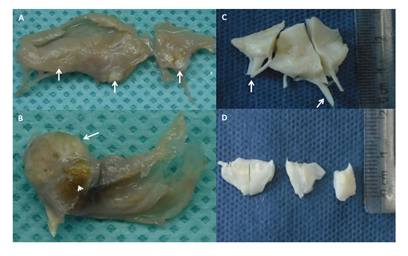
Case 1. The patient underwent bioprosthetic mitral Nº 28 and aortic valve Nº 22 replacement.
Mitral Valve photography. Diffuse thickening mainly in the free edge with small whitish nodules that correspond to calcifications (white arrows). Chordae tendineae remain thin and long.
Aortic valve photography. A large calcified nodule (white arrow) with an area of ulceration (white arrowhead). Rest of the leaflet with thickening of its free edge and some portions of the leaflet.
Case 2. Mechanical aortic St Jude Masters 21 and mechanical mitral valve St Jude Masters 25 replacement was performed.
Mitral Valve photography. The fragments are white with a fibrous appearance and marked thickening of the free edge and chordae tendineae.
Aortic valve photography. Two semilunar leaflets and a portion of the third are observed, they are white in appearance and fibrous in consistency with marked thickening that predominates on the free edge.
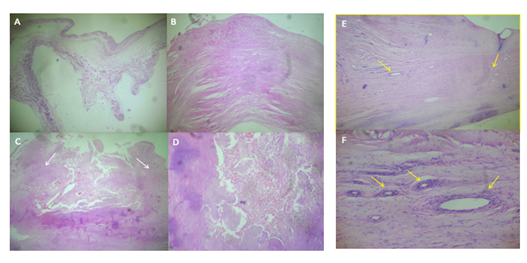
Case 1. Mitral valve. A) Area with preserved trilaminar structure, normal thickness, and replacement of the fibroelastic central layer by myxoid tissue, rich in interstitial matrix-type mucosa. H / E 2.5 x. B) Area with scar-like fibrosclerosis, which significantly increases its thickness. H / E 2.5 x. C) Ulceration zone, note the lateral fibrous edges (white arrows) that limit a central necrosis zone. H / E 2.5 x. D) Valvular necrosis zone replaced by fibrinous material with cellular debris. H / E 10x.
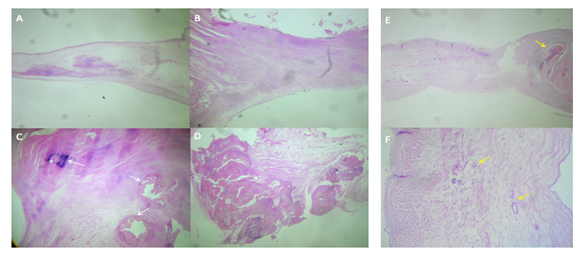
Case 2. Mitral valve. E. Increased thickness of the leaflet is observed, due to diffuse scar fibrosis, there are also numerous new blood vessels (yellow arrows). HE 10x. F. Neoformation blood vessels are observed, with wall thickening due to muscle cell hyperplasia (yellow arrows), characteristic finding of rheumatic sequelae.
Case 1. Aortic valve. A) Myxoid transformation zone, which maintains normal thickness, replacing fibroelastic tissue with interstitial matrix-type mucosa. HE 2.5 x. B) Area of scar fibrosclerosis rich in fibroblasts and collagen and significant increase of the leaflet thickness. H / E 2.5x. C) Scar fibrosclerosis zone with three foci of dystrophic calcification (white arrows) H / E 2.5x. D) Large calcified nodule. H / E 2.5 x.
Case 2. Aortic valve. E) Marked nodular thickening of the free edge due to nodular scar fibrosclerosis, to the right of the observer (yellow arrow), to the left of the observer, the proximal portion of the leaflet has proliferation of blood vessels. HE 2.5 x. F. Neoformation of blood vessels with hyperplastic walls (yellow arrows), characteristic findings of rheumatic damage. HE 10x.
References
- Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography. A scientific statement from the American Heart Association. Circulation 131 (2015): 1806-1818.
- Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of ault patients during and after cance therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular imaging. Eur Heart J-Cardiovasc Imaging 15 (2014): 1063-1093.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 