Tinea Pedis Epidemiology and Importance from the Lens of Homelessness and Low-Resource Settings
Armen Henderson1,2, Taha Rasul1,2*, Orly Morgan1,2, Christine Rafie1,2, Jackson Anderson1,2, Megan Mathew1,2, Daniel Bergholz1,2
1Department, University of Miami, Leonard M. Miller School of Medicine, Florida, USA
2Miami Street Medicine Clinic, Florida, USA
*Corresponding author: Taha Rasul, University of Miami, Leonard M. Miller School of Medicine, Florida, USA.
Received: 29 January 2024; Accepted: 13 February 2024; Published: 29 February 2024
Article Information
Citation: Armen Henderson, Taha Rasul, Orly Morgan, Christine Rafie, Jackson Anderson, Megan Mathew, Daniel Bergholz. Tinea Pedis Epidemiology and Importance from the Lens of Homelessness and Low-Resource Settings. Archives of Clinical and Biomedical Research. 8 (2024): 45-53.
View / Download Pdf Share at FacebookAbstract
Background: Tinea pedis is sometimes described as a penalty of civilization due to its propensity to spread in close-contact environments such as urban areas. Medically vulnerable urban populations, such as patients experiencing homelessness in cities, are at high risk for developing such infections. This paper reviews tinea pedis infections in homeless patients across multiple regions and explores management in low-resource settings.
Methods: We conducted a literature search to characterize tinea pedis in homeless individuals across many geographic locations and offer recommendations for treatment in low-resource settings. A total of 60 PubMed articles were found using inclusion criteria containing terms ‘tinea pedis’ and ‘homeless’. Cohort studies, cross-sectional studies, and case-control studies published from 1979-2022 were evaluated.
Results: In North America, the prevalence of tinea pedis among sheltered homeless individuals ranged from 3.2% to 13.5% compared to a frequency in the general population of approximately 10%. European studies showed a tinea pedis frequency of 4.4−34.9% in the general population and 3.2% in a homeless patient cohort. Our findings show that there is a statistically significant difference in disease burden and healthcare utilization between sheltered and unsheltered homeless patients. Findings from a medical student-run mobile clinic that provided supplies for the prevention and treatment of tinea pedis were also noted.
Conclusion: Tinea pedis remains a ubiquitous disease among homeless patients across multiple geographic locations. Treatment in low-resource settings with simple interventions, such as antifungal powder, socks, and hygiene supplies, can potentially prevent tinea pedis outbreaks and improve disease burden in the PEH population.
Keywords
<p>Tinea pedis; Patients experiencing homelessness; Epidemiology; Low-resource; Treatment; Health disparities; Health outcomes; Cutaneous fungal infections</p>
Article Details
Abbreviations:
PEH: Patients Experiencing Homelessness; HIV: Human Immunodeficiency Virus
1. Introduction
Tinea pedis is a superficial dermatophyte infection of the feet including the soles, interdigital clefts, and periungual skin [1]. It is the most common fungal infection worldwide with an estimated 10% of the total population in the United States suffering from tinea pedis infections [1]. Tinea pedis is typically caused by Trichophyton rubrum which is a dermatophyte that is endemic to many parts of the Americas, Europe, Asia, and Africa [2]. Additional dermatophytes causing tinea pedis include Trichophyton interdigitale and Epidermophyton floccosum. There are a number of risk factors for acquiring these infections including hot, humid environments, occlusive footwear, excessive sweating, and extended exposure of the skin to moisture, as well as using community pools, baths or showers [3-5]. Many of the risk factors for tinea pedis co-occur with the environmental vulnerabilities of Patients Experiencing Homelessness (PEH). In addition, PEH individuals' primary mode of transportation is usually by foot, and thus any trauma, injury, or disease affecting the feet can greatly affect their wellbeing and their ability to find food and shelter. Despite the increased risk factors for tinea pedis in the PEH population, such patients, particularly in the southeastern United States, have not appropriately been studied with regard to tinea pedis occurrence, prevention or treatment.
In order to best treat PEH with tinea pedis, it is important to be able to characterize the common forms of the disease. Chronic intertriginous is the most common tinea pedis. Initial symptoms include scaling, erosion, erythema, pruritus, and malodor. It is commonly found on the interdigital and plantar digital surfaces of the feet [6,7]. The vesiculobullous form of tinea pedis is less well known, and presents with pustules or vesicles on the plantar foot surfaces [8-10]. Bacterial infections from chronic intertriginous and vesiculobullous form tinea pedis may result in cellulitis, abscesses, ulcers, and chronic non-healing wounds. Some conditions, such as hyperhidrosis, can predispose patients to tinea infections. Patients are generally advised to keep their feet dry by a combination of clean cotton-based clothing as well as with the use of certain antiseptic powders, such as clotrimazole powder [11-14]. This is also true for the period immediately following occlusive footwear usage, as topical antifungals may be required after the shoes are taken off [15-18].
Complications are more likely in patients with multiple comorbidities and those who are immunocompromised [11]. Unfortunately, many PEH fall into these two groups, due to a combination of chronic illnesses, substance dependence, and suboptimal mental health. In addition, the frequency of skin or soft tissue infections can be compounded by nutritional deficiency, decreased access to timely care, and lack of adequate centers for hygiene [12]. As such, PEH are at a high risk for severe complications related to tinea pedis. Therefore, it is especially important to treat and prevent tinea pedis infections in the PEH population. First-line treatment for tinea pedis includes topical azoles and terbinafine. Azoles have a favorable side effect profile compared to terbinafine, which should generally be avoided in patients with pre-existing liver disease due to the potential for hepatotoxicity [13-15]. Systemic treatment via oral medication is also an option, but only if there is involvement of the dorsum of the foot, heel, and/or sole, or in the case of refractory infection with blisters. Systemic treatment with azoles should be carefully balanced with other medications due to the inhibition of cytochrome p450 metabolism [13].
Prognosis is generally favorable if treatment and hygiene measures are adopted concurrently. Tinea pedis prevention is generally a function of environmental hygiene conditions. For example, improvement of hygiene in swimming pools and bathing areas as well as frequent washing of changing rooms can help in controlling the spread of infection between individuals [11]. In certain cases linked to public baths, selective use of tolnaftate powder has been shown to also reduce levels of interdigital tinea pedis [16-20].
The adequate treatment of tinea pedis in PEH populations is an essential step toward ensuring a lack of complications, especially in the inherently high-risk environment of the street [20-22]. Certain street outreach organizations such as Miami Street Medicine have noted that in the Miami Health District, tinea pedis is a common condition among the unsheltered homeless [23]. The combination of a tropical climate, lack of public hygiene facilities, and constant need to walk long distances to reach food and temporary shelter means that the feet of PEH are subject to near constant stress and maceration [24]. PEH in tropical regions such as South Florida are often subject to rainstorms, hurricanes, and other adverse weather events. Many homeless shelters have extended waitlists and sometimes are unable to accommodate a sharp increase in patient volume during times of inclement weather. This can lead to many health issues related to exposure, which can greatly affect podiatric care and result in worsening disease burden. The objectives of the study are to review prevalence, risk factors, and complications of tinea pedis among people experiencing homelessness. The study also assesses differences of tinea pedis between sheltered and unsheltered homeless individuals, and differences of tinea pedis among people experiencing homelessness across global regions.
2. Methods
2.1 Overview
A PubMed search was conducted with queries using the key terms ‘tinea pedis’ and ‘homeless.’ Inclusion criteria allowed for meta-analyses, randomized controlled trials, clinical trials, observational studies, and reviews. The search was restricted to English-language articles.
2.2 Search strategy and information sources
A search was performed in the PubMed database, for articles spanning from Jan 1, 1979 to Mar 1, 2022 and included search terms such as: (Homeless Persons OR Homeless *OR Unsheltered OR Street people *OR Housing instability OR Housing instability OR Vulnerable populations) AND (Derm OR Skin* OR Dermatophytosis OR Fungal skin infection OR Tinea pedis OR Fungal foot infection). Articles were restricted to the English language.
2.3 Eligibility criteria
Original research, review articles, case series, and case reports which mentioned tinea pedis in housed or unhoused homeless populations were included in this review. Some inclusion criteria were (i) inclusion of Patients Experiencing Homelessness (PEH) in both sheltered and unsheltered situations, (ii) epidemiologic data focused on tinea pedis or cutaneous fungal disease directly or in association with other skin or systemic conditions. Certain commentaries, preliminary reports, and conference abstracts which were found to be relevant and met the eligibility criteria, but were not found on the PubMed search, were included as well.
2.4 Article selection
The PubMed search yielded a total of 133 articles. Two reviewers (C.R., J.A.) completed an initial review of titles and abstracts, with a third added in cases of arbitration (T.R). Data extraction was in the form of an Excel spreadsheet which stratified the material with regard to demographic characteristics, tinea pedis parameters, diagnosis, outcomes, and significance of study.
2.5 Assessing study quality
T.R. and C.R. assessed study quality through the Oxford Centre for Evidence-Based Medicine Levels of Evidence Scale [25], which rates study quality from 1 (best quality) to 5 (lowest quality). Additionally, the level of evidence is rated from A (highest level) to D (lowest level). The article was prepared using the PRISMA 2020 guidelines and a checklist is available upon request [26].
3. Results
There are greater numbers of PEH in large urban settings due to concentration of food, shelter, and resources. High density PEH populations increase each individual’s susceptibility to tinea pedis infections due to forced crowding which affects person-to-person spread [27]. Overfilled shelters as well as high-volume shared hygiene facilities greatly increase the likelihood of dermatophyte infection. For PEH, the most common location for dermatologic infections is the lower limb [18,27,28]. This is likely due to a multitude of factors relating to urban homelessness, including poor access to hygiene facilities, constant walking, improper or poorly maintained footwear, comorbid chronic illnesses, and increased likelihood of traumatic injury [29]. Our results indicated the most common lower extremity conditions for this population were immersion foot [30], pitted keratolysis [31], nail pathologies [32], and foot infections [33-37].
While some studies have tried to characterize overall prevalence of tinea pedis among various homeless populations, none of the results found in our study examined the regional variation in prevalence of infection [38]. A study from 2011-2015 in the San Francisco Bay Area found that among homeless patients at a shelter-based health clinic, approximately 21% of all dermatologic diagnoses included superficial fungal infections [18]. Among these diagnoses, tinea pedis was seen in 65% of patients, suggesting a higher prevalence compared to other cutaneous dermatophyte infections such as onychomycosis (fungal nail infection) and cutaneous candidiasis. The distribution of tinea pedis in the general population of the San Francisco Bay Area between 1974 and 1994 was estimated to be 23.4% of dermatophyte infections [33]. Stratigos et al. [19] in 1999 conducted a study in a shelter-based cohort in Boston and estimated the prevalence of tinea pedis to be 38% (Figure 1, Table 1). And so, while there has been documentation of large urban centers and the tinea pedis prevalence in their PEH populations, there has been a distinct lack of data for other geographical regions including the southeastern United States. Results did not show that epidemiological studies have been repeated on a larger, regional basis to compare tinea pedis variation between states. It is important to evaluate the geographical difference, as PEH individuals living in a tropical environment with high levels of rainfall, as that of the southeastern United States, are at increased risk due to environmental factors that can predispose them to the formation of tinea pedis.
In South Florida, current efforts are being made to quantify the prevalence of tinea pedis in the local PEH population. Miami Street Medicine, an organization that provides medical outreach programs for unsheltered PEH have reported foot fungi, including tinea pedis, in 29.6% of the dermatologic complaints encountered over an 8-month period [23]. The only major tinea pedis epidemiologic study conducted in the same region evaluated onychomycosis in a non-homeless geriatric population [29].
3.1 Lack of comprehensive data on PEH in certain global regions
Certain studies in Europe demonstrate a higher risk of tinea pedis in housed individuals compared to their unhoused counterparts [2,39]. However, there are comparatively fewer studies on European PEH populations, mostly limited to the Marseilles region of France which demonstrated a tinea pedis rate of approximately 3.2% [2]. On the other hand, the Achilles Project included a multinational evaluation of foot diseases, and concluded a foot fungal disease frequency of approximately 35% [40]. A lack of comprehensive tinea pedis epidemiologic data in PEH was noted in South American, Asian, and African studies. Although other health parameters have been documented such as HIV status and certain parasitic infections, specific data on tinea pedis is incomplete [41]. The data gap found could be due to lack of funding for public health initiatives such as epidemiologic recordings [32,33,42-47]. The papers in our study mostly listed tinea pedis as a confound of other diseases, such as a potential comorbidity in HIV patients [31,32]. Many papers considered refugees to be a confounder and left them out of the study, while data on this population could be useful as they are a subtype of PEH [34,48,49].
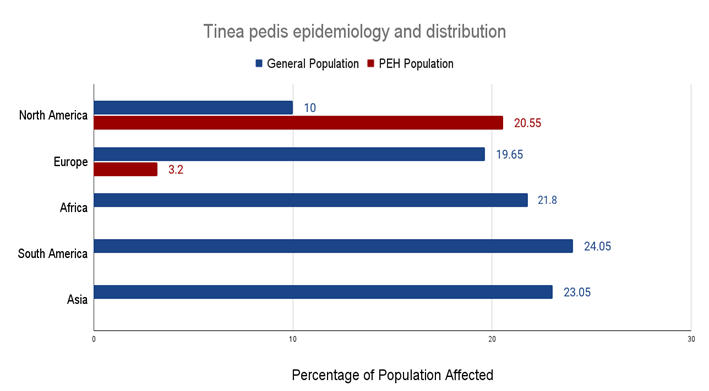
3.2 Tinea pedis epidemiology and distribution among PEH
|
Region |
General Population |
PEH Population |
|
North America |
10% [3,31] |
3.1 - 38% [14,15,16,19] |
|
Europe |
4.4% - 34.9% [27,47,57] |
3.2% [2,15] |
|
Africa |
21.1 - 22.5% [22,28] |
Not sufficiently studied |
|
South America |
2.5% - 45.6% [4,31,43,58] |
Not sufficiently studied |
|
Asia |
15.1% - 31% [8,12,33,53] |
Not sufficiently studied |
Table 1: Tinea pedis prevalence ranges by region in general populations compared to PEH populations.
3.3 Difference in outcomes between sheltered and unsheltered homeless
While the distinction between sheltered and unsheltered PEH has not yet been widely adopted in the literature, there are significant differences in demographics and health service usage between the two groups [24,50]. Evaluating unsheltered patients with sheltered patients under the generalized term ‘homeless’ can conceal important information about each group’s unique predictors of health. The research that has been done on sheltered and unsheltered PEH separately has shown significant differences. For example, Petrovich et al. [24] detailed the different levels of healthcare service use between unsheltered (94.2%) and sheltered (83.5%) PEH over a 24-month period. The study also found a statistically significant difference in emergency department usage for unsheltered (72.5%) versus sheltered (59.8%) PEH. Another study found unsheltered PEH were more likely to have chronic physical health conditions and substance abuse than sheltered PEH. Eiset et al. [54] found that unsheltered women had over a 3-times greater likelihood of fair or poor physical health, as well as over 12-times greater odds of poor mental health compared to sheltered homeless women [34]. Studies involving tinea pedis in PEH populations have been primarily conducted in shelters. No articles found in our study addressed tinea pedis rates specifically in the unsheltered homeless population [30,51].
3.4 Tinea pedis-associated infections and complications
The host response to tinea pedis infection is epidermal proliferation, causing skin scaling and thickening, as well as fissuring and maceration in the interdigital spaces, usually around the fourth or fifth toe [52-55]. Such disruptions in the epidermal barrier permit other organisms such as streptococci to colonize interdigital spaces. In particularly severe cases, tinea pedis can be associated with recurrent cellulitis [38-40]. A study by Al Hasan et al. [44] evaluated 22 episodes of lower extremity cellulitis, and found that of the patients who had tinea pedis, 85% had a concurrent infection with β-hemolytic streptococci. The study found the co-occurrence of β-hemolytic streptococci and tinea pedis in patients with cellulitis to be statistically significant. While gram-positive bacteria are typically found in interdigital spaces, tinea pedis’ bactericidal products can facilitate the growth of gram-negative organisms such as Pseudomonas, Proteus, and Klebsiella which can lead to gram-negative cellulitis [45].
Patients with weakened immune systems or other chronic diseases such as diabetes and HIV are more likely to acquire, and have greater potential to suffer from, complications unique to their population, such as diabetic foot ulcers [34-37]. The development of diabetic foot ulcers has been shown to facilitate onychomycosis and tinea pedis [56,57]. Ulcerated and exposed subcutaneous tissue is susceptible to further infection which can lead to further complications such as osteomyelitis, a bacterial or fungal infection of the bone [47].
4. Discussion
Studies also combined areas that have differences in climate and local governance structures. Most studies evaluated the entire United States without accounting for regional variation, such as the Southern, Midwestern, and Northern climate. Region specific research will likely show climate factors such as humidity and rainfall would influence the prevalence of conditions like immersion foot and tinea pedis [2,8,19]. Results did not yield studies detailing the epidemiology of tinea pedis among PEH in the southeastern United States, and studies conducted in California and Massachusetts would have very limited generalizability for a tropical climate like South Florida [2,16,19]. Moreover, there are unique structures of regional governmental and non-for-profit support. Data conducted on barriers of access to care in a different state have limited scope of application given the unique health care barriers existing regionally [30]. For example, none of the studies addressed undocumented homeless individuals, who make up an important part of the PEH population in the southeastern United States. Even more locally, in South Florida there was a moratorium on evictions that was lifted in October 2021 [40]. It is likely this has increased the prevalence of tinea pedis in the PEH population considerably due to the subsequent increased crowding of shelters [39].
In addition, studies did not typically distinguish between unsheltered and sheltered homeless patients [16,17,19,21]. Without separating these two groups, many of the studies found in the review do not reflect the unique environmental factors of each group and their subsequent risk of infection. An individual exposed to a crowded bathroom space every day in a shelter and a person who sleeps exposed to a street environment have distinct and important risk factors concerning infection. The most important predictor of homeless health has consistently been access to shelter [53,54]. With this in mind, it cannot be overlooked that many of the studies grouped sheltered and unsheltered populations together. The lack of reliable hygiene, laundry, and health facilities make it likely that unsheltered homeless patients would have higher rates of dermatophytosis. However, it is worth considering whether the crowded conditions in shelters make it easier for the spread of fungal skin infections. Underfunded shelters may also be unable to appropriately sanitize their facilities, leading to the spread of tinea pedis particularly among showers and common areas. Improved access to healthcare could offset the increased risk of person-to-person spread, thereby reducing rates of tinea pedis infection in sheltered patients as compared to their unsheltered counterparts. Research must distinguish between these two groups, as their unique living situations and risk factors could influence treatment and prevention protocols.
5. Recommendations for Future Research Directions
We recommend future research stratify data between sheltered and unsheltered PEH. Specific treatments and treatment success rates between the two groups may be different and warrants further investigation for best practice recommendations in the treatment of tinea pedis. In addition, researchers should be judicious about documentation of the epidemiology of the PEH population they are studying. It is not feasible and cannot be expected that public housing facilities or shelters will increase their epidemiology census, as their resources are already spread thin and it is not their primary priority. Research programs should sustain their own data monitoring for unsheltered patients which would have the added benefit of creating a longitudinal care relationship.
5.1 Recommendations for clinicians of PEH populations
Providing measures to maintain foot health and hygiene is paramount for PEH populations [55,56]. Overall foot health can be maintained by regular examination and cleaning, as well as by wearing clean and protective footwear [57-60]. Careful examination of the plantar surface, interdigital surfaces, and toenails should be performed to rule out pathologies that could contribute to recurrent tinea pedis such as onychomycosis or other, more serious infections [32-37]. This is particularly important in regions with high levels of humidity such as South Florida; in humid climates, tinea pedis should be evaluated among all PEH presenting at a clinic. Patients with multiple comorbidities should work towards maintenance of their illnesses in order to prevent foot complications. Here regional and national diversity plays a role, as certain countries have better access to medications that treat chronic diseases, such as insulin, for PEH populations. [15]. Among the PEH population of Florida, diabetic foot ulcers are frequently observed [14]. Pre-emptive treatment could prevent these complications from arising and potentially decrease emergency department utilization [17,21].
Outreach events such as community-based foot-washing sessions should be more frequently employed in order to assess the overall foot health of patients in unsheltered settings. Events like these provide an opportunity to connect high-risk patients to more immediate care and follow up [23]. In addition to foot washing accommodations, clean cotton socks can definitively prevent recurrences of this condition and are low cost for non-profits and shelters to provide. Preliminary data from Miami Street Medicine clinic found unsheltered PEH were receptive to distribution of socks and antifungal powder [23]. This portends well for patient adherence to foot health maintenance when given the resources.
5.2 Limitations
Every study has its limitations and ours is no exception. The data was collected from only one database, and only English language articles were included. There is also an inherent bias when studying a topic where data collection is resource dependent. Tinea pedis among PEH populations is an under-studied topic, and so the data is biased towards regions which have access to epidemiologic resources. While the research gathered includes studies conducted in various countries throughout each continent, few studies looked into regional variation within that country. Our study did take this into account, and frequency ranges were used to convey variability [14-18,58-60]. Still, when looking through the results it is clear some countries have overrepresentation and others are underrepresented. In South America, Brazil and Colombia accounted for most tinea pedis studies. Disproportionate research of certain countries may skew the ranges towards the prevalence being inaccurately high in those regions compared to others [43,45,58]. In addition, non-industrialized nations do not necessarily have the same socioeconomic conditions which lead to populations of urban-based homeless individuals [30-32]. As such, there may be different groups with similar health disparities, such as refugees or isolated rural populations.
6. Conclusion
Our study conducted a literature review to analyze the epidemiology of tinea pedis among sheltered and unsheltered homeless populations. Findings show that more is needed to address the needs of this high-risk community. Certain countries and regions were overrepresented in research found, while others were underrepresented. Miami Street Medicine is one organization in South Florida working to fill this gap, and their preliminary research on tinea pedis reflects the vastly diverse impact that the immediate environment can have on the prevalence of tinea pedis. Further research on cutaneous health conditions in PEH should evaluate the differences between sheltered and unsheltered homeless patients in order to properly tailor treatment. Among patients in low-resource settings, care can take the form of providing cotton socks, protective footwear, and antifungal powder. Although stark health disparities remain among PEH, ensuring adequate foot health can prevent complications in such a high-risk patient group.
Declarations
The views and opinions expressed in this presentation are those of the authors and do not necessarily reflect the official policy or position of the University of Miami Health System. The recommendations are based on contemporary literature as well as preliminary data from homeless health outreach programs. They are not meant to be substitutes to actual clinical guidelines.
Ethical Approval and Consent to participate
Not applicable.
Consent for publication
The authors hereby consent to the publication of our Work in all Archives of Clinical and Biomedical Research publications.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no known competing financial interests, personal relationships, or other conflicts of interest in this paper.
Funding
This review was not funded or sponsored by any external source.
Authors' contributions
Taha Rasul: Manuscript preparation, central thesis development, literature evaluation, manuscript drafting, data analysis.
Orly Morgan: Manuscript structuring, editorial correction, content structuring and correction, data analysis.
Christine Rafie: Grammatical correction, figure inclusion, content structuring and correction, data analysis.
Jackson Anderson: Content structuring and correction, data analysis.
Megan Mathew: Grammatical correction, data analysis.
Daniel Bergholz: Final paper evaluation and content management.
Armen Henderson: Advised thesis development and literature review guidelines.
Acknowledgements
We gratefully acknowledge the Miami Street Medicine Clinic for their work in improving the health of PEH in the Miami Health District. We would also like to thank Dr. Brian Morrison for his thoughtful comments on the direction of this paper.
References
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Critical reviews in microbiology 41 (2015): 374-388.
- Badiaga S, Menard A, Tissot Dupont H, et al. Prevalence of skin infections in sheltered homeless. European Journal of Dermatology: EJD 15 (2015): 382-386.
- To MJ, Brothers TD, Van Zoost C. Foot Conditions among Homeless Persons: A Systematic Review. PLoS One 11 (2016): e0167463.
- Mazza M, Refojo N, Davel G, et al. Mycology Network of the Province of Buenos Aires (MNPBA). Epidemiology of dermatophytoses in 31 municipalities of the province of Buenos Aires, Argentina: A 6-year study. Rev Iberoam Micol 35 (2018): 97-102.
- Tsunemi Y, Takehara K, Miura Y, et al. Specimens processed with an extraction solution of the Dermatophyte Test Strip can be used for direct microscopy. Br J Dermatol 177 (2017): e50-e51.
- Vlahovic TC. Onychomycosis: Evaluation, Treatment Options, Managing Recurrence, and Patient Outcomes. Clin Podiatr Med Surg 33 (2016): 305-318.
- Drago L, Micali G, Papini M, et al. Management of mycoses in daily practice. G Ital Dermatol Venereol. 152 (2017): 642-650.
- Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian Dermatol Online J 7 (2016): 77-86.
- Taheri A, Davis SA, Huang KE, et al. Onychomycosis treatment in the United States. Cutis. 95 (2015): E15-21.
- Becker BA, Childress MA. Common Foot Problems: Over-the-Counter Treatments and Home Care. Am Fam Physician 98 (2018): 298-303.
- Clebak KT, Malone MA. Skin Infections. Prim Care 45 (2018): 433-454.
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert Consensus on the Management of Dermatophytosis in India (ECTODERM India). BMC Dermatol 18 (2018): 6.
- Lipner SR, Scher RK. Onychomycosis: Clinical overview and diagnosis. J Am Acad Dermatol 80 (2019): 835-851.
- D'Souza MS, O'Mahony J, Achoba A. Exploring Foot Care Conditions for People Experiencing Homelessness: A Community Participatory Approach. J Prim Care Community Health 13 (2022): 21501319211065247.
- Adly M, Woo TE, Traboulsi D, et al. Understanding Dermatologic Concerns among Persons Experiencing Homelessness: A Scoping Review and Discussion for Improved Delivery of Care. J Cutan Med Surg 25 (2021): 616-626.
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis 89 (2012): 25-32.
- Contag C, Lowenstein SE, Jain S, et al. Survey of symptomatic dermatologic disease in homeless patients at a shelter-based clinic. Our Dermatology Online 8 (2017): 133-137.
- Wilmington M, Aly R, Frieden IJ. Trichophyton tonsurans tinea capitis in the San Francisco Bay area: increased infection demonstrated in a 20-year survey of fungal infections from 1974 to 1994. Journal of medical and veterinary mycology: bi-monthly publication of the International Society for Human and Animal Mycology, 34 (1996): 285-287.
- Stratigos AJ, Stern R, González E, et al. Prevalence of skin disease in a cohort of shelter-based homeless men. Journal of the American Academy of Dermatology, 41 (1999): 197-202.
- O’Connell J. Premature Mortality in Homeless Populations: A Review of the Literature. Nashville: National Health Care for the Homeless Council, Inc. (2005).
- To MJ, Brothers TD, Van Zoost C. Foot Conditions among Homeless Persons: A Systematic Review. PloS one, 11 (2016): e016746.
- Abd Elmegeed AS, Ouf SA, Moussa TA, et al. Dermatophytes and other associated fungi in patients attending to some hospitals in Egypt. Braz J Microbiol 46 (2015): 799-805.
- Rasul T, Bergholz D. Foot Health Needs Assessment (2002).
- Petrovich JC, Hunt JJ, North CS, et al. Comparing Unsheltered and Sheltered Homeless: Demographics, Health Services Use and Predictors of Health Services Use. Community mental health journal, 56 (2020): 271-279.
- OCEBM Levels of Evidence. Center for Evidence-Based Medicine (CEBM).
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews BMJ 372 2021: n71.
- Perea S, Ramos MJ, Garau M, et al. Prevalence and risk factors of tinea unguium and tinea pedis in the general population in Spain. J Clin Microbiol 38 (2000): 3226-3230.
- Toukabri N, Dhieb C, El Euch D, et al. Prevalence, Etiology, and Risk Factors of Tinea Pedis and Tinea Unguium in Tunisia. Can J Infect Dis Med Microbiol (2017): 6835725.
- Scherer WP, McCreary JP, Hayes WW. The diagnosis of onychomycosis in a geriatric population: a study of 450 cases in South Florida. J Am Podiatr Med Assoc 91 (2001): 456-464.
- Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet 384 (2014): 1529-1540.
- Sanders DM, Todd C, Chopra M. Confronting Africa's health crisis: more of the same will not be enough. BMJ 331 (2005): 755-758.
- Skyers N, Jarrett S, McFarland W, et al. HIV Risk and Gender in Jamaica's Homeless Population. AIDS Behav 22 (2018): 65-69.
- Aly R. Incidence of dermatophytes in the San Francisco Bay area. Dermatologica, 161 (1980): 97-100.
- Nyamathi AM, Leake B, Gelberg, L. Sheltered versus nonsheltered homeless women differences in health, behavior, victimization, and utilization of care. Journal of general internal medicine 15 (2000): 565-572.
- Scher RK, Breneman D, Rich P, et al. Once-weekly fluconazole (150, 300, or 450 mg) in the treatment of distal subungual onychomycosis of the toenail. J Am Acad Dermatol 38 (1998): S77-S86.
- Lopes JO, Alves SH, Mari CR, et al. A ten-year survey of onychomycosis in the central region of the rio grande do sul, brazil. Rev Inst Med Trop Sao Paulo 41 (1999): 147-149.
- Volk B, Tiu A, St Anna L. Clinical Inquiry: which oral antifungal works best for toenail onychomycosis? J Fam Prac 62 (2013): 100-101.
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses 51 (2008): 2-15.
- Diongue K, Ndiaye M, Diallo MA, et al. Fungal interdigital tinea pedis in Dakar (Senegal). Journal de mycologie medicale 26 (2016): 312-316.
- Florida Homelessness Statistics. U.S. Interagency Council on Homelessness.
- No Safe Place. The Criminalisation of Homelessness in U.S. Cities. A Report by the National Law Centre on Homelessness and Poverty (2014).
- White TC, Findley K, Dawson TL Jr, et al. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med 4 (2014): a019802.
- de Oliveira Pereira F, Gomes SM, Lima da Silva S, et al. The prevalence of dermatophytoses in Brazil: a systematic review. J Med Microbiol 70 (2021).
- Al Hasan M, Fitzgerald SM, Saoudian M, et al. Dermatology for the practicing allergist: Tinea pedis and its complications. Clin Mol Allergy 2 (2004): 5.
- Zhan P, Liu W. The Changing Face of Dermatophytic Infections Worldwide. Mycopathologia 182 (2017): 77-86.
- Grumbt M, Monod M, Yamada T, et al. Keratin degradation by dermatophytes relies on cysteine dioxygenase and a sulfite efflux pump. J Invest Dermatol 133 (2013): 1550-1555.
- Burzykowski T, Molenberghs G, Abeck D, et al. High prevalence of foot diseases in Europe: results of the Achilles Project. Mycoses 46 (2003): 496-505.
- Aste N, Pau M, Aste N, et al. Tinea pedis observed in Cagliari, Italy, between 1996 and 2000. Mycoses 46 (2003): 38-41.
- Gentles JC, Evans EG. Foot infections in swimming baths. Br Med J 3 (1973): 260-262.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls--a prospective study. Mycoses 57 (2014): 754-758.
- Leyden JL. Tinea pedis pathophysiology and treatment. J Am Acad Dermatol 31 (1994): s31-s33.
- Aboul-Ella H, Hamed R, Abo-Elyazeed H. Recent trends in rapid diagnostic techniques for dermatophytosis. Int J Vet Sci Med 8 (2020): 115-123.
- Sakka N, Shemer A, Barzilai A, et al. Occult tinea pedis in an Israeli population and predisposing factors for the acquisition of the disease. Int J Dermatol 54 (2015): 146-149.
- Eiset AH, Aoun MP, Haddad RS, et al. Asylum seekers' and Refugees' Changing Health (ARCH) study protocol: an observational study in Lebanon and Denmark to assess health implications of long-distance migration on communicable and non-communicable diseases and mental health. BMJ Open 10 (2020): e034412.
- Ilkit M, Tanir F, Hazar S, et al. Epidemiology of tinea pedis and toenail tinea unguium in worshippers in the mosques in Adana, Turkey. The Journal of dermatology 32 (2005): 698-704.
- Bhutani LK, Mohapatra LN, Kandhari KC. Tinea pedis--a penalty of civilization. A sample survey of rural and urban population. Mykosen 7 (1971): 335-336.
- Drakensjö IT, Chryssanthou E. Epidemiology of dermatophyte infections in Stockholm, Sweden: a retrospective study from 2005-2009. Med Mycol 49 (2011): 484-488.
- Carrascal-Correa DF, Zuluaga A, González A. Species distribution of the main aetiologic agents causing skin dermatophytosis in Colombian patients: A 23-year experience at a Mycological Reference Center. Mycoses 63 (2020): 494-499.
- Sigurgeirsson B, Olafsson JH, Steinsson JB, et al. Long-term effectiveness of treatment with terbinafine vs. itraconazole in onychomycosis: a 5-year blinded prospective follow-up study. Arch Dermatol. 138 (2002): 353-357.
- Benedict K, Jackson BR, Chiller T, et al. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 68 (2019): 1791-1797.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 