Staphylococcus aureus Lipoteichoic Acid has a Dual Effect on Inflammatory Responses in Airway Epithelial Cells via the Transcription Factor NFκB
Clare M Cooksley1, Hai Bac Tran1, Alkis J Psaltis1, Susan Lester2, Peter-John Wormald1, Sarah Vreugde1?
1Department of Surgery - Otolaryngology Head and Neck Surgery, University of Adelaide, Adelaide, Australia
2Department of Rheumatology, The Queen Elizabeth Hospital, Adelaide, Australia
*Corresponding author: Sarah Vreugde, Department of Surgery - Otolaryngology Head and Neck Surgery, The University of Adelaide, The Queen Elizabeth Hospital, 28 Woodville Road, Woodville South SA 5011, Australia
Received: 21 July 2020; Accepted: 28 July 2020; Published: 14 August 2020
Article Information
Citation: Clare M Cooksley, Hai Bac Tran, Alkis J Psaltis, Susan Lester, Peter-John Wormald, Sarah Vreugde. Staphylococcus aureus Lipoteichoic Acid has a dual effect on inflammatory responses in airway epithelial cells via the transcription factor NFκB. Archives of Clinical and Biomedical Research 4 (2020): 367-380.
View / Download Pdf Share at FacebookAbstract
Staphylococcus aureus is a major human pathogen that causes a wide array of infections ranging in severity from mild skin infections to life-threatening diseases such as sepsis. S. aureus can cause severe inflammation whilst, at the same time, modulating specific innate and humoral immune defense mechanisms. Lipoteichoic acid (LTA) is a component of the cell wall of gram-positive bacteria that is abundantly released by S. aureus. We investigated the dose-dependent effects of LTA on inflammation in airway epithelial cells. Purified S. aureus LTA was applied to airway epithelial (NuLi-1) cells in a range of concentrations, followed by assessment of expression of NFκB p50 and p65/RelA via qPCR, immunofluorescence and western blot. Multiplex analysis of cell culture supernatants was carried out to evaluate cytokine levels. NFκB p50 mRNA varied according to the LTA dose (p = 0.013) whereas NFκB p65/RelA did not (p = 0.90). A dose-dependent increase in NFκB p50 mRNA expression was seen up to 10 μg/ml LTA treatment, before falling at the 100μg/ml treatment. Western blot analysis showed nuclear and cytoplasmic NFκB p50 and cytoplasmic NFκB p65 protein reduced with increasing LTA concentrations. Multiplex analysis demonstrated that cytokine levels increased with LTA dose, but then decreased at the highest LTA dose (100 μg/ml). This study identifies LTA as having a dual dose-dependent effect on immune activation at low dosages and ablation at high dosages, associated with altered expression levels of NFκB. It adds LTA to the extensive repertoire of possible immune modulators produced by S. aureus.
Keywords
<p>Lipoteichoic acid; Staphylococcus aureus; NFκB p50; Immune evasion</p>
Article Details
Abbreviations
LTA= Lipoteichoic Acid
NFκB = Nuclear Factor kappa B
1. Introduction
Staphylococcus aureus is responsible for causing diseases ranging in severity from mild skin and soft tissue infections (SSTI) to severe and life-threatening diseases such as endocarditis, osteomyelitis, septic arthritis and sepsis. S. aureus colonizes the nares of approximately 56% of human populations (24% permanently and 31% intermittently) [1, 2]. Most infections result from endogenous nasal carriage, even though the causal events underlying the transition from colonization to infection are not well defined [3]. In particular, persistent carriers have a higher chance of developing staphylococcal infections compared to intermittent or non- S. aureus carriers [4]. The mechanisms leading to S. aureus nasal carriage are thought to be multi-factorial and involve host factors (e.g. immune function and genetic susceptibility) [5, 6], environmental factors (e.g. hospitalisation and crowding) [7] and bacterial factors (e.g. toxin production) [6, 8]. Bacterial factors promoting nasal carriage are particularly those that promote adhesion of S. aureus to epithelial cells such as clumping factor B and teichoic acids [8, 9]. Teichoic acids are characteristic of gram-positive bacteria and play fundamental roles in bacterial physiology and morphology. They include wall teichoic acids (WTAs), which are covalently linked to the peptidoglycan polymer of the bacterial cell wall, and lipoteichoic acids (LTAs), which are anchored in the bacterial membrane via a glycolipid [10, 11]. LTA is thought to play an important role in bacterial cell division and has been shown to activate the immune system of infected hosts and promote inflammation [12].
1. aureus can cause recurrent and chronic infections even in the presence of a robust immune response, implying that prior infection with S. aureus does not result in protective immunity to subsequent infections [13]. Indeed, vaccination of mice with heat killed S. aureus induces activation of dendritic cells, T-cells and B-cells. However, neither those cells nor their secreted cytokines IFN-γ and IL-17, affect bacterial clearing from organs or disease outcome [14]. Moreover, S. aureus can prime human TH17 cells to produce either IFN-γ or IL- 10 [15] and a large S. aureus-specific T-memory cell pool is commonly present in healthy adults irrespective of their carrier status [16]. Antibodies to S. aureus toxins and antigens are relatively stable over time in persistent carriers as well as in non-carriers, however, median levels of antibodies to some of those antigens are higher in persistent carriers [17]. Together, this data indicates that S. aureus elicits a strong and lasting B- and T-cell dependent immune response that is, however, ineffective at protecting against re-infection. These findings are in line with the notion that S. aureus has developed sophisticated mechanisms to evade the host adaptive and innate immune defences. These mechanisms enable the pathogen to survive and establish effective and recurrent infections in the context of a hostile antigen- specific immune response. Indeed, numerous S. aureus factors have been described that can reduce or abrogate both innate and humoral immune defence mechanisms at different levels [18]. In particular, LTA has been shown to modulate innate and adaptive immunity by suppressing the activation, proliferation and migration of T-cells [19, 20] and neutrophils [21]. However, the effect of LTA on immune activation of airway epithelial cells has not been established. This study evaluated the effect of LTA at different concentrations on immune activation of airway epithelial cells in vitro.
2. Materials and Methods Cells and Cell Culture
NuLi-1 (CRL-4011) cells were obtained from The American Type Culture Collection (ATCC). They were grown in Bronchial Epithelial Growth Medium, serum-free (BEGM Bullet kit, CC- 3170) (Lonza group, Basel, Switzerland). This was made up from BEBM basal medium and SingleQuot additives, with the exception of gentamycin-amphotericin B. In addition, medium was supplemented with 50 µg/ml of G-418 disulfate salt (Sigma-Aldrich, Castle Hill, Australia). Cells were cultured in collagen-coated cell culture flasks (ThermoFisher Scientific, Waltham, Massachusetts, USA) and for agonist stimulation experiments were seeded into Falcon 8 well glass chamber slides (Corning Inc, Mulgrave, Australia), 6-well culture plates (ThermoFisher Scientific) or 12-well culture plates (Merck Millipore, Bayswater, Australia). Multiwell plates were coated with 60µg/ml collagen IV (Sigma-Aldrich).
2.1 Treatment of NuLi-1 cells with purified S. aureus LTA
Purified S. aureus LTA (LTA-SA, Invivogen, San Diego, USA) was applied to the culture medium of NuLi-1 cells in concentrations ranging from 0.1 µg /ml to 100 µg/ml for different times and incubated at 37°C with 5% carbon dioxide and 95% humidity. Invivogen’s LTA-SA is highly purified using n-butanol extraction to ensure purity and specific bioactivity [22]. After treatment, culture medium was harvested and stored at -80°C. Cells were subjected to RNA extraction, immunofluorescence or nuclear and cytoplasmic protein extraction.
2.2 Immunofluorescence for NFκB
Cells grown in 8-well culture slides were fixed with 2.5% formalin (Sigma-Aldrich) in phosphate buffered saline (PBS) for 10 minutes before washing four times in tris buffered saline + 0.05% Tween 20 (TBST, Sigma-Aldrich) and air drying. Cells were permeabilised with 1% Sodium Dodecyl Sulphate (Sigma-Aldrich) in TBS for 5 minutes before washing 5 times with TBST. A drop of serum free block (SFB; Dako, Glostrup, Denmark) was applied to each well and incubated for 1 hour at room temperature. Cells were then incubated overnight at 4°C in primary antibody mouse anti-human NFκB p105/p50, 1:100 (Abnova Corporation, Taipei City, Taiwan) or rabbit anti-human NFκB p65, 1:40 (Santa Cruz Biotechnology Inc, Dallas, Texas, USA) diluted in 10% SFB/TBST. Excess primary antibody was removed by washing five times with TBST before incubation for 1 hour at room temperature with secondary antibody [for NFκB p105/p50, sheep anti-mouse Cy3, 1:200, (Sigma-Aldrich); for NFκB p65, donkey anti-rabbit Alexafluor 647, 1:200 (Jackson ImmunoResearch Labs Inc., PA, USA)]. After 3 washes with TBST, cells were counterstained with 4', 6-diamidino-2- phenylindole (DAPI, Sigma-Aldrich) before washing a final twice and mounting with Fluorescence Mounting Medium (Dako). Slides were stored at 4°C in dark conditions and visualised using fluorescent microscopy (Zeiss, Oberkochen, Germany).
2.3 Nuclear and cytoplasmic protein extraction
Nuclear and cytoplasmic protein fractionation was carried out on cells grown in 6-well culture plates using the Pierce NE-PER Nuclear and Cytoplasmic Protein Extraction Reagents kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
2.4 Western analysis<
Protein was quantified using the Pierce BCA protein assay kit (Thermo Fisher Scientific), according to the manufacturer’s instructions. Western analysis was performed using the Invitrogen XCell SureLock Mini Cell System (Thermo Fisher Scientific). 5 µg protein was resolved using a 10% Bis-Tris NuPAGE gel and transferred onto a nitrocellulose membrane. Membranes were probed with rabbit monoclonal anti-human NFκB p105/p50 (1:5000; Abcam Plc, Melbourne, Australia), and rabbit polyclonal anti-human NFκB p65 (1:1000; Santa Cruz) antibodies. Equal protein loading was assessed using rabbit polyclonal anti- human Profilin I antibody (1:10,000; Thermofisher Scientific) as a cytoplasmic marker and mouse monoclonal anti-human TBP antibody (1:5:000; Abcam Plc) as a nuclear marker. Secondary antibodies used were HRP-conjugated goat anti-rabbit (Abcam Plc) and goat anti- mouse (Abnova). Protein bands were developed using Invitrogen ECL chemiluminescent substrate (ThermoFisher Scientific). Luminescence was detected using the LAS-4000 Imager (Fugifilm, Tokyo, Japan). Band densitometry was performed using Multi Gauge 2.0 software (Fugifilm). Densitometry measurements of NFκB p50 (n = 3) and NFκB p65/RelA (n = 2) Western Blots were normalised to either profilin (for cytoplasmic protein) or TBP (for nuclear protein). Analysis of LTA dose was performed using a gamma (log link) generalised regression model, with blot replicates included as a blocking variable, and total intensity (across all bands) as the exposure variable. Results were expressed as intensity ratios, relative to no LTA treatment.
2.5 RNA extraction from NuLi-1 cells
Cells which had been grown in 12-well collagen coated plates were washed in PBS, followed by RNA extraction using the RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). An on-column DNAse treatment was performed using an RNase- Free DNase Set (Qiagen). RNA integrity was assessed using an Experion RNA StdSens Kit and the Experion Electrophoresis station (Bio-Rad Laboratories, Gladesville, Australia). RNA concentration was quantified using a NanoDrop 1000 Spectrophotometer (ThermoFisher Scientific).
2.6 Reverse Transcription and Real Time Quantitative PCR
RNA was reverse transcribed into cDNA using the QuantiTect Reverse Transcription kit (Qiagen) according to the manufacturer’s instructions. For each sample a control omitting the reverse transcriptase enzyme was prepared. Real time qPCR was performed using Universal Taqman Mastermix II and Taqman Gene Expression Assays (both Life Technologies by ThermoFisher Scientific). Gene expression assays used were Hs99999903_m1 (Actin-B), Hs00765730_m1 (NFκBp50) and Hs00153294_m1 (NFκB p65/RelA). Relative quantification was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories). Linear mixed model analysis was used to estimate DDCt values accounting for all sources of experimental variation [between experiment (n = 2), within experiment treatment-time replicates (n = 3) and technical qPCR replicates (n = 2)], as described [23]. Results were expressed as fold change, after normalization to both β-actin and the no LTA treatment group.
2.7 Multiplex cytokine analysis
Multiplex analysis of cell culture supernatant samples was performed on a Bio-Plex 200 system (Bio-Rad Laboratories) using a Bio-Plex Pro Human Cytokine Group I panel 8-plex assay according to the manufacturer’s instructions. Cytokines analysed were IL-2, IL-4, IL-6, IL-8, IL-10, GM-CSF, IFN-g and TNF-α. Cytokine release was assessed using an LTA dose- time factorial design, with three experimental replicates for each LTA dose-time combination. Analysis was performed by a MANOVA linear model because cytokines were measured simultaneously in a multiplex assay. Results were expressed as the change in cytokine relative to the reference level.
2.8 Cell viability assay
Viability of treated cells was measured using the Cyto Tox 96 Non-Radioactive Cytotoxicity Assay (Promega Corporation, Madison, WI, USA) according to the manufacturer’s instructions. LDH release was measured according to a LTA dose-time factorial design, with three experimental replicates, each with 2 measurement replicates, for each treatment- time combination. Analysis was performed by a random intercept linear mixed regression model, with loge(Absorbance) as the response. Results were expressed as percentage viability, relative to no LTA treatment at one hour, according to the formula: (1- coefficient)*100.
2.9 Statistics
All analyses were performed using Stata v14 (StataCorp LLC, College Station, Texas USA). Analyses were performed using generalised regression models that were appropriate for the outcome variable and the experimental structure in the data. Ordinal trends in the data (LTA dose response and time response) were interpreted using orthogonal polynomial contrasts, with p-values corrected for multiple comparisons using Sidak’s methods. When both LTA dose and time were included in the experiment, interpretation was performed on main effects only. P-values < 0.05 indicated statistical significance.
3. Results
3.1 Gene expression analysis
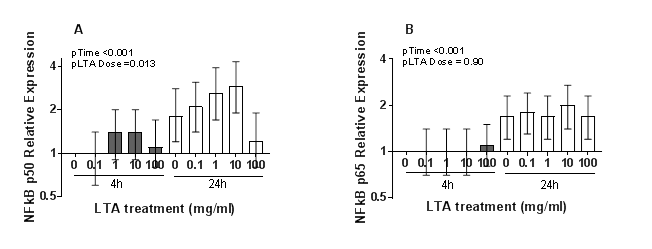
Both NFκB p50 and p65/RelA mRNA increased between 4 and 24 hours (p < 0.001). LTA treatment induced a dose-dependent increase in NFκB p50 mRNA expression up to 10 µg/ml LTA treatment, before falling at the 100µg/ml treatment (Figure 1). NFΚBp50 mRNA varied according to the LTA dose (p = 0.013) whereas NFκB p65/RelA did not (p = 0.90). While there was not a linear trend for NFκB p50 expression and LTA dose (pcorrected = 1.00), there was a significant quadrature term (pcorrected = 0.014) indicative of lower NFκBp50 expression at high LTA doses.
Figure 1: Changes in relative mRNA expression of (A) NFkB p50 and (B) NFkB p65/RelA with LTA dose and time. Data shown is the mean of 2 experimental replicates, each consisting of 3 treatment-time replicates and 2 technical qPCR replicates. Normalised Ct values were expressed relative to the no LTA treatment group at 4 hours (DDCt method). Vertical bars represent 95% confidence intervals.
3.2 NFκB protein expression
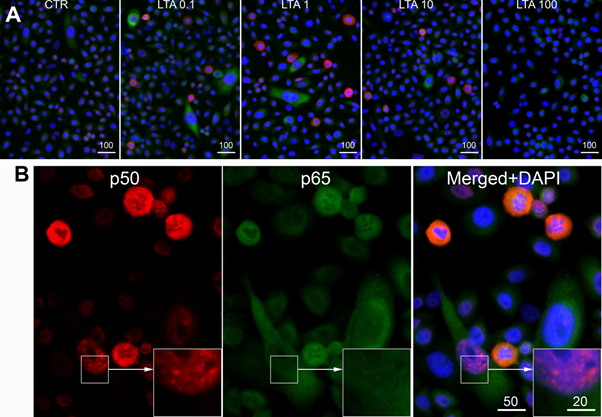
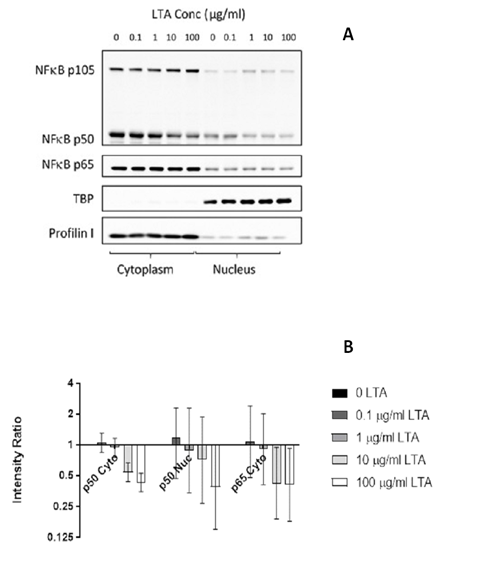
Immunofluorescence analysis showed cytoplasmic and nuclear expression of both NFkB p50 and p65/RelA in NuLi-1 cells treated with LTA and control cells (Figure 2). Western blot analysis showed a reduction in expression of both nuclear and cytoplasmic NFkB p50 protein with increasing LTA concentration, consistent with a dose-response relationship. The reduction was significant in both the cytoplasm (p < 0.001) and the nucleus (p = 0.031). Cytoplasmic NFkB p65 protein also decreased significantly with increasing LTA dose (p = 0.004), but the NFkB p65 nuclear bands were weak with no substantial changes (Figure 3).
3.3 Cytokine analysis
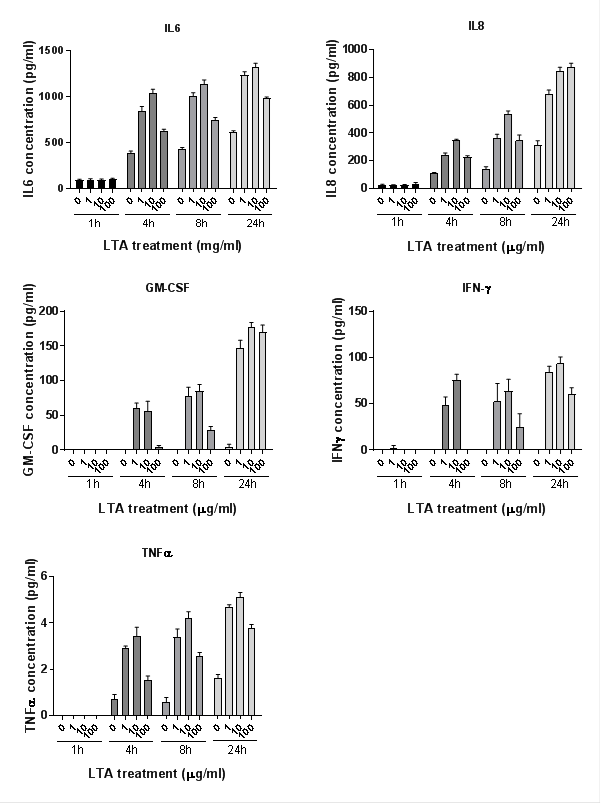
Cytokines were measured using Multiplex assay. Five of the eight inflammatory cytokines analysed were produced at detectable levels (IL-6, IL-8, GM-CSF, IFN-g and TNF-α, Figure 4). Cytokine release increased in a time-dependent manner for all cytokines (plinear < 0.001 for all). At 1 hour, levels were mostly undetectable; therefore analysis was only performed on data from 4, 8 and 24 hours. TNFα was also excluded from statistical analysis due to its presence at low concentrations. Cytokine levels increased with LTA dose, (plinear < 0.001 for all), but the level decreased at the highest LTA dose (100 µg/ml) (pquadratic < 0.001 for all).
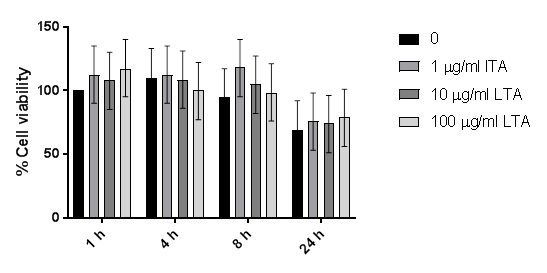
Cell viability assays showed no significant difference between LTA treatments, confirming that the changes in cytokine levels were not due to cytotoxic effects of LTA (see Supplementary data).
Figure 2: Representative images of NFκB p50 (red) and p65/RelA (green = pseudo colour of far red immunofluorescence) in NuLi-1 cells. A, Cells were treated with different doses of LTA (0; 0.1; 1; 10 and 100 µg/ml). B, Cells were treated with LTA at 10 µg/ml. Both nuclear and cytoplasmic expression of NFκB p50 (red) and p65/RelA could be seen. Scale bars are in micrometers.
Figure 3: Western blot analysis (A) and densitometry (B) of NFκB p50 and p65/RelA production in nuclear and cytoplasmic protein fractions extracted from NuLi-1 cells after 24 hour treatment with four concentrations of LTA-SA. Error bars indicate the 95% CIs. P values for reduction relative to the 0 LTA treatment were <0.001 for NFκB p50 cytoplasmic, 0.031 for NFκB p50 nuclear and 0.004 for NFκB p65 cytoplasmic. Data shown represents the mean of 3 biological replicates for p50; 2 for p65/RelA.
Figure 4: Cytokine production in NuLi-1 cells after 1, 4, 8 and 24 hours treatment with four concentrations of LTA-SA. Cytokine release increased significantly in a time-dependent manner for all cytokines (plinear < 0.001 for all) and with LTA dose, (plinear < 0.001 for all). Levels decreased significantly at the highest LTA dose (pquadratic < 0.001 for all). Error bars represent standard error. Data shown represents the mean of 3 biological replicates.
4. Discussion
This study showed that S. aureus LTA had a dose-dependent effect on the mRNA expression of NFκB p50 in airway epithelial cells with induction of expression at low concentrations and reduced expression at high concentrations. These changes in expression were associated with dose-dependent changes in the release of pro-inflammatory cytokines. Together, this data indicates that LTA has a dose-dependent dual effect on inflammation with a reduction in inflammation at high LTA concentrations associated with reduced NFκB p50 expression.
NFκB is the master regulator of inflammatory and stress responsive gene transcription and plays a pivotal role in the immune response to infection, cell proliferation and survival [24]. Dysregulation of NFκB expression has been linked to various inflammatory diseases such as cancer, autoimmune diseases and septic shock. NFκB activation depends on a number of positive and negative regulatory elements. In the absence of inflammatory stimulation, NFκB dimers are held inactive in the cytoplasm through association with IkB proteins. Inflammatory stimuli trigger a degradation of IkB proteins, releasing NFκB dimers that translocate to the nucleus, and promote transcription of target genes [25]. Recent research has demonstrated that the fold change in gene expression of the nuclear fraction of NFκB determines transcription of inflammatory genes [26]. Interestingly, and in line with those findings, our results indicate a dose-response relationship between LTA and NFκB p50 expression in both the cytoplasm and the nucleus in relation to secretion of pro- inflammatory cytokines. Five NF-κB family members have been described in mammals: RelA/p65, RelB, c-Rel, p50 (NF-κB1), and p52 (NF-κB2). They form either homodimers or heterodimers and can exert both positive and negative effects on target gene transcription. Whilst p50 lacks a C-terminal transactivation domain (TAD), it can regulate transcription through heterodimerization with TAD-containing NF-κB subunits such as RelA/p65, expression of which was also reduced in response to high LTA concentrations in this study.
LTA is a polymer of alternating units of glycolipids and hydrophilic phosphoric acid and is a major constituent found in the envelope of Gram-positive bacteria including S. aureus [27]. LTA has various roles in bacterial cell division, separation and biofilm formation [11] and has immunomodulatory properties by interacting with Toll Like Receptor (TLR) 2, CD14 [12, 28,] CD36 [29] and platelet-activating factor receptor [30]. Binding of LTA to TLR2 results in the activation of a wide array of signaling cascades, including those involving NFκB with induction of pro-inflammatory cytokine secretion. Similarly, in this study, LTA at concentrations below 10 µg/ml induced mRNA expression of NFκBp50 in association with an increased secretion of pro-inflammatory cytokines. Our results indicate however that LTA negatively affects the mRNA and protein expression of p50 NFκB at dosages above 10 µg/ml. It is known that LTA is released in substantial amounts during bacterial growth and infection and in contrast to lipopolysaccharide, which triggers severe inflammatory responses at low concentrations [31], relatively large amounts of LTA (approximately 1- 10 µg/ml) are required to elicit cellular responses in vitro [32]. These large quantities of LTA have been found also in vivo: LTA levels as high as 10 µg/ml were measured in wash fluid samples of atopic dermatitis (AD) patients with levels correlating with S. aureus colony-forming unit (cfu) counts and AD disease severity [33]. Indeed, when the number of S. aureus is around 106 cfu/mL, measurable amounts of LTA can be found in >90% of AD skin lesions [33] with levels above 106 cfu/mL indicative of clinical infection [34]. Moreover, LTA concentrations in cerebrospinal fluid of patients with S. pneumoniae meningitis were as high as 26 µg/ml with an inverse correlation of LTA concentrations and incidence of neurologic sequelae and mortality [35]. LTA has been shown to modulate the adaptive immune system by suppressing the proliferation of CD4+ T-cells [19] and by inducing a temporary functional T-cell paralysis when LTA is applied at high concentrations [20]. In line with our findings, these studies indicate a potential immune evasion mechanism of S. aureus where high LTA concentrations, frequently encountered during S. aureus infection, have a generalized effect on reducing inflammation, potentially in a NFκB-dependent way. Our findings might also be relevant in the context of bacterial sepsis where LTA has been shown to impair neutrophil migration in a TLR2 dependent way [21].
Further research is needed to determine the effect of local and circulating LTA levels on the pathophysiology of infection and whether targeting LTA might affect the balance of pro- and anti-inflammatory responses that are at play in the context of severe infections and sepsis.
5. Conclusion
We have evaluated the effect of LTA on immune activation of airway epithelial cells, which has not previously been established. LTA was shown to have a dual dose-dependent effect on immune activation at low dosages and ablation at high dosages, associated with altered expression levels of NFkB. It adds LTA to the extensive repertoire of possible immune modulators produced by S. aureus. Targeting LTA as a potential immune evasion mechanism might prove a novel therapeutic alternative in fighting staphylococcal infections.
Acknowledgments
The authors would like to thank Dr Mahnaz Ramezanpour for her technical assistance and the Garnett Passe and Rodney Williams Memorial Foundation for funding.
Conflicts of Interest
None relevant to this submission.
References
- Muthukrishnan G, Lamers RP, Ellis A, et al. Longitudinal genetic analyses of Staphylococcus aureus nasal carriage dynamics in a diverse population. BMC Infect Dis 13 (2013): 221.
- Thammavongsa V, Kim HK, Missiakas D, et al. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 13 (2015): 529-543.
- Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5 (2005): 751-762.
- Nouwen JL, Fieren MW, Snijders S, et al. Persistent (not intermittent) nasal carriage of Staphylococcus aureus is the determinant of CPD- related infections. Kidney Int 67 (2005): 1084-1092.
- Nouwen J, Boelens H, van Belkum A, et al. Human factor in Staphylococcus aureus nasal carriage. Infect Immun 72 (2004): 6685-6688.
- van Belkum A, Melles DC, Nouwen J, et al. Co-evolutionary aspects of human colonisation and infection by Staphylococcus aureus. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases 9 (2009): 32-47.
- Peacock SJ, Justice A, Griffiths D, et al. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J Clin Microbiol 41 (2003): 5718-5725.
- Weidenmaier C, Kokai-Kun JF, Kristian SA, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med 10 (2004): 243-245.
- Wertheim HF, Walsh E, Choudhurry R, et al. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med 5 (2008): e17.
- Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev 67 (2003): 686-723.
- Percy MG, Grundling A. Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu Rev Microbiol 68 (2014): 81-100.
- Schroder NW, Morath S, Alexander C, et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD- 2 are not involved. J Biol Chem 278 (2003): 15587-15594.
- Miller LG, Eells SJ, David MZ, et al. Staphylococcus aureus skin infection recurrences among household members: an examination of host, behavioral, and pathogen-level predictors. Clin Infect Dis 60 (2015): 753-763.
- Schmaler M, Jann NJ, Ferracin F, et al. T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-MyD88- dependent T cell activation. J Immunol 186 (2011): 443-452.
- Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 484 (2012): 514- 518.
- Kolata JB, Kuhbandner I, Link C, et al. The Fall of a Dogma? Unexpected High T-Cell Memory Response to Staphylococcus aureus in Humans. J Infect Dis 212 (2015): 830-838.
- Verkaik NJ, de Vogel CP, Boelens HA, et al. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis 199 (2009): 625-632.
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol 3 (2005): 948-958.
- Son YM, Song KD, Park SM, et al. Lipoteichoic acid suppresses effector T cells induced by Staphylococcus aureus-pulsed dendritic cells. J Microbiol Biotechnol 23 (2013): 1023-1030.
- Kaesler S, Skabytska Y, Chen KM, et al. Staphylococcus aureus-derived lipoteichoic acid induces temporary T-cell paralysis independent of Toll-like receptor 2. J Allergy Clin Immunol 138 (2016): 780-790 e786.
- Alves-Filho JC, Freitas A, Souto FO, et al. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci U S A 106 (2009): 4018-4023.
- Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med 193 (2001): 393-397.
- Steibel JP, Poletto R, Coussens PM, et al. A powerful and flexible linear mixed model framework for the analysis of relative quantification RT-PCR data. Genomics 94 (2009): 146-152.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 140 (2010): 883-899.
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 26 (2012): 203-234.
- Lee RE, Walker SR, Savery K, et al. Fold change of nuclear NF-kappaB determines TNF-induced transcription in single cells. Mol Cell 53 (2014): 867-879.
- Schneewind O, Missiakas D. Lipoteichoic acids, phosphate-containing polymers in the envelope of gram-positive bacteria. J Bacteriol 196 (2014): 1133-1142.
- Lotz S, Aga E, Wilde I, et al. Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. Journal of leukocyte biology 75 (2004): 467-477.
- Draing C, Sigel S, Deininger S, et al. Cytokine induction by Gram-positive bacteria. Immunobiology 213 (2008): 285-296.
- Zhang Q, Mousdicas N, Yi Q, et al. Staphylococcal lipoteichoic acid inhibits delayed- type hypersensitivity reactions via the platelet-activating factor receptor. J Clin Invest 115 (2005): 2855-2861.
- Hoetzenecker W, Echtenacher B, Guenova E, et al. ROS-induced ATF3 causes susceptibility to secondary infections during sepsis-associated immunosuppression. Nat Med 18 (2011): 128-134.
- Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev 18 (2005): 521-540.
- Travers JB, Kozman A, Mousdicas N, et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. J Allergy Clin Immunol 125 (2010): 146- 152 e141-142.
- Williams RE, Gibson AG, Aitchison TC, et al. Assessment of a contact- plate sampling technique and subsequent quantitative bacterial studies in atopic dermatitis. Br J Dermatol 123 (1990): 493-501.
- Schneider O, Michel U, Zysk G, et al. Clinical outcome in pneumococcal meningitis correlates with CSF lipoteichoic acid concentrations. Neurology 53 (1999): 1584-1587.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 