The Effects of Glucagon-Like Peptide-1 Receptor Agonists of Liraglutide on Bone Turnover Makers Among Type 2 Diabetes Mellitus Patients: A Meta-Analysis of Randomized Controlled Trials
Jing-yi Kang1, Jin-Tao Guan3*, Shan-shan Lei2*
1School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
2Department of Medicine, Zhejiang Academy of Traditional Chinese Medicine, Hangzhou, Zhejiang 310007, P.R. China
3First People’s Hospital of Taizhou, Zhejiang 318020, China
*Corresponding author(s): Jin-Tao Guan, First People’s Hospital of Taizhou, Zhejiang 318020, China
Shan-shan Lei, Department of Medicine, Zhejiang Academy of Traditional Chinese Medicine, Hangzhou, Zhejiang 310007, P.R. China
Received: 05 January 2022; Accepted: 12 January 2022; Published: 02 February 2022
Article Information
Citation: Jing-yi Kang, Shan-shan Lei, Jin-Tao Guan. The Effects of Glucagon-Like Peptide-1 Receptor Agonists of Liraglutide on Bone Turnover Makers Among Type 2 Diabetes Mellitus Patients: A Meta-Analysis of Randomized Controlled Trials. Archives of Clinical and Biomedical Research 6 (2022): 134-144.
View / Download Pdf Share at FacebookAbstract
Objective: Liraglutide are associated with a decreased risk of fracture among Type 2 Diabetes Mellitus Patients (T2DM) but the mechanism is unclear, the effect of liraglutide on Bone Mineral Density (BMD) and Bone Turnover Markers (BTMs) were taken into consideration.
Method: We searched for randomized controlled trials of liraglutide in PubMed, Embase, the Cochrane Library, Web of Science, CNKI and VIP database up to October 2021. Heterogeneity among studies was examined by Cochrane Q test.
Results: 7 pieces of eligible literature involving 478 patients were divided into the liraglutide treatment group (n = 241) and control group (n = 237) in this meta-analysis. Based on fixed effect model, liraglutide had no beneficial effect on BMD (MD: 0.00; 95% CI: (-0.01, 0.02); p=0.69, I2=0%), but there were significant increase effects of liraglutide on bone gla protein (BGP, MD: 0.63; 95% CI: (0.23, 1.03); p=0.002, I2=36%), Bone specific alkaline phosphatase (BAP, MD: 0.85; 95% CI: (0.30, 1.40); p=0.002, I2=23%) and PINP (MD: 6.90; 95%CI: (5.71, 8.09); p =0.002) compared to conventional treatment. Moreover, liraglutide decrease serum β cross-linked C-telopeptide of type I collagen (β-CTX, MD: 0.03; 95%CI: (0.01, 0.05); p = 0.0005, I2 =0%).
Conclusions: This meta-analysis demonstrated that liraglutide significantly increase OC, BAP, PINP and reduce β-CTX content compared to conventional treatment in T2DM patients, but the difference was no beneficial effect on BMD. This finding may provide additional evidence for the use of liraglutide to improve skeletal health in T2DM patients.
Keywords
<p>Bone turnover markers; BMD; Liraglutide; Meta-analysis</p>
Article Details
1. Introduction
Type 2 Diabetes Mellitus (T2DM) contribute to bone fragility, bone turnover imbalance and predisposes to increased risk of fracture, inferior bone healing and other skeletal complications [1]. In addition, some anti-diabetic drugs have notable detrimental skeletal effects. In spite of the fact that T2DM treatment has witnessed a marked change in recent years, the best method for treating T2DM complicated fracture or osteoporosis is not still know. Consequently, the presentation of an appropriate therapeutic strategy for T2DM-diagnosed osteoporosis patients or bone fragility should not only be effective in re-establishing good glycaemic control but also in minimising skeletal complications. There is increasing evidence that Glucagon-like peptide-1 receptor agonists (GLP-1RAs), now greatly prescribed for the treatment of T2DM, have beneficial skeletal effects. Liraglutide are the chief GLP-1RAs with a broad range of clinical applications. Number of clinical and Meta analysis [2, 3] showed that the incidence of fracture in diabetic patients was reduced by the treatment of liraglutide, and it was considered that the treatment of liraglutide has the effect of improving BMD, improves bone turnover markers. On the basis of these studies, many scholars have discussed the influence of bone metabolism in patients with diabetes mellitus. Although many clinical trials and observational studies investigated the association between bone metabolism and liraglutide, the results are inconsistent for the differences in the research subjects, basic data, dosage and duration of treatment, the conclusion is not uniform. In particular, whether there is a significant difference in BMD and bone resorption can be increased in patients with diabetes mellitus. The present meta-analysis aimed to assess whether liraglutide reduce bone mineral density, bone turnover markers with T2DM patients when compared to placebo or conventional therapy in a Randomized Controlled Trial (RCT). The aim of this study is to systematically provide a theoretical basis for the clinical application of liraglutide to improve skeletal health in T2DM patients.
2. Methods
2.1 Search strategies
We searched for randomized controlled trials of liraglutide in PubMed, Embase, the Cochrane Library, Web of Science, CNKI and VIP database from inception to October 2021. To identify relevant studies comparing the effects of liraglutide with those of placebo or other anti-diabetic drugs in a T2DM population, we used the following search terms: "liraglutide" and "diabetes" and "bone". Of note, preprint results from registered clinical trials were also included.
2.2 Selection of studies
Studies were included in the meta-analysis if they met the following criteria: (a) prospective, randomized, and controlled clinical studies; (b) included adult patients with T2DM; (c) compared liraglutide with either placebo or other anti-diabetic drugs in patients with T2DM, and the dosage of liraglutide ≥ 0.6mg/day; (d) reported the bone bone metabolic index in both groups, including bone mineraldensity (BMD), bone alkaline phosphatase (BAP), bone gla protein (BGP), procollagen type I N-terminal propeptide (PINP), or β cross-linked C-telopeptide of type I collagen (β-CTX). All clinical trials cover at least one of these measures; (e) duration of follow-up ≥ 12 weeks; and (f) original studies published in English and Chinese. On the other hand, studies were excluded if they did not provide original data or only included patients with type I diabetes or bone disease or special populations (eg, children or pregnant women) or no full article or average age ≥75 years.
2.3 Data extraction
JY.K and SS.L were responsible for the literature search according to inclusion criteria and exclusion criteria. After searching, duplicate studies were excluded using EndNote X7; non-eligible literature was further excluded by reviewing the full text. Any disagreement was resolved by the third investigator JT.G. Then, JY.K and SS.L were responsible for extracting data through eligible literature, including the first author, country and region, therapeutic strategies, sample size, intervention dosage, treatment duration, mean years, HbAlc with baseline.
2.4 Assessment of study quality
Study quality was assessed by the guidance of the Cochrane Handbook, which included six parts: random sequence, allocation concealment, performance bias, detection bias, attrition bias, reporting bias and other bias. The risk of bias of each study was evaluated using the Cochrane risk of bias tool [4].
2.5 Data synthesis and analysis
Statistical analysis was performed using Review Manager 5.3 software (The Cochrane Collaboration, Copenhagen, Denmark). Continuous variable data used mean difference (MD) and 95% CI were used as efficacy statistics. Heterogeneity across studies was assessed using I2 statistics. A fixed-effects model was used if no significant evidence of statistical heterogeneity or clinical diversity was found (p ≥0.05, I2 ≤ 50%); otherwise, a random-effects model was used (p < 0.05, I 2 > 50%). Publication bias was evaluated by visual inspection of funnel plot asymmetry.
3. Results
3.1 Selection of eligible literatures
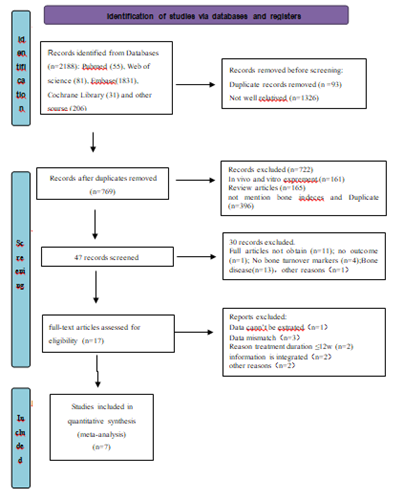
Following the searching strategy described in Figure 1, 2188 pieces of literature were initially identified based on the assessment of the titles and abstracts. We excluded 2181 pieces of literature strictly conformed to the inclusion and exclusion criteria. At last, a total of 7 pieces of eligible literature were finally included in the meta-analysis.
3.2 Characteristics of included literatures
A total of 7 pieces of eligible literature involving 478 patients were divided into the liraglutide treatment group (n = 241) and control group (n = 237). The baseline characteristics, including the study design, treatment methods of each group, sample size of each group were shown in Table 1. All included studies are of high quality.
Table 1: The main characteristics of the included studies.
3.3 Quality assessment
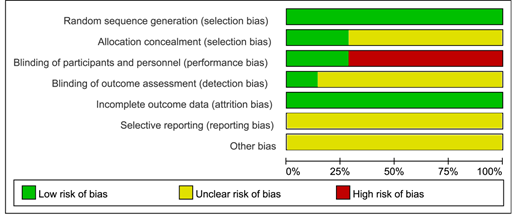
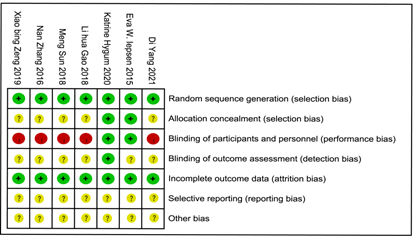
We carried a quality assessment of the 7 RCTs included in the meta-analysis according to the Cochrane Collaboration's risk of bias assessment tool, the risk of bias is shown in Figure 2 and Figure 3. All 7 RCTs grouped by random number table method. Only 1 RCT 5 was performed and assessed by blinding researchers and participants. 5 RCT [5-9] did give the number of people who were lost to follow-up or withdrew and the reason.
3.4 Meta-analysis
3.4.1 Effect on BMD
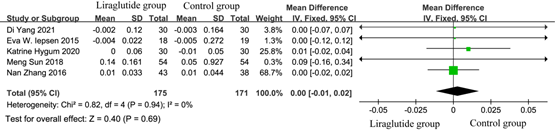
Bone mineral density (BMD) data were obtained from 5 studies [5, 7-10] involving 346 diabetic patients. Among these, 175/346 received liraglutide treatment. As shown in Figure 4, the test for the overall efficacy manifested as the following: Z = 0.40 (p= 0.69), Chi2 =0.82, df =3 (p = 0.94), and I2 = 0%. Liraglutide treatment had no effect on improved BMD of diabetic patients than conventional treatment (MD = 0.00, 95% CI: -0.01, 0.02). It indicated that liraglutide had no beneficial effect on BMD in diabetic patients.
3.4.2 Effect on bone formation indices
3.4.2.1 Effect on bone gla protein
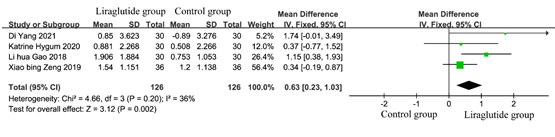
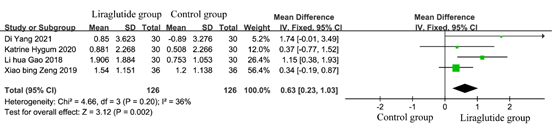
According to the inclusion criteria and exclusion criteria, a total of 4 studies met the criteria. To estimate the effect of bone gla protein in diabetic patients, the data of 252 patients from 4 studies [5-7, 11, 12], including 126 in the liraglutide treatment group and 126 in the control group were analyzed. The results of heterogeneity test showed that P=0.20, I2=36%, indicating that there was no significant heterogeneity among the studies. As shown in Figure 5, the test for the efficacy manifested as the following: Z = 3.12 (P= 0.002), Chi2 =4.66, df =3 (p =0.20). It indicated that after treatment, the bone formation index of diabetic patients was improved.
3.4.2.2 Effect on BAP
BAP from 3trials [5, 6, 12] involving 192 patients (96 in the liraglutide treatment group and 96 in the control group) were analyzed to assess the overall improvement. The results of heterogeneity test showed that P=0.27, I2= 23%, indicating that there was no significant heterogeneity among the studies, so the fixed-effect model was used for meta-analysis. As shown in Figure 6, liraglutide treatment group significantly improved BAP of diabetic than conventional treatment (MD:0.85;95% CI:0.30-1.40;P = 0.002). The results showed that BAP increased significantly in liraglutide patients after treatment.
3.4.2.3 Effect on PINP
According to the inclusion criteria and exclusion criteria, there were 4 articles included [5, 7, 9, 11]. The heterogeneity test results showed that P < 0.0001, I2 = 89%, suggesting that there was great heterogeneity among the studies. Further sensitivity analysis found that Katrine5 had a great impact on the results. After elimination, the heterogeneity decreased from 89% to 0%, and P = 0.56, then the fixed effect model was used for analysis. As shown in Figure 7, after liraglutide treatment, the bone formation index PINP of diabetics increased significantly, with statistical significance (MD: 6.90; 95%CI: 5.71-8.09; P=0.002) compared to conventional treatment.
3.4.3 Effect on bone resorption indices of β cross-linked C-telopeptide of type I collagen
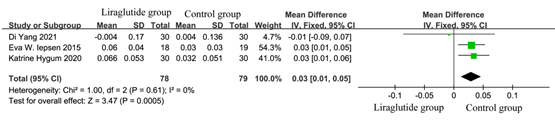
According to the inclusion criteria and exclusion criteria, there were 4 articles included [5, 7, 9, 11]. The heterogeneity test results showed that P = 0.04, I2 = 63%, suggesting that there was great heterogeneity among the studies. Sensitivity analysis found that Zeng et al 12 was the main cause of heterogeneity, which might be related to the different detected method. Zeng et al detected the content of β-CTX by enzyme-linked immunosorbent assay, while others used chemiluminescence to measure. Different detection methods lead to heterogeneity. After removing Zeng et al 11, the heterogeneity test results changed to P = 0.61, I2 =0%. As shown in Figure 8, the test for the overall efficacy was Z =3.47 (p = 0.0005), Chi 2 = 1.0, df = 2 and liraglutide treatment significantly reduce content of β-CTX (MD: 0.03; 95% CI?0.01-0.05).
4. Discussion
When we considered eligible literatures, we focused on the administration and treatment duration time. As liraglutide 0.6 mg/d subcutaneous injection was well tolerated and rarely applied in clinic, we eliminated the literatures of liraglutide 0.6 mg/d to treatment T2DM12. Treatment duration was more than 12 weeks was included in the study. It is well known that bone remodeling is a dynamic and long process. Short-term liraglutide treatment may change the markers of bone metabolism in serum, but the variety of BMD content can't be emergence. Therefore, the duration time in this research set more than 12 weeks. We further analyzed the literature and found that the treatment duration time of liraglutide was greater than or equal to 24 weeks, suggesting that the effect of liraglutide on bone needs to be observed for a long time. We performed this meta-analysis involving 7 studies of 478 patients. Categorized by the therapeutic strategies, they were divided into the liraglutide treatment group (n = 241) and the control group (n = 237). The meta-analysis results showed that compared with other therapies, liraglutide showed a significant increase in BAP, BGP, PINP and 25-OH vitamin D effects among the bone formation indices. Likewise, liraglutide were shown to cause a lower β-CTX level. Howerever, liraglutide did not show a statistically significant effect on BMD in patients with T2DM. These suggested that liraglutide had effect on maintaining the bone homeostasis in diabetic patients.
BMD measurement by dual X-ray absorptiometry (DXA) is the most accurate (more than 95%) method to evaluate of bone hardness. The BMD with or without T2DM in postmenopausal women do not differ but the biochemical marker levels of bone turnover are lower in women with T2DM [13]. BMD detection includes hip bone, neck bone, whole body bone, lumbar bone, femur in different literatures, however according to the data extract from the included literature, BMD of femur are usually detected. Our current meta-analysis revealed no significant increase in BMD of femur following the consumption of liraglutide compared with other therapies, which is in line with Gilbert et al. ALP is a widespread membrane-bound glycoprotein, total ALP in adult blood about 50% comes from liver and 50% from bone. Total ALP may be increased due to liver, gallbladder, pancreas and other diseases, which can't specifically reflect bone formation. BAP is produced by active osteoblasts, which is a more specific marker of bone formation. Our current meta-analysis revealed a significant increase of serum BAP. BGP is a non-collagenous protein synthetized by osteoblast during the mineralization of matrix and its rise shows bone formation. PINP is one of the main metabolites of type 1 procollagen transformed into type 1 collagen by protease, which can sensitively reflect the state of bone formation. Likewise, our research also showed a significant increase in BGP and PINP. These may be explained to liraglutide bind to GLP-1R in osteoblasts and active the pathway of wnt/β-catenin, which promote osteoblasts proliferation and differentiation [14, 15].
CTX is an intermolecular crosslink of type 1 collagen, including β-CTX and α-CTX, which keep the stability of the collagen structure. In the process of bone resorption, CTX is released into the blood with the degradation of type 1 collagen and excreted through urine. Therefore, the concentration of CTX in blood and urine can specifically reflect the level of bone resorption; β-CTX is a sensitive and specific bone resorption marker. Katrine study found that liraglutide significantly reduced the bone resorption index of β-CTX in T2DM patients5, however L Vedtofte et al found that bone resorption is unchanged by liraglutide in type 2 diabetes patients [5, 16]. In our study, we did not obtain a significant difference in β-CTX in T2DM patients between liraglutide treatment group and control. Due to the limit of literatures included, more clinical studies may be needed to confirm the effect of liraglutide on bone resorption. In conclusion, the present meta-analysis worthy to note that liraglutide therapy has a notable increase in bone dormation profiles such as BAP, BGP, PINP and significant decrease in β-CTX when trials lasted for more than 24 weeks, but not change in BMD, compared with other therapies in the patients, who were with T2DM. According to the meta-analysis, the authors suggest that patients in T2DM with osteoporosis who have poor glycemic control can choose liraglutide treatment. Finally, given the potential limitations of this study, a greater number of large-scale, properly performed RCTs are are still needed for further verification.
5. Data Availability
The data to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors’ Contributions
Shanshan Lei conceived and designed the experiments. Jing-Yi Kang and Jin-Tao Guan contributed significantly to analysis and manuscript preparation.
Acknowledgments
There was no founding supported.
References
- Mabilleau G, Pereira M, Chenu C. Novel skeletal effects of glucagon-like peptide-1 (GLP-1) receptor agonists. J Journal of Endocrinology 236 (2018): 17-0278.
- Cheng L, Hu Y, Li YY, et al. Glucagon-like peptide-1 receptor agonists and risk of bone fracture in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. J Diabetes/metabolism research 3168 (2019).
- Su B, Sheng H, Zhang M, et al. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists' treatment: a meta-analysis of randomized controlled trials. J Endocrine 48 (2015): 107-115.
- Zhao X, Huang K, Zheng M, et al. Effect of liraglutide on blood pressure: A meta-analysis of liraglutide randomized controlled trials. 19 (2019): 4.
- Hygum K, Harslf T, Jrgensen NR, et al. Bone resorption is unchanged by liraglutide in type 2 diabetes patients: A randomised controlled trial. Bone 132 (2020): 115197.
- Gao LH, Zhu YJ, Zhang Cl, et al. Effects of Liraglutide on bone metabolism in male obese patients with type 2 diabetes mellitus. Journal of Chinese Physician 20 (2020): 126-128.
- Di Y, Yu Z, Fabo F, et al. Effect of liraglutide and insulin glargine on bone metabolism in male patients with type 2 diabetes. Zhejiang Medical Journal 43 (2015): 26-32.
- Zhang N. Effects of Liraglutide on Lipid and Bone Metabolism and Inflammatory Factors of T2DM Patients with Obesity or Overweight, ANHUI MEDICAL UNIVERSITY (2016).
- Iepsen EW, Lundgren JR, Bolette H, et al. GLP-1 Receptor Agonist Treatment Increases Bone Formation and Prevents Bone Loss in Weight-Reduced Obese Women. Journal of Clinical Endocrinology Metabolism 100 (2015): 2909-2917.
- Sun M. The Effect of GLP-1 Analogues, Liraglutide Combined with Metformin on the Bone Mineral Density in Patients with Type 2 Diabetes Mellitus. Dalian medical university 32 (2009): 1-27.
- Zeng X, ZHU Cd, XIE Bq. Effect of Liraglutide on Bone Metabolism in Overweight Male Patients with Type 2 Diabetes. Medical Innovation of China 16 (2020): 70-74.
- Zhao X, Huang K, Zheng M, et al. Effect of liraglutide on blood pressure: A meta-analysis of liraglutide randomized controlled trials. J BMC Endocrine Disorders 7 (2019): 19.
- Osório, Joana. BMD and fracture risk in T2DM--clarifying a paradox. J Nature Reviews Endocrinology 7 (2011): 376-376.
- Li ZY, Li SL, Wang N, et al. Liraglutide, a glucagon-like peptide-1 receptor agonist, suppresses osteoclastogenesis through the inhibition of NF-kappa B and MAPK pathways via GLP-1R. Biomedicine & Pharmacotherapy (2020): 130.
- Hou HW, Xue P, Wang Y, et al. Liraglutide regulates proliferation, differentiation, and apoptosis of preosteoblasts through a signaling network of Notch/Wnt/Hedgehog signaling pathways. European Review for Medical and Pharmacological Sciences 24 (2020): 12408-12422.
- Vedtofte L, Nielsenhannerup E, Foghsgaard S, et al. Bone turnover in women with prior gestational diabetes after 52 week's treatment with liraglutide (2017).



 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 