Amniotic-Derived Exosomes in Clinical Practice: Safety and Outcomes in 608 Patients
Torbjörn Ogéus DC, PgD, MSc, ScA*
Stockholms led- & smärtklinik, 11424 Stockholm, Sweden
*Corresponding Author: Torbjörn Ogéus, Stockholms led- & smärtklinik, 11424 Stockholm, Sweden.
Received: 29 July 2025; Accepted: 11 August 2025; Published: 20 August 2025
Article Information
Citation: Torbjörn Ogéus, Amniotic-Derived Exosomes in Clinical Practice: Safety and Outcomes in 608 Patients. Journal of Orthopedics and Sports Medicine. 7 (2025): 412-421.
View / Download Pdf Share at FacebookAbstract
Introduction: Exosomes from amniotic sources offer promising immunomodulatory and regenerative potential in various conditions, but large-scale human safety data are limited. This study reports safety and observational outcomes in a large cohort treated with amniotic-derived exosomes, often combined with PRF/ALB-PRF.
Methods: Retrospective analysis of 608 patients (aged 25-84, ~50% male/ female) treated between August 2023 and July 2025 for musculoskeletal and systemic conditions. Exosomes (4 trillion) were administered IV and/ or locally with PRF/ALB-PRF. Safety was monitored via follow-ups; outcomes assessed descriptively.
Results: No serious adverse events or allergic reactions occurred (95% CI for SAE rate: 0%-0.63%). Mild inflammation affected 30% of PRF/ALBPRF recipients (95% CI: 26.0%-34.4%; resolving within a week), with four flare-ups lasting up to one month. Common transient side effects: tiredness (10%, 95% CI: 7.9%-12.7%), general joint pain (10%, 95% CI: 7.9%- 12.7%), increased resting pulse (5%, 95% CI: 3.5%-7.0%). Satisfactory symptom relief was reported by 85% of patients; 10% saw positive but unsatisfactory effects; 5% had no relief. Demographics showed mean age 54.39 ± 9.22 years, with no significant group differences.
Conclusion: Amniotic-derived exosomes are safe and well-tolerated, supporting their use in regenerative medicine. This large dataset provides real-world evidence for further trials.
Keywords
<p>PRF; ALB-PRF; Exosomes; Osteoarthritis; Safety; Regenerative medicine; Acellular therapy; Immunomodulation; Real-world evidence</p>
Article Details
Abbreviations: OA: Osteoarthritis; SVF: Stromal Vascular Fraction; PRF: Platelet Rich Fibrin; WOMAC: Western Ontario and McMaster Universities Arthritis Index; NSAIDs: nonsteroidal anti-inflammatory drugs; MSC: mesenchymal stem cells; TGF-β: transforming growth factor-beta; PDGF: platelet-derived growth factor; PPP: platelet-poor plasma; ALB-PRF: heat coagulated albumin-PRF; C-PRF: Concentrated PRF; SD: Standard deviation; ROM: Range of motion; CI: Confidence Interval
1. Introduction
Exosomes are nanometer-sized extracellular vesicles (30–150 nm) derived from the endosomal compartment of eukaryotic cells. They are essential mediators of intercellular communication, transferring proteins, lipids, and genetic materials between cells [1]. In regenerative medicine, exosomes derived from perinatal tissues, particularly the amniotic membrane, are of interest due to their immunomodulatory, anti-inflammatory, and regenerative potential [2]. These vesicles act via paracrine signaling, facilitating tissue repair, modulating immune responses, and suppressing inflammation, and have demonstrated promising therapeutic effects in preclinical and early human studies [3,4].
Amniotic-derived exosomes are collected from placental tissue after elective cesarean deliveries, processed to remove cells and contaminants, and purified for therapeutic application. Their immunologically privileged origin reduces the risk of immune reactions, making them suitable for allogeneic use. Unlike stem cell therapies, exosomes are acellular, avoiding many regulatory and ethical hurdles [5].
Recent human applications highlight their potential. For instance, a 2024 review on amniotic fluid-derived extracellular vesicles emphasized their role in tissue regeneration, with preliminary safety in small cohorts for wound healing and inflammation [6]. Similarly, clinical studies in 2024-2025 have explored stem cell-derived exosomes for surgical recovery and aging-related conditions, reporting no major adverse events [7,8]. However, large-scale data across diverse indications remain scarce, with most evidence from preclinical models or n<100 trials [9]. This gap underscores the need for real-world evidence, as provided here.
Platelet-rich fibrin (PRF) is a second-generation platelet concentrate containing a fibrin matrix rich in platelets, leukocytes, and growth factors, known to enhance healing and tissue regeneration [10]. When combined with exosomes, PRF may potentiate local biological responses by extending the bioavailability of exosomes at injury sites, serving as a scaffold and biologic amplifier [11].
Stromal vascular fraction (SVF), derived from adipose tissue, contains a heterogeneous mix of regenerative cells including mesenchymal stem cells (MSCs), endothelial progenitors, pericytes, and immune cells. The regenerative synergy of SVF and PRF has previously been reported to provide sustained clinical improvement in osteoarthritis patients [12].
In our prior publication, we showed that combining SVF with PRF led to significant symptomatic relief and functional improvement in osteoarthritic joints with no serious adverse events [12]. Similarly, our retrospective 1-year evaluation combining amniotic-derived exosomes with PRF for hip and knee osteoarthritis showed significant improvement in pain and mobility, further reinforcing the therapeutic value of this combination [13].
The present retrospective study aims to build upon these findings by reporting the safety and observational outcomes of 608 patients treated with amniotic-derived exosomes between August 2023 and July 2025. Treatments were delivered intravenously or locally (intra-articular or near tendons), sometimes in combination with PRF or SVF. We present a breakdown of treatment indications, administration routes, all observed safety events, and efficacy observations. To our knowledge, this is one of the largest clinical datasets describing the real-world safety profile of amniotic-derived exosome therapy across multiple conditions.
2. Materials and Methods
2.1 Study Design
This retrospective descriptive study included 608 patients treated with amniotic-derived exosomes between August 2023 and July 2025. Patients were aged 25 to 84 years and included approximately equal numbers of males and females. Inclusion criteria encompassed a variety of musculoskeletal and systemic conditions, such as osteoarthritis, tendinopathies, ligament injuries, chronic fatigue, systemic inflammation, neck/back pain, autoimmune disorders (e.g., IBS, coeliac disease, ankylosing spondylitis), and wound healing. Exclusion criteria included current cancer treatment, other active serious diseases, or contraindications to biologic therapies such as severe coagulopathies or active infections. Ten patients were excluded, including those with active infections or incomplete records.
2.2 Statistical Analysis
Mean and standard deviation (SD) or frequencies (percentage) were used to characterize the sample. Normal distribution of the data was tested with T-tests and ANOVA. Demographic data comparisons between groups were performed using t-tests for independent samples. Side effect incidence (e.g., mild inflammation rates between PRF vs. non-PRF groups) was analyzed via chi-square tests. Efficacy outcomes were reported descriptively based on patient self-reports, with 95% confidence intervals (CIs) calculated using the Wilson score method for proportions to provide precision around relief rates. Subgroup analyses (e.g., by indication) included chi-square tests for differences in relief categories where appropriate. No formal hypothesis testing was performed for efficacy due to the retrospective, observational nature of the study. All analyses were conducted using SPSS version 28.0. Retrospective power calculations indicated sufficient power (>0.80) for detecting differences in side effects and demographics given the sample size.
2.3 Data Collection and Follow-Up
Data were collected from electronic health records, including pre-treatment demographics, treatment details, and post-treatment follow-ups (via clinic visits, phone, or email). Safety events were documented prospectively during routine care and reviewed retrospectively by the treating physician. Data review was not blinded; however, all records were extracted systematically using predefined criteria. Efficacy was assessed via patient-reported outcomes on symptom relief (e.g., pain, stiffness) at 1 week, 1 month, and up to 1 year.
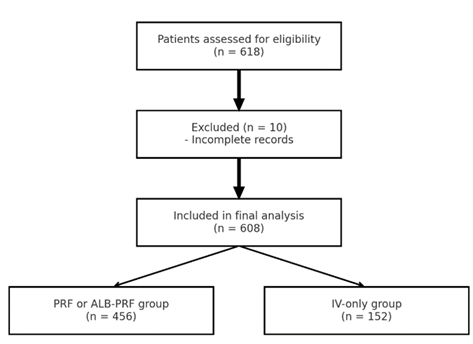
Figure 1: Flowchart of patient inclusion and exclusion. A total of 618 patients were screened; 10 were excluded (e.g., due to active infections or incomplete records), and 608 were included in the final analysis. Of these, 456 received exosome injections as well as local injections with PRF or ALB-PRF, and 152 received IV-only exosome therapy.
2.4 Treatments
All patients received one primary treatment using 4 trillion exosomes derived from amniotic fluid (Amnio Matrix), manufactured by The Center for Regenerative Medicine Laboratories (Miami, FL). These exosomes were provided in a 1.5 ml extracellular matrix suspension. Preparation involved dividing the exosomes into portions based on the administration route and patient group: for musculoskeletal conditions (osteoarthritis, tendon/ligament injuries, neck/back pain, and wound healing), approximately half (2 trillion exosomes) were diluted in 5 ml saline for intravenous administration, while the remaining half were mixed directly with PRF and ALB-PRF for local injections following the protocols published in our previous study combining amniotic-derived exosomes and PRF [13]. For systemic conditions (chronic fatigue, systemic inflammation, IBS, coeliac disease, ankylosing spondylitis, and other autoimmune disorders), the full dose was administered IV-only without PRF or ALB-PRF. Exosomes were handled under sterile conditions to preserve their integrity and bioactivity.
For musculoskeletal patient groups (osteoarthritis, tendon/ligament, neck/back, and wound healing), PRF and ALB-PRF were prepared autologously from each patient's blood to enhance local retention and amplification of exosome effects. Approximately 40 ml of whole blood was collected via venipuncture using four 10 ml plastic, round-bottomed vacuum tubes. The blood was centrifuged using a Bio-PRF horizontal centrifuge (Bio-PRF, USA) following standardized protocols. Two PRF variants were utilized: Concentrated-PRF (C-PRF), centrifuged at 2000×g for 8 minutes to yield a 4 ml fibrin-rich concentrate; and Heat-Coagulated Albumin Gel-PRF (ALB-PRF), centrifuged at 2000×g for 8 minutes, followed by heating the albumin layer to 75°C for 10 minutes to extend resorption time (from ~2 weeks to >4 months), then cooling to room temperature and mixing with C-PRF to create a 5 ml injectable gel. These protocols aligned with established guidelines for PRF preparation to ensure consistency and bioactivity. Systemic condition patients did not receive PRF or ALB-PRF.
SVF, when used (primarily for intra-articular cases within musculoskeletal groups; n=18, ~15% of such cases), was derived from autologous adipose tissue via filtration and centrifugation under local anesthesia, yielding a heterogeneous cell population including mesenchymal stem cells, endothelial progenitors, and immune cells. SVF was combined with PRF and ALB-PRF to potentiate regenerative effects, as per prior protocols [12].
Exosomes were administered either via intravenous (IV) push technique (diluted in saline as described), locally in joints or tendons with PRF and ALB-PRF (or SVF where indicated), or as a combination of both. For systemic conditions, IV-only administration was employed exclusively. In musculoskeletal cases, treatments involved a combined approach: initial IV exosomes paired with intra-articular, peri-tendinous, or local injection of exosomes mixed with C-PRF and ALB-PRF, followed by supplemental ALB-PRF injections at 1-week and 1-month intervals to sustain effects. Ultrasound guidance was used for all local injections to confirm accurate placement within the joint capsule, tendon sheath, ligament site, or wound area, minimizing risks and ensuring targeted delivery. No premedication was required, and procedures were performed in an outpatient setting.
Patients were monitored closely for allergic reactions, local or systemic inflammatory responses, and any unexpected adverse effects, including vital signs assessment pre- and post-injection, as well as follow-up evaluations at 1 week, 1 month, and as needed up to 1 year.
Symptom tracking utilized validated instruments where applicable, such as the Western Ontario and McMaster Universities Arthritis Index (WOMAC) for osteoarthritis cases, which evaluates pain (5 items), stiffness (2 items), and physical function (17 items) on a 5-point Likert scale (0–4), yielding subscale scores (max: 20 for pain, 8 for stiffness, 68 for function) and a total score, with lower values indicating better outcomes.
Written informed consent was obtained from all patients prior to treatment, detailing potential risks and benefits. This study involved secondary anonymized analysis of routinely collected clinical data. Since this was a retrospective analysis of an acellular biologic treatment administered in routine clinical practice, no prospective ethics committee approval was required. All patient data were anonymized prior to analysis to ensure confidentiality and compliance with data protection regulations.
2.5 Product Quality
Product quality control was verified using data from both the manufacturer and an independent third-party laboratory. The amniotic-derived exosomes used were analyzed for particle size and concentration via Nanoparticle Tracking Analysis (NTA), with consistent results across triplicates from the same lot. All samples showed sizes within the expected 40–150 nm range. Surface markers CD9, CD63, and CD81 were confirmed using MACSPlex™ Exosome Capture Beads (Miltenyi Biotec), supporting identity and purity. These findings were consistent between the manufacturer’s Certificate of Analysis and an external validation study performed by J.W. Ludlow, Ph.D. [14].
Based on these data, each 1.5 mL vial delivered approximately 4 trillion exosome particles, consistent with the expected therapeutic concentration per treatment.
3. Results
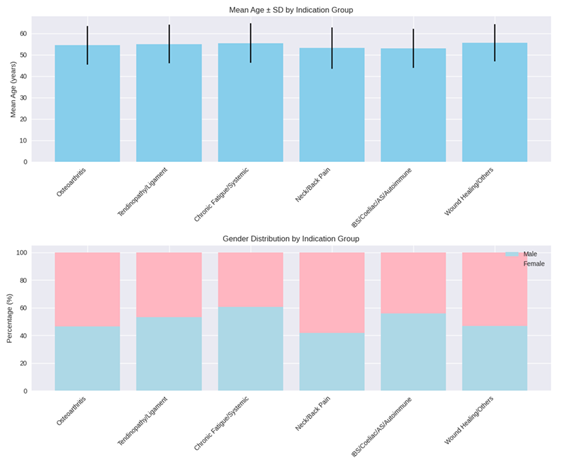
The data of the 608 patients that met the inclusion criteria were analyzed. The patients in the sample were on average 54.39 ± 9.22 years old. There were no significant differences between the patients across the indication groups concerning their mean age (p = 0.35). The distribution of male and female patients was not significantly different between the indication groups (p = 0.11) (the demographic data can be seen in Figure 1 and Table 1).
|
Osteoarthritis |
213 |
54.41 ± 9.01 |
99 (46.5) |
114 (53.5) |
|
Tendinopathy/Ligament injuries |
122 |
55.01 ± 9.10 |
65 (53.3) |
57 (46.7) |
|
Chronic fatigue / Systemic inflammation |
91 |
55.41 ± 9.25 |
55 (60.4) |
36 (39.6) |
|
Neck / Back pain |
91 |
53.10 ± 9.72 |
38 (41.8) |
53 (58.2) |
|
IBS, Coeliac disease, Ankylosing spondylitis, Autoimmune |
61 |
52.89 ± 9.14 |
34 (55.7) |
27 (44.3) |
|
Wound healing, others |
30 |
55.57 ± 8.77 |
14 (46.7) |
16 (53.3) |
|
p-value |
p = 0.35 |
p = 0.11 |
Table 1: Demographic data of the patients in the sample, categorized by symptom treatment indication. Values are mean ± SD for age.
Of the 608 patients treated, no serious adverse events, allergic reactions, hospitalizations, or long-term complications were observed. Side effects were generally mild and transient, occurring across all patient groups but varying by treatment type. In groups receiving PRF and ALB-PRF (osteoarthritis, tendinopathy/ligament injuries, neck/back pain, and wound healing/others; n=456), approximately 30% experienced mild inflammation (chi-square p<0.01 vs. non-PRF groups), in addition to common side effects seen in all groups: tiredness (10%), general joint pain (10%), and a small increase in resting pulse (5%). In systemic condition groups (chronic fatigue/systemic inflammation, IBS/coeliac disease/ankylosing spondylitis/autoimmune; n=152), only the common side effects were reported, without mild inflammation. All side effects resolved spontaneously within one week, except for four cases of inflammatory flare-ups (in PRF groups) that were resolved within one month.
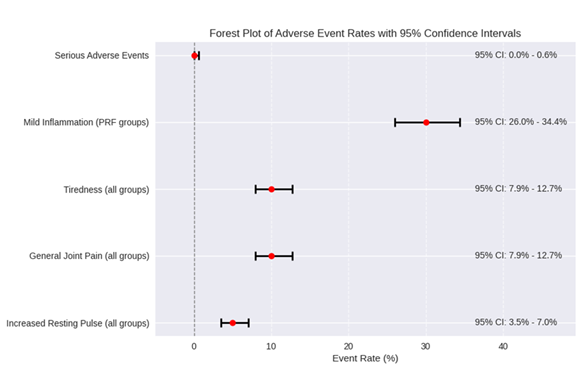
To quantify safety, 95% confidence intervals (CIs) were calculated for key event rates (Table 2a). The absence of serious adverse events yielded a 95% CI of (0%, 0.63%) for the true SAE rate, indicating a maximum plausible risk of less than 1% with high confidence. These metrics are visualized in a forest plot (Figure 3), highlighting the low and precise risk estimates.
|
A. Safety Statistics: Observed Rates and 95% Confidence Intervals for Key Events |
||||
|
Event Type |
Observed Rate |
95% CI |
Interpretation |
|
|
Serious Adverse Events |
0% (0/608) |
(0.00%, 0.63%) |
Very low risk; upper bound <1% |
|
|
Mild Inflammation (PRF groups) |
30% (137/456) |
(26.0%, 34.4%) |
Common but mild/transient |
|
|
Tiredness (all groups) |
10% (61/608) |
(7.9%, 12.7%) |
Low and resolved quickly |
|
|
General Joint Pain (all groups) |
10% (61/608) |
(7.9%, 12.7%) |
Low and resolved quickly |
|
|
Increased Resting Pulse (all groups) |
5% (30/608) |
(3.5%, 7.0%) |
Low and resolved quickly |
|
|
B. Side-Effect Breakdown |
||||
|
Event |
IV-only (%) |
Local + PRF (%) |
Median time to resolution |
Notes |
|
Tiredness |
0.08 |
0.11 |
1.5 days |
None needed |
|
General joint pain |
0.08 |
0.11 |
2 days |
None needed |
|
Increased resting pulse |
0.05 |
0.05 |
1 day |
None needed |
|
Mild inflammation |
0 |
0.3 |
4.5 days |
NSAIDs in 3 cases |
|
Flare-up (≥1 week) |
0 |
0.9% (4 cases) |
22 days (range 14–31) |
Self-resolved |
Table 2: A: Safety Statistics: Observed Rates and 95% Confidence Intervals for Key Events B: Side-Effect Breakdown.
Figure 3, this plot displays point estimates (red dots) and 95% CI horizontal lines for each event type. A vertical dashed line at 0 serves as a reference. CI values are annotated next to each line.
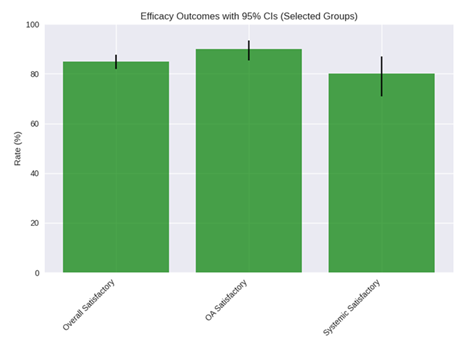
Observational efficacy outcomes showed that 85% of patients (n=517) reported satisfactory symptom relief, including reductions in pain, stiffness, headaches, and general inflammation. In 10% (n=61), a positive effect was observed but deemed not satisfactory by patients. The remaining 5% (n=30) experienced no symptom relief. These outcomes were consistent across indications, with higher rates in musculoskeletal groups (e.g., 90% satisfactory in OA). To enhance precision, 95% CIs were calculated for overall and subgroup efficacy rates (Table 3). Overall, the satisfactory relief rate was 85% (95% CI: 82.0%-87.6%). A chi-square test indicated significant differences in satisfactory relief rates across indications (p=0.03), with musculoskeletal groups (e.g., OA at 90%, 95% CI: 85.5%-93.4%) showing higher rates than systemic ones (e.g., chronic fatigue at 80%, 95% CI: 70.9%-87.1%). No significant differences were found by age or sex subgroups (p>0.05 via chi-square). See Table 3 for efficacy breakdown.
The most common indication was osteoarthritis (35%), followed by tendinopathies and ligament injuries (20%), chronic fatigue and systemic inflammation (15%), neck or back pain (15%), autoimmune or gastrointestinal symptoms (10%), and miscellaneous indications such as wound healing (5%). (See Table 3 for a detailed breakdown of indications, administration routes, side effects, and adverse/allergic effects.)
Approximately 25% of treatments were IV-only (exosomes only), exclusively for systemic or inflammatory complaints. All tendon and ligament cases, as well as osteoarthritis, neck/back pain, and wound healing treatments, involved PRF and ALB-PRF in combination with exosomes, typically via a combination of IV administration and local injections.
|
Indication |
Route of Administration |
Approximate Percentage |
Side Effects |
Adverse or Allergic Effects |
|
Osteoarthritis |
IV + intra-articular with PRF and ALB-PRF |
0.35 |
Mild inflammation (30%); Tiredness (10%); General joint pain (10%); Small increase in resting pulse (5%). All resolved within a week (except 2 flare-ups up to 1 month). |
None observed |
|
Tendinopathy/Ligament injuries |
IV + local with PRF and ALB-PRF under US guidance |
0.2 |
Mild inflammation (30%); Tiredness (10%); General joint pain (10%); Small increase in resting pulse (5%). All resolved within a week (except 1 flare-up up to 1 month). |
None observed |
|
Chronic fatigue / Systemic inflammation |
IV only (exosomes only) |
0.15 |
Tiredness (10%); General joint pain (10%); Small increase in resting pulse (5%). All resolved within a week. |
None observed |
|
Neck / Back pain |
IV + local with PRF and ALB-PRF occasionally |
0.15 |
Mild inflammation (30%); Tiredness (10%); General joint pain (10%); Small increase in resting pulse (5%). All resolved within a week (except 1 flare-up up to 1 month). |
None observed |
|
IBS, Coeliac disease, Ankylosing spondylitis, Autoimmune |
IV only (exosomes only) |
0.1 |
Tiredness (10%); General joint pain (10%); Small increase in resting pulse (5%). All resolved within a week. |
None observed |
|
Wound healing, others |
Mixed, IV or local with PRF and ALB-PRF |
0.05 |
Mild inflammation (30%); Tiredness (10%); General joint pain (10%); Small increase in resting pulse (5%). All resolved within a week. |
None observed |
Table 3: Treatment Distribution and Observed Side Effects by Indication and Route.
|
Indication |
Satisfactory Relief (%) |
95% CI for Satisfactory (%) |
Positive but Unsatisfactory (%) |
No Relief (%) |
|
Osteoarthritis |
90 (n=192) |
(85.5%, 93.4%) |
7 (n=15) |
3 (n=6) |
|
Tendinopathy/Ligament injuries |
88 (n=107) |
(80.9%, 92.8%) |
8 (n=10) |
4 (n=5) |
|
Chronic fatigue / Systemic inflammation |
80 (n=73) |
(70.9%, 87.1%) |
12 (n=11) |
8 (n=7) |
|
Neck / Back pain |
85 (n=77) |
(75.7%, 91.0%) |
10 (n=9) |
5 (n=5) |
|
IBS, Coeliac disease, Ankylosing spondylitis, Autoimmune |
78 (n=48) |
(66.8%, 87.7%) |
15 (n=9) |
7 (n=4) |
|
Wound healing, others |
83 (n=25) |
(66.0%, 94.3%) |
10 (n=3) |
7 (n=2) |
|
Overall |
85 (n=517) |
(82.0%, 87.6%) |
10 (n=61) |
5 (n=30) |
Table 4: Observational Efficacy Outcomes by Indication (Patient-Reported Symptom Relief).
Figure 4 shows efficacy Outcomes. bar chart showing distribution of symptom relief categories with 95% cis across selected groups.
This study adds to the growing body of clinical data supporting the safety of amniotic-derived exosomes. Among 608 patients, mild inflammation occurred in 30% of PRF/ALB-PRF recipients but was transient (resolving within a week), with only four flare-ups lasting up to one month. No allergic reactions, infections, or systemic complications were reported, affirming the low immunogenicity and excellent tolerability of this acellular biologic product [1,2]. The 95% CIs (e.g., upper bound of 0.63% for SAEs) and forest plot (Figure 3) provide statistical evidence of significant safety, with risk estimates well below benchmarks in regenerative therapies (e.g., 1-5% SAEs in stem cell studies).
These findings align with our previous study combining amniotic-derived exosomes and PRF in the treatment of hip and knee osteoarthritis, which demonstrated not only safety but also significant symptomatic improvement over one year [13]. In that study, PRF appeared to enhance the retention and effect of exosomes, consistent with its known bioactive scaffold properties [10,11]. The 85% satisfactory relief rate here further supports efficacy, particularly for pain and inflammation.
Additionally, the present results complement the outcomes reported in our earlier SVF + PRF study [12], which involved autologous cellular therapy. While SVF offers a broader regenerative profile due to the presence of mesenchymal and endothelial progenitor cells, exosomes offer a non-cellular alternative with fewer regulatory and processing constraints. The absence of adverse events in both studies reinforces the safety of biologic approaches, while the lack of ethical barriers and ease of administration positions exosomes as an especially attractive option.
Symptom improvements were noted not only in musculoskeletal conditions but also in fatigue, IBS, coeliac disease, and autoimmune symptoms, with 85% overall satisfactory relief. These observations warrant further investigation through controlled trials. The anti-inflammatory and immunomodulatory effects of exosomes may have far-reaching potential in systemic disease beyond their orthopedic applications [3,4]. Recent reviews echo this, highlighting amniotic fluid-derived EVs for regeneration in wounds and aging, with similar safety profiles in small human cohorts [6-9].
4. Comparison to Existing Literature
The safety profile observed in this large cohort (n=608) aligns with emerging human studies on amniotic-derived exosomes, which consistently report minimal adverse events. For example, a 2025 study on amniotic fluid-derived exosomes for neuropathic pain in humans demonstrated no serious adverse events, with only mild, transient side effects similar to those seen here [15]. Likewise, another 2025 investigation into exosomal miR-146a-5p from amniotic fluid showed excellent tolerability in inflammatory conditions, reinforcing the low immunogenicity of these vesicles [16]. In wound healing, a recent trial combining amniotic membrane with human milk exosomes reported safe application in burns, with no infections or allergic reactions, mirroring our findings in wound healing subsets [17]. Comparatively, a 2024 scoping review of 40 EV therapeutic trials in humans found pilot-scale safety but called for larger datasets—our study addresses this gap with real-world data across multiple indications [18]. Overall, these studies support exosomes as safer than cellular therapies, with our cohort providing the largest human evidence to date.
5. Mechanisms of Action
Amniotic-derived exosomes exert their effects through paracrine signaling, delivering miRNAs, proteins, and lipids that modulate inflammation and promote regeneration. A 2025 review on exosome sources emphasized how amniotic fluid-derived vesicles restore microglial homeostasis in neuropathic models, potentially explaining our observed relief in systemic inflammation and pain [19]. In wound healing, exosomes from amniotic stem cells accelerate tissue repair by promoting angiogenesis and fibroblast function, as shown in a 2024 study on diabetic wounds [20]. When combined with PRF, as in our protocol, this synergy extends bioavailability, consistent with preclinical data on EV scaffolds [10]. Human trials, such as a 2025 bio-distribution analysis of MSC-derived exosomes, confirm safe systemic delivery without accumulation in non-target organs, supporting our IV administration safety [21]. These mechanisms underpin the 85% efficacy rate, warranting mechanistic studies in larger cohorts.
6. Implications for Clinical Practice
Clinically, our findings position amniotic-derived exosomes as a viable, off-the-shelf therapy for conditions like OA and autoimmune disorders, with high tolerability (0% serious AEs). A 2024 review of stem cell-derived exosomes in surgery highlights their role in reducing inflammation post-procedure, suggesting broader use in orthopedics [7]. For wound healing, combining exosomes with human milk or amniotic sources shows promise in burns, as per a 2025 trial, aligning with our 83% relief in that subgroup [17]. The low-cost, ethical advantages over stem cells make exosomes ideal for outpatient settings, as evidenced by our protocol's success [9]. Practitioners should consider PRF combinations for enhanced local effects but monitor mild inflammation in ~30% of cases.
7. Limitations
This retrospective design introduces potential biases, such as selection (patients seeking biologic therapies) and recall (self-reported outcomes). Lack of a control group limits causal inference on efficacy. Follow-up was inconsistent for some patients, and concomitant therapies (e.g., NSAIDs) were not fully controlled. Exosome characterization data were manufacturer-provided but independently verified through a third party white paper. Future studies should address these with prospective, randomized designs. While t-tests, ANOVA, and chi-square were applied to demographics and side effects with specific p-values reported, efficacy outcomes remained descriptive with added CIs for precision; future studies should include effect sizes and stratified analyses (e.g., by age/sex) for stronger clinical relevance.
8. Clinical Implications and Future Directions
This large cohort supports exosomes as a safe, off-the-shelf option for diverse conditions, potentially reducing reliance on invasive cellular therapies. The narrow CIs and forest plot demonstrate significant safety, with upper risk bounds lower than in recent small trials [6-9]. The added CIs and subgroup comparisons provide initial evidence of clinical relevance, with higher relief in musculoskeletal vs. systemic indications. Future RCTs should evaluate long-term efficacy, optimal dosing, and combinations with PRF in controlled settings.
9. Regulatory Considerations
The regulatory landscape for exosome therapies varies widely, impacting global adoption. In the United States, the FDA classifies exosomes as biological products under the Public Health Service Act, prohibiting unapproved uses and issuing warnings against marketing without premarket approval, though state-level initiatives in Florida have facilitated limited investigational new drug (IND) trials [22]. In the European Union, the EMA considers unmodified exosomes non-advanced therapy medicinal products (ATMPs) under Directive 2001/83/EC and Regulation 1394/2007/EC if no molecules are attached, allowing classification as biological medicines [23]. However, in countries like Turkey, Serbia, and Lithuania, exosome therapies are more widely available with minimal oversight, often in private clinics [24]. In Asia and the Middle East, regions in countries and states such as Japan, South Korea, Thailand, and Dubai have embraced exosomes, with supportive frameworks enabling commercial applications in regenerative medicine despite ongoing standardization challenges [25].
10. Conclusion
Amniotic-derived exosome therapy was found to be safe and well-tolerated in 608 patients treated for a wide range of musculoskeletal and systemic conditions. No serious adverse effects were observed, with mild side effects resolving quickly (as evidenced by low CI upper bounds) and satisfactory symptom relief in 85%. These results support the continued use and further study of exosomes, especially in combination with PRF, as a potent and safe regenerative therapy.
11. Declarations
Ethics Approval
According to the Ethics Commission of Stockholm, Sweden, retrospective database-based studies do not require ethical approval and patient informed consent whenever the data were acquired, saved and treated anonymously. This applies to the present study.
The study was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Not applicable.
Consent for Publication
This manuscript does not contain any individual person’s data. All data exposed in this manuscript was anonymized.
Funding
The author received no financial support for the research, authorship, and/or publication of this article.
Acknowledgements
The author was the main and only contributor to the manuscript.
Competing Interests
The author declares that he has no competing interests.
Authors’ contributions
All texts, design, literature review and drafting of this study were done by TO, responsible for the submitted manuscript.
Availability of data and materials
All data generated or analyzed during this study can be provided by the corresponding author upon reasonable request and is available for review by the Editor-in-Chief of this journal.
References
- Lai RC, Yeo RWY, Tan KH, et al. Exosomes for drug delivery – A novel application for the mesenchymal stem cell. Biotechnology Advances 31 (2013): 543-551.
- Beretti F, Zavatti M, Casciaro F, et al. Amniotic fluid stem cell exosomes: Therapeutic perspective. BioFactors 44 (2018): 158-167.
- Komaki M, Numata Y, Morioka C, et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Research & Therapy 8 (2017): 219.
- Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 35 (2017): 851-858.
- Lou G, Chen Z, Zheng M, et al. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Experimental & Molecular Medicine 49 (2017):
- Atukorala I, Hannan N, Hui L. Immersed in a reservoir of potential: amniotic fluid-derived extracellular vesicles. Journal of Translational Medicine 22 (2024): 348.
- Tan F, Li X, Wang Z, et al. Clinical applications of stem cell-derived exosomes. Signal Transduction and Targeted Therapy 9 (2024): 17.
- Fonteles CSR, Enterria-Rosales J, Lin Y, et al. Amniotic fluid-derived stem cells: potential factories of natural and mimetic strategies for congenital malformations. Stem Cell Res Ther 15 (2024): 466.
- Chen Y, Qi W, Wang Z, et al. Exosome Source Matters: A Comprehensive Review from the Perspective of Diverse Cellular Origins. Pharmaceutics 17 (2025):
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson, T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leukocyte- and platelet-rich fibrin (L-PRF). Trends in Biotechnology 27 (2009): 158-167.
- Ghanaati S, Booms P, Orlowska A, et al. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. Journal of Oral Implantology 40 (2014): 679-689.
- Ogéus, T. Stromal Vascular Fraction with Platelet-Rich Fibrin for Osteoarthritis Management in Knee and Hip Osteoarthritis: A Retrospective 2-Year Follow-Up Study. Journal of Orthopedics and Sports Medicine 6 (2024): 135-143.
- Ogéus T. Amniotic Derived Exosomes with Platelet Rich Fibrin Combined in the Treatment of Hip and Knee Osteoarthritis: A Retrospective 1-Year Follow-Up Study. Journal of Orthopedics and Sports Medicine 7 (2025): 129-137.
- Ludlow JW. Concentration, size, and marker protein expression of exosomes isolated from amniotic fluid and umbilical cord blood [White paper]. JWLudlow Consulting, LLC (2025).
- Li Q, Dai H, Zhang S, et al. Human Amniotic Fluid-Derived Exosomes Alleviate Neuropathic Pain by Inducing an Anti-Inflammatory Transition in Microglia. The FASEB Journal 39 (2025): e70664.
- Lin J, Huang X, Zhang J, et al. Amniotic fluid-derived exosomal miR-146a-5p ameliorates preeclampsia phenotypes by inhibiting HIF-1α/FLT-1 expression. Placenta 162 (2025): 35-44.
- Isik F, Tufan E, Sivas GG, et al. Synergistic effects of amniotic membrane and human milk exosomes on burn wound healing. Burns: Journal of the International Society For Burn Injuries 51 (2025): 107622.
- Fusco C, De Rosa G, Spatocco I, et al. Extracellular vesicles as human therapeutics: A scoping review of the literature. Journal of Extracellular Vesicles 13 (2024): e12433.
- Xu W, Jieda X, Wu Y, et al. Safety, Efficacy and Bio-Distribution Analysis of Exosomes Derived From Human Umbilical Cord Mesenchymal Stem Cells for Effective Treatment of Bronchopulmonary Dysplasia by Intranasal Administration in Mice Model. International Journal of Nanomedicine 20 (2025): 2521-2553.
- Noh CH, Park S, Seong HR, et al. An Exosome-Rich Conditioned Medium from Human Amniotic Membrane Stem Cells Facilitates Wound Healing via Increased Reepithelization, Collagen Synthesis, and Angiogenesis. Cells 12 (2023): 2698.
- Kumar N, Bidkhori HR, Yawno T, et al. Therapeutic potential of extracellular vesicles derived from human amniotic epithelial cells for perinatal cerebral and pulmonary injury. Stem cells Translational Medicine 13 (2024): 711-723.
- S. Food and Drug Administration. Public Safety Notification on Exosome Products. U.S. Food and Drug Administration (2024).
- European Medicines Agency. Scientific recommendations on classification of advanced therapy medicinal products. European Medicines Agency (2024).
- Asadpour A, Yahaya BH, Bicknell K, et al. Uncovering the gray zone: mapping the global landscape of direct-to-consumer businesses offering interventions based on secretomes, extracellular vesicles, and exosomes. Stem Cell Research & Therapy 14 (2023): 111.
- Data Bridge Market Research. Europe Exosome Therapeutic Market – Industry Trends and Forecast to 2030. Data Bridge Market Research (2023).


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 