Visceral Adipose Tissue-A Common Link to the Development of Nonalcoholic Fatty Liver Disease and Metabolic Syndrome
Chileka Chiyanika, Winnie CW Chu*
Department of Imaging and Interventional Radiology, The Chinese University of Hong Kong, Hong Kong, China
*Corresponding author: Chu Chiu Wing Winnie, Department of Imaging and Interventional Radiology, Faculty of Medicine, The Chinese University of Hong Kong, Room 27026, Department of Imaging & Interventional, Radiology, G/F, Treatment Block and Children Wards, Prince of Wales Hospital, Shatin, Hong Kong SAR, China
Received: 24 July 2020; Accepted: 03 August 2020; Published: 23 September 2021
Article Information
Citation: Chileka Chiyanika, Winnie CW Chu. Visceral Adipose Tissue-A Common Link to the Development of Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Archives of Clinical and Biomedical Research 5 (2021): 742-755.
View / Download Pdf Share at FacebookAbstract
Metabolic syndrome and Non-alcoholic fatty liver disease are common findings in obesity. In both conditions, despite many proposed mechanisms to their development, changes in adipose tissue vis-à-vis visceral adipose tissue as a highly metabolically active tissue seem to be a common pathway to their development in both the lean and obese populations. In this review, we detail how the changes that occur in adipose tissue are linked to the development of both metabolic syndrome and non-alcoholic fatty liver disease.
Keywords
Adipose tissue; Visceral adipose tissue; Subcutaneous adipose tissue; Metabolic syndrome; Non-alcoholic fatty liver disease
Adipose tissue article; Visceral adipose tissue article; Subcutaneous adipose tissue article; Metabolic syndrome article; Non-alcoholic fatty liver disease article
Article Details
1. Introduction
Obesity is emerging as one of the main causes limiting life expectancy in developed countries [1]. It is linked to an increased risk of metabolic syndrome, while non-alcoholic fatty liver disease (NAFLD) is its most common complication [2]. Interestingly, a proportion of 30% of obese individuals do not develop NAFLD and metabolic aberrations; meanwhile a proportion of 20-30% of lean individuals develop NAFLD and associated conditions [3], suggesting that the development of these complications might be related to adipose tissue distribution and functions. In fact, it has been shown that visceral adipose tissue in particular plays a critical role in the genesis of metabolic diseases and NAFLD independent of generalized obesity [4, 5]. This review will therefore consider the linkage that exists among adipose tissue in particular, visceral adipose tissue, the development of metabolic syndrome and that of NAFLD.
2. Adipose Tissue
Adipose tissue is a connective tissue as well as an endocrine organ, which is involved in energy homeostasis, glucose and lipid metabolism. Adipose tissue has the capability of expanding (either in the form of hypertrophy which is the increase in adipocytes volume or hyperplasia which is the increase in adipocytes number). Adipose tissue has different behaviour under different physiological conditions. For example, in excess nutrition (energy) conditions it stores the excess energy in the form of triglycerides, while during starvation or fasting conditions it supplies energy to other tissues through the process of lipolysis [6]. Adipose tissue is divided into white adipose tissue (WAT) and brown adipose tissue (BAT).
2.1 White adipose tissue (WAT)
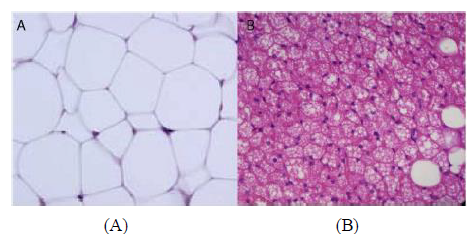
WAT is the more abundant of the two and is distributed into subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). WAT is characterised with large single lipid droplets (unilocular) and contains few mitochondria inside (Figure 1A) [7]. WAT secretes a number of different hormones that play various roles in energy metabolism and endocrine function. Among many other hormones secreted by this tissue are: adiponectin, leptin and resistin [8, 9]. Interestingly, Lee et al demonstrated that SAT was strongly associated with leptin while VAT was strongly associated with adiponectin [10]. These outcomes probably explain the biological functional differences between the two WAT subtypes. In fact, VAT is more highly metabolically active than SAT and has been associated with metabolic disorders [4, 5]. VAT depots include mesenteric, omental, perirenal and peritoneal regions [11, 12]. Contrary to the association of VAT with metabolic disorders, SAT on the other hand has been attributed to offer “protection” from metabolic disorders. Kim et al showed that an increase in the SAT area was significantly associated with regression of NAFLD [13] while Kwon et al. showed that SAT area was not associated with the incidence of metabolic syndrome [14].
2.2 Brown adipose tissue (BAT)
BAT is characterized by less lipid droplets, highly irrigated with blood vessels, innervated with noradrenergic fibres, high content of uncoupling protein 1 (UCP1) and mitochondrial contents [15] (Figure 1B). UCP1 is also expressed by WAT at the mitochondrial level but only to a lesser degree [16], which is thought to induce “white to beige fat’ transition, being referred to as “browning of white fat” or “synthesis of beige fat [17, 18].” The functions of both classical brown and beige adipose tissues are for thermogenesis and energy balance, with contribution to glucose homeostasis, mitigating insulin resistance and clearing triglycerides [19, 20].
3. Metabolic Syndrome
This is a constellation of metabolic abnormalities that includes: central obesity, insulin resistance, hypertension, and dyslipidaemia [22-24]. This syndrome is strongly associated with the increased risk for cardiovascular disease and type 2 diabetes mellitus [23] and its prevalence is high in the obese population [25]. Notably, the prevalence varies with respect to gender, ethnicity/race, age and the criteria used for its diagnosis. Table 1 shows the summary of different criterion used to diagnose metabolic syndrome.
AACE, American Association of Clinical Endocrinologists; ACR, albumin-creatinine ratio; AHA, American Heart Association; BMI, body mass index; BP, blood pressure; EGIR, European Group for Study of Insulin Resistance; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; IDF, International Diabetes Federation; NCEP ATP III, National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III); NHLBI, National Heart, Lung, and Blood Institute; T2DM, type 2 diabetes mellitus; TG, triglycerides; WC, waist circumference; WHO, World Health Organization. Υ Waist circumference: for Europids, sub-Saharan Africans, Eastern Mediterranean and Middle East populations >94 cm in men and >80 cm in women; for South Asians, Chinese, Japanese, Central and South American >90 cm in men and >80 cm in women. Φ Glucose concentration conversion factor: 1milligrams per decilitre = 0.0555 millimoles per litre. Ψ Triglyceride concentration conversion factor: 1milligrams per decilitre = 0.0113 millimoles per litre. β HDL-cholesterol concentration conversion factor: 1milligrams per decilitre = 0.02586 millimoles per litre. Adapted from McCracken et al. [23].
Table 1: Various diagnostic criterion for metabolic syndrome [26-32].
4. Link Between Visceral Adipose Tissue and Metabolic Syndrome
Several theories linking adipose tissue to the development of metabolic syndrome have been proposed but the widely accepted ones are: insulin resistance with fatty acid flux [23], neurohormonal activation, low-grade chronic inflammation and oxidative stress [33-35].
4.1 Insulin resistance hypothesis
Insulin inhibits lipolysis and hepatic gluconeogenesis, at the same time it increases glucose uptake in the muscle and liver. In an event of insulin resistance in the adipose tissue, the insulin-mediated inhibition of lipolysis is impaired resulting in an increase in the circulating free fat acids (FFAs). This increase in circulating FFAs further inhibits the antilipolytic effect of insulin [36]. This increase in circulating FFAs results in two simultaneous but independent processes: (1) they inhibit protein kinase activation in the muscle leading to reduced glucose uptake, and (2) they increase protein kinase activation in the liver leading to the promotion of gluconeogenesis and lipogenesis. The net effect of this chain reaction results in excess levels of insulin circulating in the blood relative to the level of glucose [24]. Overtime, the compensation fails and insulin secretion diminishes. Additionally, the FFAs are toxic to the beta cells in the pancreatic islet of Langerhans as they decrease the secretion of insulin [37]. Insulin resistance further adds to the genesis of hypertension due to vasoconstriction caused by FFAs and the loss of vasodilator effects of insulin [38]. Moreover, insulin resistance has been found to increase serum viscosity that induces thrombophilia, and release of proinflammatory cytokines from the adipose tissue, which contributes to, increased risk of cardiovascular diseases [39].
Visceral adipose tissue does contribute to insulin resistance. Visceral lipolysis contributes to an elevated supply of FFAs to the liver via the celiac, superior mesenteric, inferior mesenteric arteries and the portal vein. This increase in FFAs ensues in an amplfied triglycerides formation and the manufacture of apolipoprotein B containing triglyceride-rich very low-density lipoprotein cholesterol (LDL-C) in the liver [40]. The elevated levels of apolipoprotein B are an indirect effect of insulin resistance following an obliterated lipid metabolism in the liver. This elevation in the levels of apolipoprotein B corresponds to elevated levels of LDL-C and a reduction in high-density lipoprotein cholesterol (HDL) [24].
4.2 Neurohormonal activation hypothesis
As discussed earlier, adipose tissue is not only a connective tissue but an endocrine organ as well. It secretes hormones in particular leptin and adiponectin. Adiponectin regulates glucose levels and breaks down fatty acids. This protein has been associated with metabolic syndrome and cardiovascular disease [41]. Similarly, leptin regulates energy balance by inhibiting hunger that in turn reduces fat storage in adipocytes by acting on cell receptors in the arcuate nucleus of the hypothalamus. The onset of obesity increases leptin levels, and this increase is directly related to increased cardiovascular risks [24]. In antagonism to the effects of leptin, adiponectin on the other hand provides counter effects of leptin as an anti-inflammatory and anti-atherogenic adipokine. Thus, adiponectin has been considered a protective factor against the development of diabetes mellitus, hypertension and acute myocardial infarction [42, 43]. Obesity correlates with reduced adiponectin and higher leptin levels that eventually increase the cardiovascular risks.
Neurohumoral activation increases the activity of the sympathetic nervous system, renin-angiotensin system, vasopressin and atrial natriuretic peptide [44]. Of interest is the renin-angiotensin system that has also been shown to contribute to the development of metabolic syndrome. Adipose tissue produce angiotensin II following the activation of angiotensin-converting enzyme [24]. Obesity and insulin resistance have been associated with increased production of angiotensin II [45]. Activation of angiotensin II leads to the generation of reactive oxygen species through the activation of nicotinamide adenine dinucleotide phosphate oxidase [46]. The generation of reactive oxygen species result in multiple effects including among many others the expression of lectin-like oxidized low-density lipoprotein receptor-1 on the endothelium and vascular smooth muscle cells [47]. These elements: renin-angiotensin system, reactive oxygen species and low-density receptor-1 have an intertwined positive response loop that initiates a vicious cycle of inflammation, endothelial damage and fibroblast proliferation which contributes to the onset of metabolic syndrome cluster of abnormalities; hypertension, dyslipidaemia, diabetes, cardiac hypertrophy and cardiovascular disease [48].
4.3 Low-grade chronic inflammation and oxidative stress hypothesis
As the adipose tissue undergo hyperplasia and hypertrophy (related to inflammation) in response to excess nutrition, the tissue cells tend to outgrow their blood supply resulting in hypoxia [49]. In turn, cell necrosis with macrophage infiltration and the production of adipocytokines ensue [23]. Among the adipocytokines produced are: Interleukin-6 (IL-6), tumour necrosis factor alpha (TNF-α) and prothrombotic mediator plasminogen activator inhibitor-1 (PAI-1) [50].
4.3.1 Interleukin-6: Interleukin-6 as a proinflammatory cytokine plays an important role in the pathogenesis of insulin resistance and type 2 diabetes mellitus [51]. Its production has been shown to increase with the increase in body fat and insulin resistance [24]. The effect of IL-6 in the liver for instance, increases the production of C-reactive protein (CRP) [24]. High CRP levels have been associated with the development of metabolic syndrome, diabetes mellitus and cardiovascular disease [51-53].
4.3.2 Tumour necrosis factor alpha: Tumour necrosis factor alpha (TNF-α) is secreted by the adipose tissue. Its production is exponential to the increase in adipose tissue mass [24]. TNF-α induces phosphorylation and inactivation of insulin receptors in the adipose tissue including muscle cells, increases FFAs production through lipolysis and inhibits adiponectin release [54]. Increased levels of TNF-α are correlated with components of metabolic syndrome i.e. obesity and insulin resistance [55].
4.3.3 Prothrombotic mediator plasminogen activator inhibitor-1: Prothrombotic mediator plasminogen activator inhibitor-1 (PAI-1) inhibits tissue plasminogen activator and is a prothrombotic protein [23]. Although the mechanism of PAI-1 in the pathogenesis of metabolic syndrome are not yet clearly understood, obesity induced oxidative stress has been suggested. Oxidative stress is a “phenomenon caused by an imbalance between production and accumulation of oxygen reactive species in cells and tissues and the ability of a biological system to detoxify these reactive products [56].” It has been shown that circulating PAI-1 are increased in obese subjects with metabolic syndrome [57].
5. Link Between Visceral Adipose Tissue and NAFLD
NAFLD is defined as liver fat content ≥ 5% of hepatocytes by histology or intrahepatic triglyceride content ≥5.5% by MRI in non-alcoholics (i.e. 30 g/d of alcohol in men and 20 g/d in women) [58]. NAFLD is a chronic liver disease and a predominant marker for: type 2 diabetes mellitus, chronic kidney disease, cardiovascular disease, metabolic syndrome and liver related deaths [59, 60]. The pathophysiology of NAFLD and its progression is induced by multiple factors, in a “multiple parallel hit” model, encompassing an interplay at an individual level of multiple genetic, behavioral, environmental factors and adipose tissue dysfunction. (Comprehensively reviewed by Azzu et al. [1], Fang et al. [61] and Yu et al. [62]).
Of interest briefly is the adipose tissue dysfunction. The state of “increased fat” as commonly observed in obesity 63], has been found to be a primary trigger of metabolic disorders [64]. The onset of obesity stimulates remodelling in the adipose tissue as a response to the changes in the energy status [65, 66]. This remodelling induces dysregulation of the adipose tissue derived cytokines, hormones and metabolites resulting in metabolic stresses and disorder in metabolic organs [67-69]. Actually, these inflammatory adipokines and cytokines that result due to dysregulation of the adipose issue impede with adipocyte differentiation and insulin signalling, lipid accumulation and increase adipocyte lipolysis. This results in a poor ability of the stressed and hypertrophic adipocytes to take up and release free fatty acids, thus, inducing redistribution of fat in other areas (ectopic) like visceral adipose tissue, skeletal muscle, liver, pancreas, and heart [70]. When lipid supply exceeds oxidative capacity in these tissues, intracellular lipid accumulation occurs, risking an obliteration of organ function [70]. Interestingly, greater rates of lipolysis, increased insulin resistance and increased cytokines release have been associated with hypertrophied subcutaneous adipocytes while visceral adipocyte hypertrophy has been associated with dyslipidaemia [71]. The later is understood to be one of the many mechanisms to the development of NAFLD through the excess delivery of “toxic” free fat acids directly into liver through the portal circulation.
Given these proposed mechanisms, the evidence is overwhelming to link adipose tissue vis-à-vis VAT as the most metabolic adipose tissue subtype to both NAFLD and metabolic syndrome. Actually, a number of studies have shown the association between increased VAT volumes with NAFLD. For instance, VAT area was found to be independently associated with fatty liver disease [72, 73]. At the same time it has been shown that increase in VAT mass irrespective of the ethnicity and method used to measure VAT volume has at least 2 times greater risk to the development of NAFLD [72, 74]. These outcomes reiterate the critical mediatory role VAT plays to the development and complication of NAFLD.
Similarly, multiple studies have also shown that the increase in VAT volume (irrespective of the method used to measure VAT mass) is strongly associated with metabolic syndrome in all BMI categories [14, 75, 76]. Moreover, many other studies have shown that VAT area/volume adds a risk and is an independent predictor to the development of metabolic syndrome. For instance, Bi et al. [77] and Nakao et al. [78] found that VAT area was an independent predictor of metabolic syndrome. Bi et al. [77] further showed the risk of metabolic syndrome increased 3-fold with each standard deviation of VAT area. Lu et al. [79] involving 3259 subjects with normal BMI demonstrated that subjects with high VAT amounts presented a much higher risk for metabolic syndrome. Likewise, Ding et al. [80] showed that lean subjects with metabolic dysfunction had increased VAT volume compared to the controls, with nearly 4-folds greater risk for NAFLD, 20-30% lower glucose disposal rates/insulin sensitivity and 30-40% greater insulin secretion rates.
Given this overwhelming evidence linking visceral adipose to metabolic dysfunction and fatty liver disease, It can almost be safely concluded therefore that increased VAT mass whether arising from adipocyte hypertrophy or hyperplasia and obliteration in its intrinsic functions as a connective tissue and as an endocrine organ has a causal effect in the development of metabolic syndrome and NAFLD. Interventions targeted at VAT loss or inhibition of metabolic functions of VAT may be helpful to ameliorate metabolic syndrome and NAFLD in both the lean and obese population.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Azzu V, Vacca M, Virtue S, et al. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in non-alcoholic fatty liver disease. Gastroenterology 158 (2020): 1899-1912.
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology 15 (2018): 11.
- Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Archives of Internal Medicine 168 (2008): 1617-1624.
- Li L, Liu D, Yan H, et al. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obesity reviews 17 (2016): 510-519.
- Janghorbani M, Salamat MR, Aminorroaya A, et al. Utility of the visceral adiposity index and hypertriglyceridemic waist phenotype for predicting incident hypertension. Endocrinology and Metabolism 32 (2017): 221-229.
- Birsoy K, Festuccia WT, Laplante M. A comparative perspective on lipid storage in animals. Journal of Cell Science 126 (2013): 1541-1552.
- Cannon B, Nedergaard J. Studies of thermogenesis and mitochondrial function in adipose tissues. In: Adipose Tissue Protocols. Springer (2008): 109-121.
- Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Archives of Medicine Science 9 (2013): 191-200.
- Szablewski L. Introductory chapter: Types of adipose tissue. In: Adipose Tissue. IntechOpen (2018).
- Lee JJ, Britton KA, Pedley A, et al. Adipose tissue depots and their cross-sectional associations with circulating biomarkers of metabolic regulation. Journal of the American Heart Association 5 (2016): e002936.
- Gesta S, Tseng Y, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell 131 (2007): 242-256.
- Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obesity Reviews 13 (2012): 30-39.
- Kim D, Chung GE, Kwak M, et al. Body fat distribution and risk of incident and regressed nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology 14 (2016): 132-138.
- Kwon H, Kim D, Kim JS. Body fat distribution and the risk of incident metabolic syndrome: a longitudinal cohort study. Scientific reports 7 (2017): 1-8.
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiology Review 84 (2004): 277-359.
- Shabalina IG, Petrovic N, de Jong JM, et al. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell reports 5 (2013): 1196-1203.
- Petrovic N, Walden TB, Shabalina IG, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. Journal of Biological Chemistry 285 (2010): 7153-7164.
- Ishibashi J. Medicine. Beige can be slimming. Science 328 (2010): 1113-1114.
- Kaisanlahti A, Glumoff T. Browning of white fat: agents and implications for beige adipose tissue to type 2 diabetes. Journal of Physiology and Biochemistry 75 (2019): 1-10.
- Jeremic N, Chaturvedi P, Tyagi SC. Browning of white fat: novel insight into factors, mechanisms, and therapeutics. Journal of Cell Physiology 232 (2017): 61-68.
- Ràfols ME. Adipose tissue: cell heterogeneity and functional diversity. Endocrinología y Nutrición (English Edition) 61 (2014): 100-112.
- Aganovic I, Dusek T. Pathophysiology of Metabolic Syndrome. EJIFCC 18 (2007): 3-6.
- McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clinical journal of Dermatology 36 (2018): 14-20.
- Rochlani Y, Pothineni NV, Kovelamudi S, et al. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Therapeutic advances in cardiovascular disease 11 (2017): 215-225.
- Park Y, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Archives of Internal Medicine 163 (2003): 427-436.
- Alberti, Kurt George Matthew Mayer, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 15 (1998): 539-553.
- Balkau B. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabetic Medicine 16 (1999): 442-443.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Journal of American Medical Association 285 (2001): 2486-2497.
- Einhorn, Daniel. American College of Endocrinology position statement on the insulin resistance syndrome. Endocrine practice 9 (2003): 5-21.
- Alberti KG, Zimmet P, Shaw J, et al. The metabolic syndrome--a new worldwide definition. Lancet 366 (2005): 1059-1062.
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112 (2005): 2735-2752.
- Alberti K. International diabetes federation task force on epidemiology and prevention; hational heart, lung, and blood institute; American heart association; wo. Circulation 120 (2009): 1640-1645.
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37 (1988): 1595-1607.
- Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Comprehensive Physiology 3 (2013): 1-58.
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The lancet 365 (2005): 1415-1428.
- Boden G, Shulman G. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. European Journal of Clinical Investigation 32 (2002): 14-23.
- Tooke J, Hannemann M. Adverse endothelial function and the insulin resistance syndrome. Journal of Internal Medicine 247 (2000): 425-431.
- Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52 (2003): 2882-2887.
- Juhanâ€Vague I, Alessi M, Mavri A, et al. Plasminogen activator inhibitorâ€1, inflammation, obesity, insulin resistance and vascular risk. Journal of Thrombosis and Haemostasis 1 (2003): 1575-1579.
- Lewis GF, Steiner G. Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Diabetes Care 19 (1996): 390-393.
- Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation 104 (2001): 3052-3056.
- Ouchi N, Ohishi M, Kihara S, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 42 (2003): 231-234.
- Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. Journal of American Medical Association 291 (2004): 1730-1737.
- Middlekauff HR. The treatment of heart failure: the role of neurohumoral activation. Internal medicine 37 (1998): 112-122.
- Vaneckova I, Maletinska L, Behuliak M, et al. Obesity-related hypertension: possible pathophysiological mechanisms. Journal of Endocrinology 223 (2014): 63-78.
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. American Journal of Physiology-Cell Physiology 292 (2007): 82-97.
- Gobal F, Deshmukh A, Shah S, et al. Triad of metabolic syndrome, chronic kidney disease, and coronary heart disease with a focus on microalbuminuria: death by overeating. Journal of the American College of Cardiology 57 (2011): 2303-2308.
- Dai Y, Mercanti F, Dai D, et al. LOX-1, a bridge between GLP-1R and mitochondrial ROS generation in human vascular smooth muscle cells. Biochemical and Biophysical Research Communications 437 (2013): 62-66.
- Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinology and Metabolism Clinics of North America 37 (2008): 753-768.
- Kaur J. A comprehensive review on metabolic syndrome. Cardiology Research and Practice (2014): 943162.
- Bao P, Liu G, Wei Y. Association between IL-6 and related risk factors of metabolic syndrome and cardiovascular disease in young rats. International journal of clinical and experimental medicine 8 (2015): 13491-13499.
- Bernberg E, Ulleryd MA, Johansson ME, et al. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in ApoE−/− mice. Atherosclerosis 221 (2012): 359-365.
- Bastard J, Jardel C, Bruckert E, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. The Journal of Clinical Endocrinology & Metabolism 85 (2000): 3338-3342.
- Hotamisligil GS, Murray DL, Choy LN, et al. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proceedings of the National Academy of Sciences of the United States of America 91 (1994): 4854-4858.
- Tsigos C, Kyrou I, Chala E, et al. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism - Clinical and Experimental 48 (1999): 1332-1335.
- Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health. Oxidative medicine and cellular longevity (2017).
- Alessi M, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arteriosclerosis, Thrombosis, and Vascular Biology 26 (2006): 2200-2207.
- Wong VW, Adams LA, De Lédinghen V, et al. Noninvasive biomarkers in NAFLD and NASH-current progress and future promise. Nature Reviews Gastroenterology and Hepatology 15 (2018):461-478.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55 (2012): 2005-2023.
- Lee Y, Cho Y, Lee B, et al. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes and metabolism journal 43 (2019): 31-45.
- Fang YL, Chen H, Wang CL, et al. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From "two hit theory" to "multiple hit model". World Journal of Gastroenterology 24 (2018): 2974-2983.
- Yu J, Marsh S, Hu J, et al. The pathogenesis of nonalcoholic fatty liver disease: interplay between diet, gut microbiota, and genetic background. Gastroenterology research and practice (2016).
- Symonds ME. Adipose Tissue Biology. Springer (2012).
- Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. The Journal of Clinical Endocrinology & Metabolism 5 (1976): 299-311.
- Leff T, Granneman JG. Adipose Tissue in Health and Disease. John Wiley & Sons (2010).
- Choe SS, Huh JY, Hwang IJ, et al. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Frontiers in endocrinology 7 (2016): 30.
- Chawla A, Nguyen KD, Goh YS. Macrophage-mediated inflammation in metabolic disease. Nature Reviews Immunology 11 (2011): 738-749.
- Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. International Scholarly Research Notices inflammation (2013).
- Huh JY, Park YJ, Ham M, et al. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Molecules and Cells 37 (2014): 365-371.
- Snel M, Jonker JT, Schoones J, et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. International journal of endocrinology (2012).
- Hoffstedt J, Arner E, Wahrenberg H, et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 53 (2010): 2496-2503.
- Yu SJ, Kim W, Kim D, et al. Visceral Obesity Predicts Significant Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Medicine (Baltimore) 94 (2015): e2159.
- Ko Y, Wong T, Hsu Y, et al. The correlation between body fat, visceral fat, and nonalcoholic fatty liver disease. Metabolic syndrome and related disorders 15 (2017): 304-311.
- Van der Poorten D, Milner K, Hui J, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 48 (2008): 449-457.
- Faria G, Gonçalves A, Cunha R, et al. Beyond central adiposity: liver fat and visceral fat area are associated with metabolic syndrome in morbidly obese patients. International Journal of Surgery 14 (2015): 75-79.
- Lopes HF, Corrêa-Giannella ML, Consolim-Colombo FM, et al. Visceral adiposity syndrome. Diabetology & metabolic syndrome 8 (2016): 40.
- Bi X, Seabolt L, Shibao C, et al. DXA-measured visceral adipose tissue predicts impaired glucose tolerance and metabolic syndrome in obese Caucasian and African-American women. European Journal of Clinical Nutrition 69 (2015): 329-336.
- Nakao YM, Miyawaki T, Yasuno S, et al. Intra-abdominal fat area is a predictor for new onset of individual components of metabolic syndrome: MEtabolic syndRome and abdominaL ObesiTy (MERLOT study). Proceedings of the Japan Academy, Series B 88 (2012): 454-461.
- Lu YC, Lin YC, Yen AM, et al. Dual-energy X-ray absorptiometry-assessed adipose tissues in metabolically unhealthy normal weight Asians. Scientific Reports 9 (2019): 17698-019-53557-9.
- Ding C, Chan Z, Chooi YC, et al. Regulation of glucose metabolism in nondiabetic, metabolically obese normal-weight Asians. American Journal of Physiology-Endocrinology and Metabolism 314 (2018): 494-502.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 