Protocatechuic Acid Induces Expression of Osteogenic Genes in Human Osteoblast Cultures: Implications for the Treatment of Osteoporosis and Fractures
Lanny L. Johnson1*, Peter Chen2, Darryl D’Lima PhD3
1President Pcabioscience, LLC, Las Vegas, NV, USA
2Institute for Biomedical Sciences, San Diego, CA, USA
3Professor, Molecular & Cell Biology Scripps Research Institute, CA, USA
*Corresponding Author: Lanny L. Johnson, President Pcabioscience, LLC, Las Vegas, NV, USA.
Received: 09 October 2025; Accepted: 15 October 2025; Published: 22 October 2025
Article Information
Citation: Lanny L Johnson, Peter Chen, Darryl D Lima. Protocatechuic Acid Induces Expression of Osteogenic Genes in Human Osteoblast Cultures: Implications for the Treatment of Osteoporosis and Fractures. Journal of Orthopedics and Sports Medicine. 7 (2025): 494-499.
View / Download Pdf Share at FacebookAbstract
Background: Osteoporosis, a condition characterized by a pathologic decrease in bone density, is a common disorder in the elderly. Although anti-resorptive agents that target osteoclasts (e.g., bisphosphonates) are available to treat this condition, very few of the current drugs were of anabolic nature and designed to target osteoblasts. Protocatechuic acid (PCA, 3,4-dihydroxybenzoic acid), a nutraceutical is one such reagent.
Purpose: This proof-of-concept study was designed to determine whether PCA, an abundant natural phenolic acid, promotes gene expression in human osteoblasts in culture.
Methods: Osteogenic and anti-osteogenic gene expression was evaluated by quantitative reverse transcription-polymerase chain reaction in osteoblasts cultured for 12 days with increasing concentrations (1 μM – 100 μM) of PCA.
Results: PCA induced osteopontin (SSP1), bone sialoprotein (IBSP), tissue nonspecific alkaline phosphatase (ALPL), and bone morphogenetic protein 6 (BMP6), but not RUNX2 expression over levels detected in untreated osteoblast cultures (negative control). Interestingly, peak SSP1 expression observed in response to the highest PCA concentrations (50 μM – 100 μM) exceeded levels detected in cultures maintained in commercial osteogenic medium (positive control). By contrast, although PCA induced the expression of IL1B, both TNF and MMP1 were downregulated compared to untreated controls.
Conclusion: The results of this study revealed that PCA had a prominent impact on osteogenic and anti-osteogenic gene expression in human osteoblasts. Further research will be needed to determine if PCA or a biochemical derivative might be used clinically as part of an effective regimen to treat osteoporosis, bone metabolism and fractures.
Keywords
<p>Protocatechuic acid; Osteoblasts; Osteogenesis; Osteoporosis; Osteopenia; Fractures</p>
Article Details
1. Introduction
Osteoporosis, a common disorder among the elderly, frequently leads to an increased risk of severe fractures, debilitating pain, and poor quality of life [1-3]. Although bisphosphonate drugs (e.g., alendronate, risedronate, and others), stable derivatives of pyrophosphate that target osteoclast activity, are highly effective treatments for this disorder [4,5], the results of recent studies suggest that other pathways associated with bone development might also be exploited [6]. In addition to osteoclasts, osteoblasts, osteocytes, and their progenitors may also be suitable targets for drug therapy. Unlike osteoclasts, which are derived from monocytes/macrophages and break down bone as part of the natural remodeling process, osteoblasts and osteocytes are synthetic cells that promote bone growth and mineral deposition. Osteoblasts develop from mesenchymal and pre-osteoblast progenitor cells found in various skeletal niches via a process involving several key signaling and transcriptional regulators, including RUNX1 and RUNX2 [7,8]. Osteoblasts in bone can then develop into osteocytes, which are cells that maintain bone mineralization, transduce signals, and function as mechanosensors to facilitate bone remodeling [9,10]. While a few currently available drugs (e.g., teriparatide) can be used to promote bone deposition in patients diagnosed with osteoporosis, it is by injection and not without many potential side effects. There is clearly a need for more research into the possibility of targeting and amplifying this pathway, especially one with few side effects. Natural products research has provided new insights into osteoporosis treatment strategies. For example, the abundant naturally occurring phenolic acid, protocatechuic acid (PCA; 3,4-dihydroxybenzoic acid), has been used for centuries in traditional Chinese and other alternative medicinal regimens as an anti-inflammatory, anti-bacterial, and analgesic agent [11]. PCA is safe, non-toxic by any measure and non-allergenic, non-mutagenic. The pharmacodynamics and pharmacokinetics are known. Recent results suggest that PCA may also be an effective treatment for osteoporosis. Although most of the publications featuring PCA focus on its role in arresting osteoclast activity and preventing ongoing bone resorption [12-15], we are particularly interested in recent reports that highlight its impact on osteoblast activation and development [16-18]. Collectively, the results of these latter studies suggest that osteoblasts and their progenitors targeted by PCA may be effective targets for the development of new strategies to treat osteoporosis. Interestingly, results from our recent study revealed that human mesenchymal stem cells responded to PCA with increased expression of several genes implicated in osteogenesis, including SSP1 and RUNX2 (manuscript in preparation). Thus, this study aimed to determine the impact of PCA on the osteogenic differentiation of isolated human osteoblasts. If positive, the results from this study might be expanded to conditions other than osteoporosis and may ultimately be used to improve fracture healing and other aspects of regenerative medicine.
2. Materials and Methods
2.1 Cells and reagents
Primary human osteoblasts and human osteoblast basal medium were obtained from Cell Applications (San Diego, CA, USA). Cells were expanded in cultures with basal medium supplemented with human osteoblast growth supplement containing 0.15 g/L calcium as per the manufacturer’s instructions. Human osteoblast differentiation medium (osteogenic medium, 0.2 g/L calcium) was also purchased from Cell Applications. Crystalline PCA (3,4-dihydrobenzoic acid) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions were prepared in dimethyl sulfoxide at 648 mM and stored at -80 °C pending use. Alizarin red S was purchased from Pfaltz & Bauer (Waterbury, CT, USA, catalog no. 58005) and dissolved in distilled water to a stock concentration of 2 mg/mL, with pH adjusted with 1 M HCl to 4.1-4.3.
2.2 Tissue culture
Osteoblasts were expanded and transferred to 24-well plates at 104 cells/well, and cultured overnight before initiating the experiments. Triplicate wells were either inoculated with PCA to final concentrations ranging from 1 to 100 µM, transferred to human osteoblast differentiation medium (positive control; no PCA), or left untreated (negative control). Cultures were maintained for 12 days, with media changed every three days. Each experiment was performed three to four times.
2.3 RNA extraction and quantitative reverse transcription polymerase chain reaction
RNA was extracted from cultured cells on day 12 using RNeasy kits (Qiagen, Germantown, MD, USA). No obvious cytotoxicity was noted (i.e., floating or detached cells). cDNA was synthesized from 50 ng of RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Waltham, MA, USA). Expression of osteogenic genes (osteopontin [secreted phosphoprotein-1, SPP1], RUNX family transcription factor 2 [RUNX2], tissue nonspecific alkaline phosphatase [ALPL], and bone sialoprotein [integrin-binding sialoprotein, IBSP]), anti-osteogenic genes (tumor necrosis factor alpha [TNF], interleukin (IL)-1beta [IL1B], IL-6 [IL6], and matrix metalloproteinase-1 [MMP1]), and the growth factor bone morphogenetic protein 6 (BMP6) were evaluated by reverse-transcription quantitative polymerase chain reaction (RT-qPCR) using pre-validated TaqMan® gene expression reagents (Applied Biosystems). GAPDH was used to normalize gene expression.
2.4 Calcium assay
Cells cultured for 12 days as described above were stained with Alizarin red.
2.5 Statistical evaluation
The qRT-PCR findings are presented as mean fold-change over the negative control calculated using the ΔΔCt method.
3. Results
In the following experiments, we examined the expression of osteoblast-associated developmental genes (SSP1, RUNX2, ALPL, and BSP), anti-osteogenic cytokines (TNF, IL6, IL1B, and MMP1), and the growth factor, BMP6, in human osteoblasts cultured for 12 days with PCA.
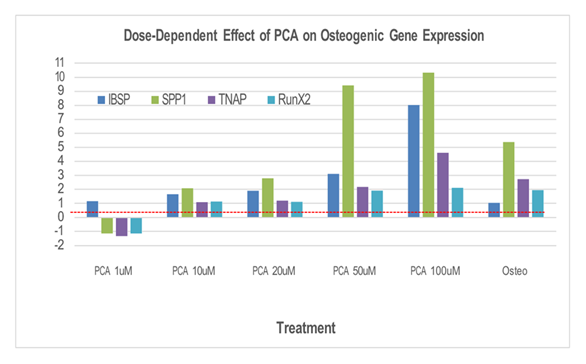
As shown in Figure 1, we observed a prominent dose-dependent increase in the expression of SSP1, ranging from 2 to greater than 10-fold over background levels in response to 10 and 100 µM PCA, respectively. Interestingly, peak SSP1 expression observed in response to PCA was higher than that detected in cultures maintained in commercial osteogenic medium (positive control). Interestingly, although no increase in IBSP expression was observed in cultures maintained in commercial osteogenic medium alone, similar dose-dependent increases in IBSP and ALPL expression were observed in the experimental cultures, peaking at 8-fold and 4 to 5-fold, respectively, over background levels in response to 100 µM PCA. By contrast, we observed only minimal increases in RUNX2 expression in response to PCA, reaching levels that largely matched those observed in the positive control cultures (i.e., 2-fold over background).
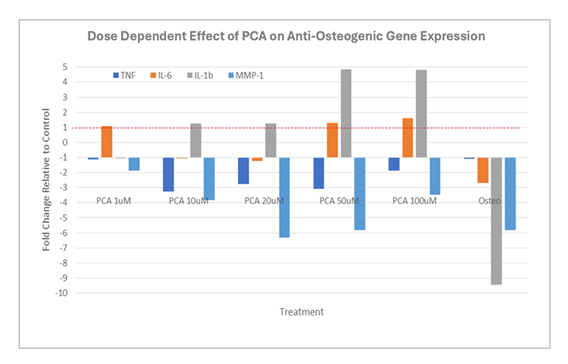
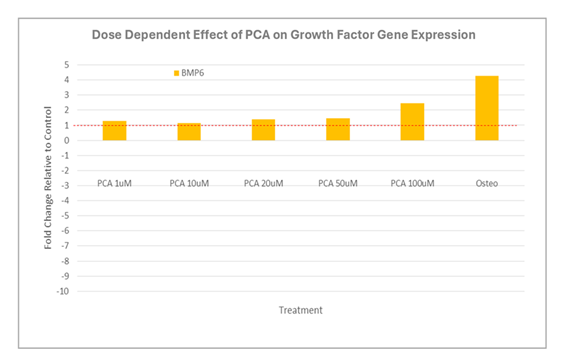
Expression of IBSP, SPP1, TNAP, and RUNX2 (fold over untreated [negative control]) in human osteoblasts after 12 days in culture with increasing concentrations of PCA or commercial osteogenic medium (positive control). Abbreviations: Osteo, commercial osteogenic medium; PCA, protocatechuic acid. We then examined the impact of increasing concentrations of PCA on the expression of key anti-osteogenic genes (Figure 2). As shown, we observed an overall down-regulation of osteogenic gene expression, notably, prominent decreases in IL1B and MMP1, in osteoblast cultures maintained in commercial osteogenic medium. Interestingly, although PCA also induced a net down-regulation of MMP1, a prominent increase in IL1B expression (to nearly 5-fold over background) was observed under otherwise identical conditions. Similarly, while osteogenic medium had little to no impact on TNF expression, moderate down-regulation was observed in response to PCA. By contrast, PCA-induced expression of BMP6 largely paralleled the response observed to commercial osteogenic medium (Figure 3).
Expression of TNF, IL6, IL1B, and MMP1 (fold over untreated [negative control]) in human osteoblasts after 12 days in culture with increasing concentrations of PCA or commercial osteogenic medium (positive control). Abbreviations: Osteo, commercial osteogenic medium; PCA, protocatechuic acid.
Expression of BMP6 (fold over untreated [negative control]) in human osteoblasts after 12 days in culture with increasing concentrations of PCA or commercial osteogenic medium (positive control). Abbreviations: Osteo, commercial osteogenic medium; PCA, protocatechuic acid. Although calcium deposition was detected in the positive control, this could not be detected in any of the cultures containing PCA (data not shown).
4. Discussion
Despite many recent advances and the availability of new treatment options, osteoporosis remains a serious problem in the elderly, with a significant impact on quality of life. Natural products may represent an untapped source of effective therapies for this disorder. Among these, PCA, a natural antioxidant and anti-inflammatory agent found in abundance in many fruits, vegetables, and grains, and common to the human diet might be developed and exploited as a treatment for this disease [11,13,19]. Several groups have already explored this possibility, including Wu et al. [14], who reported that PCA inhibited osteoclast development and promoted apoptosis, and Jang et al. [12] and Park et al. [15], who explored the impact of PCA in experimental models of bone loss in vivo. Others have explored the impact of PCA on the development and activation of osteoblasts both in vitro and in experimental animal model systems [16-18]. In this study, we examined the impact of PCA on gene expression in short-term cultures of isolated human osteoblasts. Overall, our results suggest that human osteoblasts responded positively to PCA with increased expression of SSP1, ALPL, and IBSP, but interestingly, not RUNX2. Osteopontin (encoded by SSP1) is a key differentiation marker of the osteoblast lineage and is expressed in response to osteotropic hormones as well as growth factors, mechanical stress, and inflammatory cytokines [20-24]. Osteopontin is synthesized and released by both osteoblasts and osteocytes into the extracellular matrix, where it promotes tissue mineralization and bone formation and thus may play a prominent role in osteoblast to osteocyte conversion [25,26]. Cultured human osteoblasts also expressed ALPL and IBSP in response to increasing concentrations of PCA. IBSP is the main structural protein of bone and is expressed primarily by osteoblasts [27]. By contrast, and in addition to its contributions to tissue mineralization, Nakamura et al. [28] reported that tissue nonspecific alkaline phosphatase also served as a critical regulator of osteoblast differentiation, potentially by controlling the expression of RUNX2.
Interestingly, PCA had no impact on the RUNX2 expression in osteoblast cultures. RUNX2 is a master regulator of osteogenesis, controlling transcription of many genes (including Spp1 and Ibsp, among others), that play critical roles in the conversion of pluripotent progenitors to mature osteoblasts. However, and despite its essential role in promoting early osteoblast development, RUNX2 is down-regulated to facilitate the development of mature osteoblasts [29] and is not among the major transcripts identified in differentiated osteocytes [30]. Our findings are consistent with these earlier observations. There is a substantial literature that considers the interactions of cytokines with and their synthesis by cells comprising the skeletal system (i.e., osteoimmunology) (reviewed in [31,32]). While there are several reports documenting the activities of several pro-osteoblastic mediators (e.g., interleukin-10 (IL-10), IL-11, IL-18, interferon-γ (IFN-γ) as well las cytokine synthesis (IL-1B, IL-6) in osteoblasts [33] as a group, most of these mediators (e.g., TNF-α, IL-1α, IL-4, IL-7, IL-12, IL-13, IL-23, IFN-α, and IFN-β, among others) suppress osteoblast differentiation and/or promote osteoclast activity [34-37]. Similarly, BMP6, a member of the transforming growth factor-beta (TGF-β) cytokine superfamily, also regulates osteoclast activity and promotes bone remodeling [38]. Our study has several limitations. First, although we reported the differential expression of several osteogenic and anti-osteogenic genes, we did not document protein expression or cell morphology. Likewise, although our findings suggest that PCA may have a pronounced impact on gene expression in human osteoblasts, the results will need to be validated in longer-term cultures and in vivo experimental models. These issues can be addressed in future studies.
5. Conclusion
Collectively, our findings on the impact of PCA on isolated human osteoblasts add to the growing literature focused on developing new drugs and alternative targets for osteoporosis therapy. Ultimately, PCA and/or an effective derivative may be available for use as an alternative therapeutic agent for patients diagnosed with osteoporosis and osteopenia, as well as other disorders of the skeletal system.
Acknowledgments
This study was performed on independent contract with the Institute for Biomedical Sciences, 3120 Biomedical Sciences Way, La Jolla, CA 92093 (Suppl. File 1).
Conflicts of Interest
This study was entirely funded by Pcabioscience, LLC of Las Vegas, NV. Dr. Johnson is the owner. Dr. Johnson holds US patent 10,143,670 December 4, 2018, regarding fracture healing. Dr. Johnson provided the hypothesis. The study was performed on an independent contract basis with Pcabioscience’s payment to the Institute for Biomedical Sciences, 3120 Biomedical Sciences Way, La Jolla, CA 92093. Drs. Chen and D’Lima were independent contractors functioning under work for hire status and who designed and executed this study without any financial interests. Drs. Chen and D’Lima have no conflict of interests.
References
- Arceo-Mendoza RM, Camacho PM. Postmenopausal osteoporosis: A Review of Latest Guidelines. Endocrinology and Metabolism Clinics of North America 50 (2021): 167-178.
- Muñoz M, Robinson K, Shibli-Rahhal A. Bone health and osteoporosis prevention and treatment. Clinical Obstetrics & Gynecology 63 (2020): 770-787.
- Armas LAG, Recker RR. Pathophysiology of osteoporosis. Endocrinology and Metabolism Clinics of North America 41 (2012): 475-486.
- Chen JS, Sambrook PN. Antiresorptive therapies for osteoporosis: a clinical overview. Nature Reviews Endocrinology 8 (2011): 81-91.
- Billington E, Aghajafari F, Skulsky E, et al. Bisphosphonates. BMJ (2024): e076898.
- Li H, Xiao Z, Quarles LD, et al. Osteoporosis: mechanism, molecular target and current status on drug development. Current Medicinal Chemistry 28 (2020): 1489-1507.
- Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Annals of the New York Academy of Sciences 1192 (2010): 437-443.
- Zhu S, Chen W, Masson A, et al. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discovery 10 (2024).
- Delgado-Calle J, Bellido T. The osteocyte as a signaling cell. Physiological Reviews 102 (2021): 379-410.
- Schaffler MB, Cheung W-Y, Majeska R, et al. Osteocytes: master orchestrators of bone. Calcified Tissue International 94 (2013): 5-24.
- Song J, He Y, Luo C, et al. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol Res 161 (2020): 105-109.
- Jang S-A, Song HS, Kwon JE, et al. Protocatechuic acid attenuates trabecular bone loss in ovariectomized mice. Oxidative Medicine and Cellular Longevity 29 (2018): 7280342.
- Qu Z, Zhao S, Zhang Y, et al. Natural compounds for bone remodeling: Targeting osteoblasts and relevant signaling pathways. Biomedicine & Pharmacotherapy 180 (2024): 1174-1190.
- Wu YX, Wu TY, Xu BB, et al. Protocatechuic acid inhibits osteoclast differentiation and stimulates apoptosis in mature osteoclasts. Biomedicine & Pharmacotherapy 82 (2016): 399-405.
- Park S, Kim J, Cheon Y, et al. Protocatechuic acid attenuates osteoclastogenesis by downregulating JNK/C-FOS/NFATC1 signaling and prevents inflammatory bone loss in mice. Phytotherapy Research 30 (2016): 604-612.
- Rivera-Piza A, An YJ, Kim DK, et al. Protocatechuic acid enhances osteogenesis, but inhibits adipogenesis in C3H10T1/2 and 3T3-L1 cells. Journal of Medicinal Food 20 (2017): 309-319.
- Xu G, Pei QY, Ju CG, et al. Detection on effect of different processed Cibotium barometz on osteoblasts by CCK-8. Zhongguo Zhong Yao Za Zhi 38 (2013): 4319-4323.
- Feng X, Feng W, Wu P, et al. Exploration the mechanism underlying protocatechuic acid in treating osteoporosis by HIF-1 pathway based on network pharmacology, molecular docking, molecular dynamics simulation and experimental verification. Journal of Functional Foods 122 (2024): 106-531.
- Liu RH. Potential synergy of phytochemicals in cancer Prevention: Mechanism of action. Journal of Nutrition 134 (2004): 3479S-3485S.
- Zhu S, Chen W, Masson A, et al. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discovery 10 (2024).
- Zohar R, Cheifetz S, McCulloch Ca, et al. Analysis of intracellular osteopontin as a marker of osteoblastic cell differentiation and mesenchymal cell migration. European Journal of Oral Sciences 106 (1998): 401-407.
- Mardiyantoro F, Chiba N, Seong C-H, et al. Two-sided function of osteopontin during osteoblast differentiation. The Journal of Biochemistry (2024).
- Sodek J, Chen J, Nagata T, et al. Regulation of osteopontin expression in osteoblasts. Annals of the New York Academy of Sciences 760 (1995): 223-241.
- Si J, Wang C, Zhang D, et al. Osteopontin in bone metabolism and bone diseases. Medical Science Monitor 26 (2020): e919159.
- Morinobu M, Ishijima M, Rittling SR, et al. Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. Journal of Bone and Mineral Research 18 (2003): 1706-1715.
- Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: How osteoblasts become osteocytes. Developmental Dynamics 235 (2005): 176-190.
- Kraenzlin ME, Seibel MJ. Measurement of biochemical markers of bone resorption. In: Elsevier eBooks (2006): 541-563.
- Nakamura T, Nakamura-Takahashi A, Kasahara M, et al. Tissue-nonspecific alkaline phosphatase promotes the osteogenic differentiation of osteoprogenitor cells. Biochemical and Biophysical Research Communications 524 (2020): 702-709.
- Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell and Tissue Research 339 (2009): 189-195.
- Youlten SE, Kemp JP, Logan JG, et al. Osteocyte transcriptome mapping identifies a molecular landscape controlling skeletal homeostasis and susceptibility to skeletal disease. Nature Communications 12 (2021).
- Cai L, Lv Y, Yan Q, et al. Cytokines: The links between bone and the immune system. Injury 55 (2023): 111-203.
- Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone–immune interactions in health and disease. Nature Reviews Immunology 19 (2019): 626-642.
- Han Y, You X, Xing W, et al. Paracrine and endocrine actions of bone—the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Research 6 (2018).
- Amarasekara DS, Kim S, Rho J. Regulation of osteoblast differentiation by cytokine networks. International Journal of Molecular Sciences 22 (2021): 28-51.
- Xu J, Yu L, Liu F, et al. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: a review. Frontiers in Immunology 14 (2023).
- Lacey DC, Simmons PJ, Graves SE, et al. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis and Cartilage 17 (2008): 735-742.
- Zhou P, Zheng T, Zhao B. Cytokine-mediated immunomodulation of osteoclastogenesis. Bone 164 (2022): 116-540.
- Kamiya N. The Role of BMPs in Bone Anabolism and their Potential Targets SOST and DKK1. Current Molecular Pharmacology 5 (2012): 153-163.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 