Multimodal Approaches for Pain Management and Improving Functional Outcomes Following Amputation
David Parvizi, Sugeeth Kandikattu, Ramtin Sahafi, Artin Allahverdian, Blake Han, Marcel P Fraix, Devendra K Agrawal*
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California 91766, USA
*Corresponding Author: Devendra K Agrawal, Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, California 91766, USA.
Received: 03 September 2025; Accepted: 10 September 2025; Published: 18 September 2025
Article Information
Citation:
David Parvizi, Sugeeth Kandikattu, Ramtin Sahafi, Artin Allahverdian, Blake Han, Marcel P Fraix, Devendra K Agrawal. Multimodal Approaches for Pain Management and Improving Functional Outcomes Following Amputation. Journal of Orthopedics and Sports Medicine. 7 (2025): 449-463.
View / Download Pdf Share at FacebookAbstract
In the United States, limb amputation affects around 2.3 million people, with projected numbers rising due to the higher levels of diabetes and peripheral vascular disease. Amputee patients not only encounter mobility loss but also residual limb pain, psychological stress, and disparities in access to healthcare. This article presents a critical review of the current literature on functional outcomes and pain management for patients who underwent limb amputation. In terms of pharmacological therapies, gabapentinoids and antidepressants provide minimal relief for post amputation pain, whereas surgical techniques including targeted muscle reinnervation and regenerative peripheral nerve interfaces have resulted in much larger improvements in decreasing neuroma-related and phantom limb pain. The non-pharmacological therapies, including mirror therapy, graded motor imagery, and virtual reality are some of the most effective interventions for reduction of phantom limb pain. Functional improvement can be optimized through microprocessor-controlled prosthetics, osseointegration, and early rehabilitation with an emphasis on resistance training, mobility, and psychological support. Newer techniques including neuromodulation and artificial intelligence-enhanced prosthetic control also play a part in rehabilitation; however, evidence is limited due to their novel introduction to the rehabilitation space. Overall, a multidisciplinary and individualized multimodal approach to rehabilitation remains the gold standard to improve pain control, quality of life, and functional restoration.
Keywords
<p>Amputation; Heterotopic ossification; Microprocessorcontrolled prosthesis; Mirror therapy; Neuromodulation; Phantom limb pain; Prosthetics; Regenerative peripheral nerve interface; Rehabilitation; Residual limb pain; Spinal cord stimulation; Targeted muscle reinnervation</p>
Article Details
1. Introduction
In the United States, as of 2024, there are an estimated 2.31 million Americans who are currently living with limb loss, with 90% of them being lower extremity amputations [1]. Each year there are about 150,000 to 185,000 amputations done in the United States. Most of these cases are usually due to non-traumatic causes such as peripheral artery disease and diabetes. The most recent data shows the prevalence of limb loss in the United States to be an estimated 1.6 million Americans living with amputations as of 2005, with the rates doubling by 2050 due to rising rates of diabetes and obesity [2]. Incidence of amputations had a decline throughout the early 2000s; however, a reversal of this trend continued from 2009 to 2015 as the number of young adults with diabetes had increased [3]. The rate of amputation is not evenly distributed and is skewed by race. Hispanics, African Americans, and individuals of lower socioeconomic status are among the group with the highest rates of amputations. African Americans in the United States have a 2-4-fold higher risk of lower extremity amputations compared with white patients. Hispanics also have an elevated rate of amputation with an odds ratio for a major amputation of 1.6 compared to white patients [4]. Moreover, African American and Hispanic patients are less likely to receive a limb-salvage intervention, such as revascularization, compared to white patients. They are also more likely to receive an above the knee amputation which are more highly associated with adverse outcomes and lower rates of prosthesis use [5]. These disparities are related to delayed presentation, lower rate of insurance coverage, structural inequities, and less access to multidisciplinary care [6].
Amputation results in significant functional and psychosocial consequences. These include loss of mobility, increased dependency, decreased quality of life, and higher rates of depression and mental health issues. The annual cost of amputations costs the United States 10 billion dollars per year, which reflects not only on the direct medical expenses for patients but also the economic toll it takes on our healthcare system. 5-year mortality after a major amputation approaches 60% which is comparable to many of the worst cancers [7]. Depression, anxiety, and social isolation are rated as the three most impactful clinical conditions that amputations have on psychological health. The American Heart Association published that the rates of major depressive disorder reach 30% as well as associated body image dysmorphia and post-traumatic stress symptoms [8]. The combination of these psychological symptoms leads to worsening mobility, lower prosthesis use, and diminished participation in social and recreational activities leading to a worsening of quality of life. Negative perceptions of amputees and lack of social support are the two strongest predictors of functional recovery and increased disability post-amputation. However, these factors can be targeted and modified with psychological and rehabilitative interventions [9].
Effective management of pain and restoration of function for patients who underwent limb amputation requires evidence-based, multimodal, and individualized interventions. The United States Department of Veterans Affairs and Department of Defense have laid out guidelines for clinical practice which emphasizes a standardized protocol for pain assessment using validated tools. This assessment includes pain characteristics and its impact on patient function, as well as recommending a multidisciplinary approach to pain management throughout all phases of rehabilitation post-amputation. The broad scope approach is to transition away from narcotic pharmacological interventions to the integration of physical, psychological, and mechanical modalities with frequent adjustments based on patient response and preferences [10]. Residual limb pain and phantom limb pain both remain relevant and challenging to tackle. Systematic reviews have shown there is no singular intervention that can act as a gold standard. Intervention would consist of peripheral nerve blocks, preoperative pain control, and pharmacologic agents such as ketamine are the three most used treatments, however, none of these interventions are considered to work superiorly to one another [11]. Non-pharmacologic therapies such as image-based procedures have shown the most evidence for phantom limb pain, however, the evidence across studies vary necessitating further research [12]. Two emerging treatments include peripheral nerve stimulation and targeted muscle reinnervation which have shown promise, yet current evidence is also limited and varied across studies [13]. The US Department of Veterans Affairs has outlined that functional restoration can be achieved most efficiently through open and closed chain kinetic exercises, progressive resistance, and early mobility training. Combining these together, improvements have been found in gait, strength, cardiovascular fitness, and daily activities [10]. Altogether, the current consensus supports a multimodal, individualized, and multidisciplinary approach to managing pain and restoring function in amputees. Ongoing research is needed to optimize standard of care protocols and address the gaps in our knowledge.
The purpose of this article is to integrate recent literature on interventions and therapeutic approaches for functional recovery and pain control in amputees and compare the effectiveness of surgical, prosthetic, rehabilitative, pharmacological, and psychological interventions.
2. Methods
The PubMed, Embase, and Cochrane databases were searched using the terms “amputation,” “rehabilitation,” “prosthesis,” “phantom limb pain,” “functional restoration,” and “interventions.” Any randomized controlled trial, cohort study, or systematic review in pediatric or adult populations published in English during the period of 2000-2025 were included. Titles and abstracts were screened for relevance by the authors, and full texts were reviewed for final inclusion. Case reports, abstracts, and the level of evidence were also noted based on the study design. Studies were categorized by intervention aimed at restoring function or managing pain (pharmacologic, surgical, prosthetic, rehabilitation, psychological). Primary outcomes were pain severity, functional recovery, and health-related quality of life.
3. Pathophysiology and Challenges
3.1 Phantom Limb Pain
Phantom limb pain (PLP) is a common occurrence observed in patients after amputation, with anywhere between 40-80% of amputees experiencing it, depending on population [14-16]. Large cross-sectional and survey studies across all populations confirm that most of the amputees experience PLP at some point, with lifetime prevalence estimates ranging from 76% to 87% [17,18]. PLP arises from complex interactions between peripheral and central nervous system mechanisms following amputation. Peripheral mechanisms include ectopic activity in injured nerve fibers and dorsal root ganglia (DRG), neuroma formation, and local inflammatory changes. Ectopic activity from axotomized primary afferent neurons in the DRG can generate abnormal sensory input, which has been shown to drive PLP; targeted anesthetic blockade of the DRG is known to rapidly extinguish PLP, supporting a peripheral origin in various cases [19-21]. In addition, peripheral sensitization involves upregulation of sodium channels and inflammatory mediators, contributing to spontaneous pain and hyperexcitability [22,23].
Central mechanisms are characterized by maladaptive plasticity within the spinal cord and brain. At the spinal level, loss of normal afferent input due to the loss of a limb leads to dorsal horn sensitization and NMDA receptor hyperactivation, facilitating central sensitization and amplifying pain signals [20,21]. In the brain, amputation induces reorganization of the primary somatosensory cortex, with adjacent cortical areas invading the deafferented territory. This cortical remapping is strongly associated with PLP severity and persistence, as demonstrated by neuroimaging studies [16,22-24]. The maintenance of phantom limb representation and altered body schema further contribute to the experience of PLP [23-25]. While peripheral input does modulate PLP, it is insufficient to cause it in isolation, suggesting that delicate interplay between peripheral and central mechanisms in modulating PLP [26-28].
3.2 Residual Limb Pain
Residual limb pain (RLP) is another pain-related mechanism frequently experienced by patients. Some may confuse residual limb pain with phantom limb pain due to overlapping clinical presentations and subjective descriptions by patients. However, they are distinct entities with different pathophysiological mechanisms and diagnostic features. While PLP is defined as pain perceived in the missing (amputated) limb, residual limb pain is localized to the remaining stump or residual limb [16,28]. Differentiation relies on careful history: phantom limb pain is typically described as pain in the absent limb, often with neuropathic qualities (burning, shooting), while residual limb pain is felt in the physical stump and may be provoked by palpation or prosthetic use [16,28,29].
Residual limb pain is a heterogeneous syndrome with multiple causes and pathophysiological mechanisms behind it. The pathophysiology of residual limb pain is multifactorial. Neuroma-related pain, for example, arises from abnormal axonal sprouting and ectopic neural activity at the nerve stump, leading to peripheral sensitization [30-32]. Mechanical factors (prosthetic fit, bone spurs) cause nociceptive pain via tissue injury or inflammation [29,33]. Ischemic pain results from inadequate perfusion and tissue hypoxia. Central sensitization may also contribute, especially in chronic cases [28,34].
Neuromas are one common type of residual limb pain after amputation. They are defined as disorganized growths of transected nerve endings, presenting as focal, often severe pain at the nerve end, sometimes with a positive Tinel sign [30,32,35]. Neuromas primarily form when transected peripheral nerves attempt to regenerate but lack a distal target, resulting in a disorganized proliferation of axons, Schwann cells, and connective tissue at the nerve end [36-39].
The risk for neuroma increases the more proximal the amputation level is and with younger age, while diabetes and hypothyroidism appear protective, possibly due to impaired nerve regeneration and sensation [39]. Pathophysiologically, neuroma pain is driven by ectopic discharges from regenerating axons, upregulation of sodium channels, neuroinflammatory mediators, and local mechanical sensitivity [27,28]. This leads to peripheral sensitization and, in more severe or chronic cases, may evolve to central sensitization.
Ischemia is another complication after amputation that causes debilitating residual limb pain. It is characterized by deep, aching pain due to poor vascular supply, especially in patients with vascular disease [40]. Ischemia is common, particularly in patients with underlying peripheral vascular disease or compromised local blood flow. Vascular etiologies are considered especially relevant in lower extremity amputees, where vascular disease is a common indication for amputation and a risk factor for post-amputation pain and ischemic changes [40].
Pathophysiologically, ischemia results from inadequate perfusion of the residual limb, leading to tissue hypoxia, accumulation of metabolic byproducts, and activation of nociceptive pathways [40,41]. This mechanism is distinct from neuroma-related pain, which is focal and neuropathic, and from pain due to poor prosthetic socket fit, which is mechanical and positional. Infection, another cause of residual limb pain, is characterized by inflammatory signs and may coexist with ischemia, further complicating diagnosis [41].
Additional causes of pain after amputation include infection, heterotopic ossification, and bone spurs [29,33]. Infection in the residual limb typically manifests as cellulitis, abscess, or osteomyelitis. The mechanism behind this is bacterial colonization of the surgical site, often facilitated by poor wound healing, vascular compromise, or prosthetic-related skin breakdown. Infection triggers a local inflammatory response, leading to tissue edema, increased pressure, and activation of nociceptors, resulting in pain. Chronic infection can progress to osteomyelitis, where bacterial invasion of bone induces persistent inflammation and bone destruction, further amplifying pain and complicating prosthetic use [33,41].
Heterotopic ossification is the abnormal formation of bone within soft tissues, most commonly occurring after trauma, surgery, or burns. In amputees, heterotopic ossification is driven by a dysregulated inflammatory response, with sustained release of osteoinductive cytokines (e.g., BMPs, TNF-α) from macrophages and other immune cells. These cytokines induce mesenchymal stem cells and fibro-adipogenic progenitors to differentiate into osteoblasts, resulting in the deposition of ectopic bone. Mechanical loading, hypoxia, and neurogenic factors further promote heterotopic ossification. Heterotopic ossification can cause nerve impingement, restrict soft tissue mobility, and hinder proper prosthetic fitting, leading to pain and functional impairment [42-47].
Osteophytes, also known as bone spurs, are a similar abnormally bony manifestation due to intrinsic issues after amputation. Osteophytes develop at the cut ends of bone following amputation due to abnormal bone remodeling and mechanical stress. They are characterized by focal outgrowths of bone that can irritate surrounding soft tissues, compress nerves, and create pressure points within the prosthetic socket. This leads to localized pain, especially during weight-bearing or prosthesis use [33,41].
In addition to intrinsic issues causing residual pain after amputation, there are various external and/or mechanical factors that can compromise comfort and healing after amputation. Poor prosthetic fit is one example, and it is the leading cause of post-amputation functional pain [33,48]. The mechanism behind suboptimal prosthetic fitting is more mechanical than pathophysiological: ill-fitting sockets can cause abnormal pressure distribution, shear forces, and friction at the stump-socket interface, leading to skin breakdown, soft tissue injury, edema, and chronic pain, which is typically positional and improves with prosthetic adjustment [29,30,32,33,35]. Inadequate prosthetic alignment or suspension may cause abnormal gait and additional musculoskeletal pain, further reducing prosthesis use and quality of life [49,50]. Additional causes of pain related to poor prosthetic fit include volume fluctuations of the residual limb, changes in body weight, and socket material properties [33,48].
Other relevant external factors include excessive prosthesis use, poor limb hygiene, and environmental conditions (e.g., heat, humidity) that exacerbate skin irritation. Chronic mechanical irritation can also promote local inflammation, contributing to persistent pain and potentially sensitizing peripheral nociceptors [48,50]. Improper alignment or suspension of the prosthesis may lead to gait issues and continued musculoskeletal pain, further reducing compliance with the prosthesis and quality of life [48,49].
3.3 Challenges and Knowledge Gaps in the Pathophysiology of Limb Pain
There are still various knowledge gaps regarding the pathophysiology and causes of PLP. While central mechanisms such as maladaptive plasticity and cortical reorganization have understood well in the literature, the relative contributions and interactions of peripheral, spinal, and supraspinal processes are not fully understood [20,22,23,27,51-53]. The medical literature highlights that functional neuroimaging and clinical studies support central reorganization as a key driver, but the failure of peripherally focused treatments to consistently resolve PLP suggests that central mechanisms may predominate in chronic cases [20,22,52]. Moreover, the role of aberrant activity in residual muscles and its relationship to PLP, as demonstrated by recent electromyography studies, is a novel area requiring further exploration [53]. Additionally, the impact of proprioceptive memory, body representation mismatch, and contextual factors such as prosthesis use and compensatory behavior on PLP development and maintenance remains poorly characterized [23,25,51]. Outstanding questions include how peripheral and central changes interact over time, whether there are critical windows for intervention, and why some patients develop severe PLP while others do not, despite similar injuries [21,27].
Moreover, there is a lack of standardized biomarkers and predictive models for PLP risk, severity, and treatment response [27,28,34]. The impact of genetic, psychosocial, and environmental factors is recognized but not well quantified, and optimal timing of preventive intervention modalities are unknown [27,28]. The heterogeneity of patient experiences and the absence of mechanism-based therapies highlight the demand for longitudinal, multimodal studies using advanced neuroimaging, electrophysiology, and molecular profiling to clarify the dynamic interplay of peripheral and central changes [22,23,53]. Future research should also address the efficacy of emerging interventions such as brain-computer interfaces and virtual reality in modulating cortical representations and alleviating PLP [23].
Regarding RLP, knowledge gaps hover around the diverse etiologies and their overlapping clinical presentations. The mechanisms by which neuromas, ischemia, poor prosthetic fit, infection, heterotopic ossification, and bone spurs interact to produce chronic pain are poorly defined [28,34]. The contribution of peripheral sensitization, local inflammation, and mechanical factors to persistent RLP, and how these processes may trigger or exacerbate central sensitization, requiring additional investigation [20,28]. The role of pre-amputation pain, amputation site, co-morbidities, and prior history of chronic pain as risk factors for RLP is established but underlying neurobiological pathways require further investigation [34].
There is limited understanding of how external factors such as prosthetic design, limb hygiene, and environmental conditions modulate RLP severity and chronicity [28]. The lack of standardized diagnostic criteria and objective assessment tools for differentiating RLP subtypes impedes targeted management [30]. Future research should focus on developing more robust clinical algorithms, integrating imaging and electrophysiological modalities, and elucidating the molecular and biomechanical pathways linking external factors to persistent pain. Multidisciplinary studies are necessary to clarify the interactions between peripheral pathology, mechanical stress, and central sensitization, and to optimize individualized prevention and treatment strategies [28,34].
4. Interventions for Pain Management
4.1 Pharmacologic Therapies
Treatment for amputations can vary depending on the degree of injury as well as the stage of the injury. Common pharmacological treatments for amputations are non-steroidal anti-inflammatory drugs (NSAIDs), opioids, gabapentinoids, and antidepressants [54].
Non-steroidal anti-inflammatory drugs function by inhibiting cyclooxygenase (both COX-1 and COX-2), which decreases the level of prostaglandins which is beneficial for mitigating inflammation. NSAIDs are best used for post operative pain but are not ideal for neuropathic pain such as phantom limb pain [55]. Limitations include the typical side effects of NSAIDs which are gastrointestinal impairment, cardiovascular risk, and renal impairment [56].
Opioids function by binding to m-opioid receptors in both the brain and the spinal cord to increase pain threshold, alter pain perception, and inhibit pain transmission. This treatment is most beneficial for intense acute-post surgical amputation pain and can also be used for refractory neuropathic pain [57]. Limitations for this treatment are opioid tolerance, addiction, and respiratory depression [58].
Gabapentoids function by binding to the α2δ subunit of presynaptic voltage-gated calcium channels in the CNS which assist in reducing excitatory neurotransmitter release and decreasing central sensitization and neuropathic firing [59]. This treatment is the primary method for treating neuropathic pain and for phantom limb pain. Limitations for these will be sedation, dizziness, and ataxia [60].
Tricyclic antidepressants (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) function by inhibiting serotonin and norepinephrine reuptake which can enhance the descending pathways from the brain stem to the spinal cord decreasing the neuropathic pain. For amputations, this treatment is beneficial for phantom limb pain as well as neuropathic residual pain [61]. These also benefit the patients by improving sleep and help treat mood disorders that are concurrent with chronic pain syndromes. Limitations include anticholinergic effects, arrhythmias, nausea, and insomnia [62].
N-Methyl-D-aspartate (NDMA) receptor antagonists also play a role in management of post amputation pain. Low dose ketamine has been shown to have a potentially useful role in patients with phantom limb pain (PLP) and complex regional pain syndrome [63]. Ketamine provides analgesic properties by inhibition of central sensitization mechanisms mediated by NMDA receptors, which are involved in conditions of chronic neuropathic pain followed by amputation [64]. Low-dose intravenous ketamine can be used to treat acute pain and has modestly demonstrated short-term reduction in pain intensity with the most pronounced effects being in the immediate postoperative period and in pain resistant to conventional methods of pain control. The American College of surgeons has recommended ketamine as a perioperative supplemental add-on to pain regimen, rather than being the primary method of treatment. However, the evidence of long-term pain management with use of ketamine in post-amputation patients remains a question as the literature for its support is weak [65].
Adverse effects of NMDA antagonists, especially ketamine, include dysphoria, hallucinations, dissociation, sedation, sensory disturbances, and a drug “high”. Tolerance is also an issue with repeated administration and high-dose regiments which can lead to serious risks and are not currently recommended [66]. Moreover, caution should be taken with patients who suffer from any form of cardiovascular disease as NMDA antagonists can result in a transient increase in blood pressure. Other NDMA antagonists such as memantine, dextromethorphan, amantadine, and magnesium have not shown any consistent improvement and can lead to serious neuropsychiatric or gastrointestinal adverse events [67]. In conclusion, low dose ketamine may provide some acute pain relief in the perioperative period, however, its limited long-term use and large side effect profile makes it an adjunct for select patients rather than a standard of care pain therapy. Its impact on long-term function remains unclear and more research must be done to fully determine its uses.
Utilizing these different medications together offer the best possible treatment for amputation related pain. For acute amputation pain and residual limb pain, both NSAIDs and opioids are optimal for mitigating the pain. For neuropathic pain, gabapentin and TCAs/SNRIs are used for phantom limb pain as well as other non-pharmacological pain treatments that will be discussed later.
4.2 Interventional and Surgical Approaches
Aside from pharmacological treatments, there are innovative and effective interventional and surgical approaches to treat amputation related pain.
Targeted muscle reinnervation is done by surgically rerouting transected peripheral nerves into nearby motor nerves of residual muscles. This is done to provide a specific target for regenerating axons to reduce the aberrant production of and formation of neuroma. It also creates motor endplates to generate more organized signaling and decrease as well as enhance prosthetic control which can amplify electromagnetic signals [68]. This treatment can be very beneficial for residual limb pain as well as for phantom limb pain. Limitations for this treatment is that it is surgically advanced which increases difficulty of application, not 100% effective, and prone to having break-through pain [69].
Regenerative peripheral nerve interface is done by implanting transected nerve endings into free muscle grafts. By implanting functional nerve endings into muscle grafts, it benefits by regenerating axons and preventing neuroma formation. This will organize nerve signals into the muscle which can reduce pain and even enhance prosthetic control. This has been a popular treatment for both neuroma related residual limb pain and phantom limb pain in amputees [70]. Some limitations for this treatment modality is that it depends on the viability of the muscle grafts and that it has not been properly studied due it being a novel technique that is not widely utilized [71].
Neuroma excision is a rudimentary treatment compared to the others in this paper. This treatment is utilized by surgical excision of painful neuromas. The key issue with this treatment is that these neuromas tend to regenerate and exacerbate the problem in the long run [72]. The limitations of this modality include surgical risk and the reformation of neuromas which can lead to poor treatment for phantom limb pain and other neuropathic pain [73]. This treatment is most beneficial when combined with targeted muscle reinnervation and/or regenerative peripheral nerve interface. This procedure involves burying the nerve into the muscle and has been shown to produce better results [74].
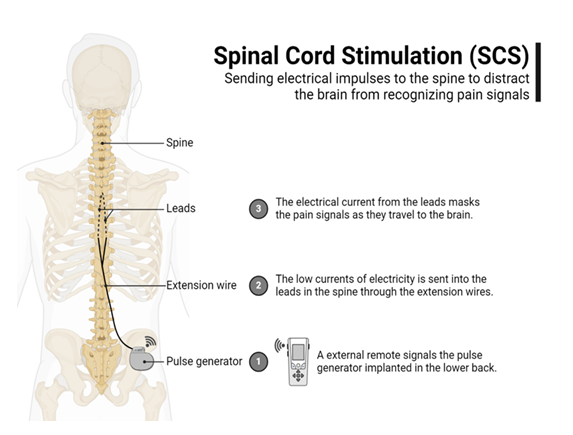
Spinal cord stimulation (SCS) is an advanced technique where electrodes are placed in the epidural space to transmit electrical signals to the dorsal columns of the spinal cord (Figure 1). This will activate the inhibitory interneurons which modulate the ascending pain signals which can mitigate/alter cortical pain perception. This is commonly used for chronic, refractory phantom limb pain and as well as for other neuropathic pain (e.g., stump pain) [75]. Some limitations for this modality include the surgical risk of implantation, infection, and lead migration [76].
Like the SCS, the dorsal root ganglion stimulation also used electrodes to provide focal modulation of pain signals at the dorsal root ganglion which serves as a collection of sensory neuron cell bodies at the spinal nerve roots. The benefit of this treatment is that it is more specific/precise than the SCS and can also be used for neuropathic pain and phantom limb pain [77]. This treatment carries the same risks as a SCS, and it is usually utilized when a SCS fails to improve the pain or is too localized.
Targeted muscle reinnervation involves surgically transferring transected peripheral nerves to motor nerve branches of nearby muscles. By doing this, physiologic targets for nerve regeneration are provided and can prevent the formation of a painful neuroma and disrupt aberrant nerve signaling, which are both indicated in PLP. This technique works by disrupting the constant cycle of ectopic neural activity and central sensitization that is found in PLP [78].
4.3 Non-Pharmacologic and Adjunctive Therapies
In many instances, non-pharmacologic and adjunctive treatments are used in combination with pharmacological and surgical intervention. There are many non-pharmacologic treatments that have been proven to show improvement in an amputee's neuropathic pain.
Physical therapies including desensitization techniques such as massage, transcutaneous electrical nerve stimulation (TENS), and compression garments all play a supplemental role in restoring function and pain management for post-amputation patients. However, evidence for their use is limited and outcomes vary depending on the patient.
Massage therapy can be used for short-term pain relief and edema reduction but there is no definitive evidence for functional improvement or opioid reduction in amputees. The American Academy of Orthopedic Surgeons states that massage therapy can be beneficial for pain alleviation, but studies have not shown a benefit in functional ability due to small sample sizes and limited studies [79].
Transcutaneous electrical nerve stimulation is done by placing electrodes on the stump of the amputation to deliver electrical stimulation which has been shown to activate alpha-beta fibers which closes the gates of pain transmission. This is a great treatment, but it is temporary and needs to be utilized frequently. Studies have shown that TENS can reduce pain intensity at rest and during movement for patients with PLP and stump pain. Moreover, TENS has also shown promise to facilitate perceptual embodiment of prosthetic limbs [80]. However, like massage therapy, systematic reviews do not show any high-quality randomized controlled trials with definitive evidence to support its effectiveness in post-amputation pain. TENS is a safe and economical adjunct to pain management for these patients and is recommended, however, its evidence is still unclear [81]. Compression garments can also serve in reducing edema and aid in desensitization/normalization of sensation in the residual limb, particularly in complex regional pain syndrome. Together, these physical therapies and desensitization techniques discussed can provide some symptomatic relief and support rehabilitation as an adjunct to conventional therapies. However, the lack of high-quality evidence and randomized controlled trials limit our understanding of its effectiveness.
Mirror therapy is done by having the patient place a mirror to reflect their existing limb, so it appears to the brain that they have both of their limbs. While visualizing this, the patient performs movements on the intact limb while watching the reflection which creates an illusion of the amputated limb moving/functioning normally. This is beneficial because it helps retain the cortical maps and neuronal connections for the amputated limb and reduces aberrant pain signals. Graded motor imagery goes hand in hand with mirror therapy where it takes a stepwise approach to treating the neuropathic pain. It starts with laterality recognition where the patient is to identify the left and right limbs, next is to visualize moving of the missing limb, and lastly is to use mirror therapy to complete the cortical mapping to mitigate/redirect the pain signals. This has been shown to greatly help neuropathic pain in a non-invasive and in-expensive way. Some limitations here are requirement for patient participation, early utilization of therapy, and maintenance of patient emotion to limit emotional triggers.
Virtual reality (VR) therapy and immersive rehabilitation is a very new treatment that has been utilized recently due to the advancements in technology. This functions with a similar pathology as mirror therapy but in a more advanced and immersive manner. Using VR headsets and computer-generated environments to stimulate the presence and movement of missing limbs. It utilized the headset to engage in visual, auditory, and proprioceptive feedback to restore the body representation and reduce cortical matching [82]. This has been shown to greatly help neuropathic pain more so than mirror therapy, but some limitations include being very expensive and need for access to technology [83].
Certain treatments that have not shown complete efficiency and efficacy but are still utilized for patients that do not trust or have access to more advanced modalities. These are not trusted treatments, but many patients have stated that these modalities have been beneficial for them. Acupuncture is known to modulate endorphin release and alter central pain processing, but it has been shown to produce variable results and is highly patient dependent [84]. Transcutaneous electrical nerve stimulation is done by placing electrodes on the stump of the amputation to deliver electrical stimulation which has been shown to activate alpha-beta fibers which closes the gates of pain transmission [85]. This is a great treatment, but it is temporary and needs to be utilized frequently. Lastly, desensitization techniques are where gradual exposure is used on the stump to various sensations such as textures, messages, and vibrations [86]. This can be useful for reducing residual limb pain and phantom limb pain, but limitations include requiring daily practice as well as being ineffective in some patients as well.
Lastly, if all else fails or if patients need additional management, psychological therapies are utilized to mitigate the pain that patients are experiencing. Cognitive behavioral therapy is the most common/effective treatment where psychiatrists help reframe the patient’s perception of pain and improve coping strategies [87]. Mindfulness and mediation techniques are beneficial for reducing attention to the pain and improving emotional regulation to avoid triggers and to decrease sympathetic arousal [88]. Hypnosis can also be used to modulate pain perception, but this is only used in very select instances and with specific patients [89]. Overall, all these psychological treatments are used for chronic neuropathic pain, phantom limb pain, and for associated comorbidities such as anxiety, PTSD, and sleep issues [90]. These treatments are best used in a multimodal rehabilitation program where patients are being treated with an array of treatments that are most beneficial to them, but some limitations for these include active patient participation, delayed effects, and patient resistance to psychological approaches [91], improving functional capability. Adverse effects are rare and limited to transient emotional discomfort or frustration with therapy [92].
Mindfulness and mediation techniques are beneficial for reducing attention to the pain and improving emotional regulation to avoid triggers and to decrease sympathetic arousal. Hypnosis can also be used to modulate pain perception, but this is only used in very select instances and with specific patients. Overall, all these psychological treatments are used for chronic neuropathic pain, phantom limb pain, and for associated comorbidities such as anxiety, post-traumatic stress disorder (PTSD), and sleep issues. These treatments are best used in a multimodal rehabilitation program where patients are being treated with an array of treatments that are most beneficial to them, but some limitations for these include active patient participation, delayed effects, and patient resistance to psychological approaches.
4.4 Interventional for functional restoration
Modern technological advancements for prosthetic devices and functional restoration procedures post-amputation have come a long way and now include devices such as microprocessor-controlled ankle and knee units, powered prosthetic joints, and sensory feedback systems. Microprocessor-controlled prosthetic knees (MPKs) and microprocessor-controlled prosthetic ankles (MPAs) contain accelerometers, gyroscopes, and torque sensors that are linked to a microprocessor which continuously monitors gait dynamics in real time. These have been shown to improve gait symmetry, reduce falls, and decrease energy expenditure, which all contribute to poor outcomes post-amputation. By controlling gait dynamics, MPKs and MPAs both reduce the burden of high compensation of the contralateral limb which reduces musculoskeletal pain and joint degeneration [93]. Clinical trials have shown that MPKs and MPAs have consistently led to improved quality of life and patient satisfaction with additional cost-effectiveness by reducing fall-related injuries and further hospitalizations [94].
Sensory feedback systems are now able to provide instant proprioception and tactile sensation through nerve stimulation leading to improved mobility and improved embodiment of the prostheses which has been shown to reduce functional and psychosocial stressors [95]. Moreover, surgical interventions now have a place in functional restoration. Innovations such as targeted muscle reinnervation and osseointegration have increasingly become more utilized to augment residual limb shape for prosthetic control and reduce neuropathic and phantom limb pain. These innovations have shown significant improvements in quality of life and enhanced prosthetic use [96]. Although these advancements have shown significant improvements in quality of life and improved outcomes for prosthetic joint replacement, African Americans and Hispanic patients are much more likely to be uninsured and have obstacles to functional rehabilitations leading to poorer outcomes and decreased quality of life [97].
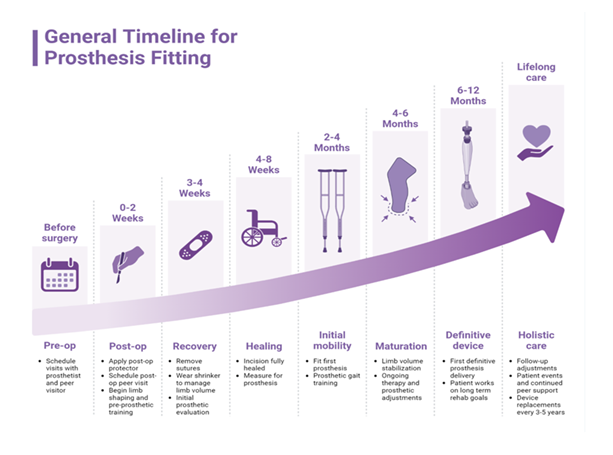
Figure 2: This image outlines a general timeline for prosthesis fitting, starting from pre-surgery preparations to lifelong care. It shows a progressive journey through stages such as post-op care, healing, initial mobility, and prosthetic maturation, ultimately leading to the fitting of a definitive device and ongoing support.
Suggested rehabilitation protocols in the United States for amputee patients place an emphasis on early mobilization and systematic pre-prosthetic training to address the high level of functional and psychosocial impact amputation has on its patients (Figure 2). This strategy also reduces the burden of post-amputation pain and optimizes outcomes through newer prosthetic technologies [98]. The US Department of Defense and Veterans Affairs both emphasize the need for early mobility training following amputation. Exercises such as early weight-bearing and gait re-education promote independence, strength, bone density, and cardiovascular conditioning. Pre-prosthetic rehabilitation aims to strengthen the residual limb by preventing edema and contracture (Figure 2). This can be done through kinetic chain and progressive resistance training which also have a positive impact in allowing patients to more easily participate in activities of daily living without the prosthesis to optimize confidence and reintegration into real-world environments. Chronic pain experienced post-amputation has a detrimental effect on psychosocial adjustment and functional ability. Patient-specific, multimodal pain management with incorporation of non-narcotic pharmacologic therapies have also proven to improve functionality and psychological stress. The American Heart Association has recommended the inclusion of mental and behavioral health interventions such as cognitive behavioral therapy and psychoeducation to treat depression, anxiety, and negative illness perception which all play a role in modifiable risk factors for poor rehabilitation outcomes [99]. As a result, multidisciplinary rehabilitation including physical, occupational, and behavioral health are endorsed to improve psychological and functional outcomes post-amputation.
4.5 Outcomes and Evidence Synthesis
The primary target of interventions for the patients with amputations is the management and reduction of pain. Non-pharmacologic interventions have been well studied, and of these, mirror therapy is the most supported and effective for the management of PLP. In a recent network meta-analysis, mirror therapy was determined to be the single most effective intervention for PLP and was also shown to provide a superior benefit when added to other therapies [100]. In a similar randomized controlled trial, the combination of phantom exercises and mirror therapy significantly reduced pain scores and improved the bodily pain domain of the SF-36 questionnaire, but other quality of life parameters did not change significantly [101]. In contrast, pharmaceutical interventions such as amitriptyline, duloxetine, and pregabalin have not demonstrated any significant benefit or superiority to placebo in several randomized controlled trials [66].
The functional outcomes that can be considered post-amputation are mobility and independence, independence in activities of daily living, quality of life, and return to work. There is moderate evidence supporting exercise-based rehabilitation as an effective intervention for the improvement of functional performance, although direct evidence is limited for the mobility and outcomes in the activities of daily living [102]. The return to work is another significant measure of outcomes for the patients with lower limb amputation. In a long-term study of veterans with lower limb amputation, around 64% of male patients were employed within 2 years of the amputation event. Strong predictors for a positive employment outcome included younger age at amputation, being married, and adherence to prosthesis wearing; however, work status was not associated with overall quality of life or functional status [103].
The most effective intervention for pain reduction remains mirror therapy and other image-based therapies. The most effective for improving general functionality was not as clear, but likely to be multimodal, as well. The least effective appeared to be the pharmaceuticals as monotherapy. Newer modalities such as graded motor imagery, virtual reality, and peripheral nerve stimulation show the most promise but have been studied in only one or two randomized controlled trials [81]. It is important to note that there are significant limitations in the available literature. Most randomized controlled trials suffer from small sample sizes, such as the 24-participant trial of phantom exercises [81,103], which do not provide sufficient statistical power and can lead to erroneous conclusions. Heterogeneity of study design, patient characteristics, outcomes, and even intervention itself are significant factors that reduce the comparability between studies. Most interventions are followed up over weeks to months but rarely over years, and long-term effectiveness and recurrence of pain are not clear [81,103]. These significant limitations should be addressed with large multicenter trials and standardized outcome reporting for future work in amputee rehabilitation.
5. Future Directions
It is likely that neuromodulation and advanced prosthetic technologies will be at the forefront of these new directions. Enhanced neuromodulation techniques and brain–computer interfaces (BCIs) hold great promise for improving amputee motor control and potentially restoring sensory function. Targeted muscle reinnervation and osseointegration are two advanced surgical techniques that reroute residual nerves to remaining muscles. By providing new targets for residual nerves, Targeted muscle reinnervation allows for more intuitive control of prosthetic limbs and can improve sensory feedback. Non-invasive neuromodulation techniques like transcranial magnetic stimulation are also being explored as means to modulate cortical plasticity, reduce phantom limb pain, and improve motor recovery in amputees [104]. Personalized prosthetics with integrated sensory feedback are likely to become a focus as well. Myoelectric prostheses, which respond to electrical activity from residual muscles, have the potential to offer more precise, user-responsive control. By incorporating feedback mechanisms (such as tactile, pressure or thermal cues), these prostheses can provide sensory information about the user’s environment or interactions, enhancing the embodiment of the prosthesis and its functional use [105]. These advances in prosthetic technology not only aim to restore mobility but also to improve overall quality of life for individuals with limb loss.
Artificial intelligence and robotics are also areas where we expect to see more developments in rehabilitation approaches in the future. AI algorithms have the potential to adapt to an individual’s unique movement patterns and adjust the prosthetic response in real-time. Robotic exoskeletons and robotic-assisted devices can provide repetitive, task-specific training to promote faster functional recovery and adaptation [106]. These tools show promise in improving independence, gait function, and upper- and lower-limb functional outcomes in amputees.
High-quality randomized controlled trials and standardized outcome measures remain another area of need in the field. Many studies in amputee rehabilitation are limited by small sample sizes, heterogeneous participant populations, non-standardized protocols, and short-term follow-up. The lack of uniformity in measurement makes it challenging to compare outcomes across studies or generalize findings to the larger population. Standardized and validated outcome measures of mobility, prosthesis use, activities of daily living, and quality of life are needed to accurately assess the efficacy of new interventions and direct future research [107].
Taken together, the advances in neuromodulation, advanced prosthetic technologies, artificial intelligence-enabled personalized support, and high-quality randomized controlled trials represent promising future directions for amputee rehabilitation. However, these areas will require close interdisciplinary collaboration between biomedical engineers, clinicians, and researchers. We hope that the critical discussion in this article is informative and would advance the conversations while spurring further innovation and discovery.
6. Conclusion
In summary, management of pain and return of function in amputee patients’ needs to be individualized, evidence-driven, and multidisciplinary. Amongst the various treatments discussed throughout this paper, multimodal treatments of phantom limb pain—integrating pharmacologic agents, regional anesthetic interventions, and particularly non-pharmacologic interventions such as mirror therapy and graduated motor imagery—have had the most robust evidence to suppress phantom limb pain. In regard to functionality, microprocessor-controlled prosthetic knees and ankles, targeted muscle reinnervation, peripheral nerve interfaces that regenerate, and exercise-driven rehabilitation have had the greatest benefit to enhance mobility, independence, and quality of life. However, there is no one size fits all treatment. The most beneficial rehabilitation is achieved by individualizing treatment to manage the patient's specific medical, psychosocial, and functional needs by integrated multidisciplinary care. Despite encouraging developments in prosthetic devices, neuromodulation, and rehabilitative interventions, existing literature is not sufficient to conclude how effective these modalities are, due to low numbers of patients, heterogeneity of studies, and short-term follow-up times. Further well-designed randomized controlled studies are required to enhance evidence, clarify protocols, and improve equitable access to these interventions. Ultimately, improvement of the lives of amputee patients will rely upon integration of innovative technologies, rehabilitative treatment centered around the patient and continued collaborative models of care.
7. Key Points
- • Greater than 2.3 million people in the United States live with limb loss with majority of them being lower extremity amputations
- • Risk for amputations and negative outcomes are worse for African American, Hispanic, and lower socio-economic status patients due to inequalities in access for limb-salvage procedures and rehabilitation programs
- • Most pain experiences post-amputation is phantom limb pain, about 40-80% of amputees, while residual limb pain including neuroma, ischemia, and prosthetic issues are also highly prevalent, both contribute to diminished quality of life
- • Phantom limb pain occurs due to peripheral and central mechanisms which underlies the necessity for multimodal rehabilitation
- • Medicants including gabapentinoids, tricyclic antidepressants, and ketamine provide minimal and variable relief, opioids are reserved for acute causes of pain but carry significant risk factors
- • Surgical/interventional approaches include targeted muscle reinnervation and regenerative peripheral nerve interfaces which have limited evidence but show strong promise for improving prosthetic control and pain management
- • Nonpharmacological therapies include mirror therapy, graded motor imagery, and virtual reality which are low cost, safe, and demonstrate the most consistent benefit for phantom limb pain compared to traditional pharmacological modalities
- • Functional restoration can be achieved with microprocessor-controlled prostheses, osseointegration, and structural rehabilitation to improve gait, reduce falls, and enhance independent living
- • Cognitive behavioral therapy, mindfulness, and psychoeducation in combination with each other are effective for addressing pain perception, depression, and anxiety to improve rehabilitation outcomes
- • Neuromodulation and sensory feedback systems are two of the most promising areas for future directions but require larger standardized trials to conclude their effectiveness
Funding:
The research work of DKA is supported by the R25AI179582 grant from the National Institutes of Health, USA. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Competing interests:
All authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Consent for publication:
All authors have read the manuscript and consented for publication.
References
- Rivera JA, Churovich K, Anderson AB, et al. Estimating recent US limb loss prevalence and updating future projections. Arch Rehabil Res Clin Transl 6 (2024): 100376.
- Sparling T, Iyer L, Pasquina P, et al. Cortical reorganization after limb loss: bridging the gap between basic science and clinical recovery. J Neurosci 44 (2024): e1051232024.
- Barnes JA, Eid MA, Creager MA, et al. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler Thromb Vasc Biol 40 (2020): 1808-1817.
- Armstrong DG, Tan T, Boulton AJM, et al. Diabetic foot ulcers: a review. JAMA 330 (2023): 62-75.
- Gallagher KA, Mills JL, Armstrong DG, et al. Current status and principles for the treatment and prevention of diabetic foot ulcers in the cardiovascular patient population: a scientific statement from the American Heart Association. Circulation 149 (2024): e232-e253.
- Allison MA, Armstrong DG, Goodney PP, et al. Health disparities in peripheral artery disease: a scientific statement from the American Heart Association. Circulation 148 (2023): 286-296.
- Kassavin D, Mota L, Ostertag-Hill CA, et al. Amputation rates and associated social determinants of health in the most populous US counties. JAMA Surg 159 (2024): 69-76.
- American Heart Association. Management of peripheral artery disease. Circulation (2023). Available at: https://www.ahajournals.org/doi/10.1161/CIR.0000000000001178
- American Heart Association. Global perspectives on vascular health. Circulation (2021). Available at: https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000967
- Webster JB, Crunkhorn A, Sall J, et al. Clinical practice guidelines for the rehabilitation of lower limb amputation: an update from the Department of Veterans Affairs and Department of Defense. Am J Phys Med Rehabil 98 (2019): 820-829.
- Kent ML, Hsia HJ, Van de Ven TJ, Buchheit TE. Perioperative pain management strategies for amputation: a topical review. Pain Med 18 (2017): 504-519.
- Leal NTB, de Araújo NM, Silva SO, et al. Pain management in the postoperative period of amputation surgeries: a scoping review. J Clin Nurs 32 (2023): 7718-7729.
- Heyns A, Dusar FR, Arienti C, et al. Overview of Cochrane systematic reviews for rehabilitation interventions in persons with amputation: a mapping synthesis. Eur J Phys Rehabil Med 61 (2025): 351-357.
- Limakatso K, Bedwell GJ, Madden VJ, Parker R. The prevalence and risk factors for phantom limb pain in people with amputations: a systematic review and meta-analysis. PLoS One 15 (2020): e0240431.
- Spezia MC, Dy CJ, Brogan DM. Phantom limb pain management. J Hand Surg 50 (2025): 208-215.
- Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol 1 (2002): 182-189.
- Stankevicius A, Wallwork SB, Summers SJ, et al. Prevalence and incidence of phantom limb pain, phantom limb sensations and telescoping in amputees: a systematic rapid review. Eur J Pain 25 (2021): 23-38.
- Diers M, Krumm B, Fuchs X, et al. The prevalence and characteristics of phantom limb pain and non-painful phantom phenomena in a nationwide survey of 3,374 unilateral limb amputees. J Pain 23 (2022): 411-423.
- Vaso A, Adahan HM, Gjika A, et al. Peripheral nervous system origin of phantom limb pain. Pain 155 (2014): 1384-1391.
- Granata G, Di Iorio R, Ilari S, et al. Phantom limb syndrome: from pathogenesis to treatment. A narrative review. Neurol Sci 45 (2024): 4741-4755.
- Kuffler DP. Origins of phantom limb pain. Mol Neurobiol 55 (2018): 60-69.
- Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest 128 (2018): 2168-2176.
- Makin TR, Flor H. Brain (re)organisation following amputation: implications for phantom limb pain. Neuroimage 218 (2020): 116943.
- Andoh J, Milde C, Tsao JW, Flor H. Cortical plasticity as a basis of phantom limb pain: fact or fiction? Neuroscience 387 (2018): 85-91.
- Giummarra MJ, Moseley GL. Phantom limb pain and bodily awareness: current concepts and future directions. Curr Opin Anaesthesiol 24 (2011): 524-531.
- Liu H, Andoh J, Lyu Y, et al. Peripheral input and phantom limb pain: a somatosensory event-related potential study. Eur J Pain 24 (2020): 1314-1329.
- Stone AB, Hollmann MW, Terwindt LE, Lirk P. Chronic post amputation pain: pathophysiology and prevention options for a heterogenous phenomenon. Curr Opin Anaesthesiol 36 (2023): 572-579.
- Doshi TL, Dolomisiewicz E, Caterina MJ, et al. Postamputation pain: a multidisciplinary review of epidemiology, mechanisms, prevention, and treatment. Reg Anesth Pain Med 50 (2025): 175-183.
- Clarke C, Lindsay DR, Pyati S, Buchheit T. Residual limb pain is not a diagnosis: a proposed algorithm to classify postamputation pain. Clin J Pain 29 (2013): 551-562.
- List EB, Krijgh DD, Martin E, Coert JH. Prevalence of residual limb pain and symptomatic neuromas after lower extremity amputation: a systematic review and meta-analysis. Pain 162 (2021): 1906-1913.
- Zhang J, Lai S, Li J, et al. Early postoperative rapid rehabilitation yields more favorable short-term outcomes in patients undergoing patellar realignment surgery for recurrent patellar dislocation: a prospective randomized controlled study. Am J Sports Med 52 (2024): 2205-2214.
- Buch NS, Qerama E, Brix Finnerup N, Nikolajsen L. Neuromas and postamputation pain. Pain 161 (2020): 147-155.
- Stover G, Prahlow N. Residual limb pain: an evidence-based review. NeuroRehabilitation 47 (2020): 315-325.
- Münger M, Pinto CB, Pacheco-Barrios K, et al. Protective and risk factors for phantom limb pain and residual limb pain severity. Pain Pract 20 (2020): 578-587.
- Chang BL, Mondshine J, Fleury CM, et al. Incidence and nerve distribution of symptomatic neuromas and phantom limb pain after below-knee amputation. Plast Reconstr Surg 149 (2022): 976-985.
- Lee EY, Sammarco MC, Spinner RJ, Shin AY. Current concepts of the management of painful traumatic peripheral nerve neuromas. J Am Acad Orthop Surg 33 (2025): 178-186.
- Hwang CD, Hoftiezer YAJ, Raasveld FV, et al. Biology and pathophysiology of symptomatic neuromas. Pain 165 (2024): 550-564.
- Lu C, Sun X, Wang C, et al. Mechanisms and treatment of painful neuromas. Rev Neurosci 29 (2018): 557-566.
- Lans J, Groot OQ, Hazewinkel MHJ, et al. Factors related to neuropathic pain following lower extremity amputation. Plast Reconstr Surg 150 (2022): 446-455.
- Murray AL, Morgenroth DC, Czerniecki JM. Residual limb claudication in a traumatic transtibial amputee. PM R 5 (2013): 152-154.
- Subedi N, Heire P, Parmer V, et al. Multimodality imaging review of the post-amputation stump pain. Br J Radiol 89 (2016): 20160572.
- Rosenberg NM, Bull AMJ. Application of a mechanobiological algorithm to investigate mechanical mediation of heterotopic bone in trans-femoral amputees. Sci Rep 8 (2018): 14196.
- Atkinson GJ, Lee MY, Mehta MK. Heterotopic ossification in the residual lower limb in an adult nontraumatic amputee patient. Am J Phys Med Rehabil 89 (2010): 245-248.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg 14 (2006): S191-S197.
- Cao G, Zhang S, Wang Y, et al. Pathogenesis of acquired heterotopic ossification: risk factors, cellular mechanisms, and therapeutic implications. Bone 168 (2023): 116655.
- Raasveld FV, Liu WC, Renthal WR, et al. Heterotopic ossification is associated with painful neuromas in transtibial amputees undergoing surgical treatment of symptomatic neuromas. Plast Reconstr Surg 155 (2025): 185-193.
- Facchin A, Lemaire S, Toner LG, et al. When bone forms where it shouldn’t: heterotopic ossification in muscle injury and disease. Int J Mol Sci 26 (2025): 7516.
- Paterno L, Ibrahimi M, Gruppioni E, et al. Sockets for limb prostheses: a review of existing technologies and open challenges. IEEE Trans Biomed Eng 65 (2018): 1996-2010.
- Döring K, Trost C, Hofer C, et al. How common are chronic residual limb pain, phantom pain, and back pain more than 20 years after lower limb amputation for malignant tumors? Clin Orthop Relat Res 479 (2021): 2036-2044.
- Chamessian A, Van de Ven T, Buchheit T, et al. Differential expression of systemic inflammatory mediators in amputees with chronic residual limb pain. Pain 158 (2017): 68-74.
- Di Pino G, Piombino V, Carassiti M, et al. Neurophysiological models of phantom limb pain: what can be learnt. Minerva Anestesiol 87 (2021): 481-487.
- Culp CJ, Abdi S. Current understanding of phantom pain and its treatment. Pain Physician 25 (2022): E941-E957.
- Therrien AS, Howard C, Buxbaum LJ. Aberrant activity in an intact residual muscle is associated with phantom limb pain in above-knee amputees. J Neurophysiol 125 (2021): 2135-2143.
- Nikolajsen L, Hansen CL, Nielsen J, et al. The effect of ketamine on phantom pain: a central neuropathic disorder maintained by peripheral input. Pain 67 (1996): 69-77.
- Harden RN, McCabe CS, Goebel A, et al. Complex Regional Pain Syndrome: Practical Diagnostic and Treatment Guidelines, 5th Edition. Pain Med 23 Suppl 1 (2022): S1-S53.
- Gibuła-Tarłowska E, Wiszniewska A, Turyk M, et al. Ketamine-From an Anesthetic to a Psychiatric Drug: Mechanisms of Action, Clinical Applications and Potential Risks. Molecules 30 (2025): 2824.
- Thompson T, Whiter F, Gallop K, et al. NMDA receptor antagonists and pain relief: A meta-analysis of experimental trials. Neurology 92 (2019): e1652-e1662.
- Ferraro MC, Cashin AG, Visser EJ, et al. Ketamine and other NMDA receptor antagonists for chronic pain. Cochrane Database Syst Rev 8 (2025): CD015373.
- Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res 6 (2013): 121-136.
- Ahuja V, Thapa D, Ghai B. Strategies for prevention of lower limb post-amputation pain: A clinical narrative review. J Anaesthesiol Clin Pharmacol 34 (2018): 439-449.
- Payne R. Limitations of NSAIDs for pain management: toxicity or lack of efficacy? J Pain 1 Suppl 3 (2000): 14-18.
- Huse E, Larbig W, Flor H, et al. The effect of opioids on phantom limb pain and cortical reorganization. Pain 90 (2001): 47-55.
- Arthur A, Kapural L, Chiacchierini RP, et al. Post-Amputation Pain: Combined Analyses of Randomized Controlled Trials Evaluating Opioids and Gabapentinoids versus Placebo. J Pain Res 17 (2024): 3449-3453.
- Bone M, Critchley P, Buggy DJ. Gabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over study. Reg Anesth Pain Med 27 (2002): 481-486.
- Jiang S, Zhou MM, Xia R, et al. Gabapentin for phantom limb pain after amputation in pediatric oncology: a systematic review protocol. Syst Rev 10 (2021): 26.
- Subedi B, Grossberg GT. Phantom limb pain: mechanisms and treatment approaches. Pain Res Treat 2011 (2011): 864605.
- Nagoshi Y, Watanabe A, Inoue S, et al. Usefulness of milnacipran in treating phantom limb pain. Neuropsychiatr Dis Treat 8 (2012): 549-553.
- Hall N, Eldabe S. Phantom limb pain: a review of pharmacological management. Br J Pain 12 (2018): 202-207.
- Cheesborough JE, Smith LH, Kuiken TA, et al. Targeted muscle reinnervation and advanced prosthetic arms. Semin Plast Surg 29 (2015): 62-72.
- Bjorklund KA, Alexander J, Tulchin-Francis K, et al. Targeted Muscle Reinnervation for Limb Amputation to Avoid Neuroma and Phantom Limb Pain in Patients Treated at a Pediatric Hospital. Plast Reconstr Surg Glob Open 11 (2023): e4944.
- Leach GA, Dean RA, Kumar NG, et al. Regenerative Peripheral Nerve Interface Surgery: Anatomic and Technical Guide. Plast Reconstr Surg Glob Open 11 (2023): e5127.
- Lin Z, Yu P, Chen Z, et al. Regenerative peripheral nerve interface reduces the incidence of neuroma in the lower limbs after amputation: a retrospective study based on ultrasound. J Orthop Surg Res 18 (2023): 619.
- Sehirlioglu A, Ozturk C, Yazicioglu K, et al. Painful neuroma requiring surgical excision after lower limb amputation caused by landmine explosions. Int Orthop 33 (2009): 533-536.
- Maslow JI, LeMone A, Scarola GT, et al. Digital Nerve Management and Neuroma Prevention in Hand Amputations. Hand (N Y) 18 (2023): 838-844.
- Senger JB, Hardy P, Thorkelsson A, et al. A Direct Comparison of Targeted Muscle Reinnervation and Regenerative Peripheral Nerve Interfaces to Prevent Neuroma Pain. Neurosurgery 93 (2023): 1180-1191.
- McAuley J, van Gröningen R, Green C. Spinal cord stimulation for intractable pain following limb amputation. Neuromodulation 16 (2013): 530-536.
- Corbett M, South E, Harden M, et al. Brain and spinal stimulation therapies for phantom limb pain: a systematic review. Health Technol Assess 22 (2018): 1-184.
- Srinivasan N, Zhou B, Park E. Dorsal Root Ganglion Stimulation for the Management of Phantom Limb Pain: A Scoping Review. Pain Physician 25 (2022): E1174-E1182.
- Kim SY, Kim YY. Mirror therapy for phantom limb pain. Korean J Pain 25 (2012): 272-274.
- Falbo KJ, Phelan H, Hackman D, et al. Graded motor imagery and its phases for individuals with phantom limb pain following amputation: A scoping review. Clin Rehabil 38 (2024): 287-304.
- Guémann M, Olié E, Raquin L, et al. Effect of mirror therapy in the treatment of phantom limb pain in amputees: A systematic review of randomized placebo-controlled trials does not find any evidence of efficacy. Eur J Pain 27 (2023): 3-13.
- Ambron E, Miller A, Kuchenbecker KJ, et al. Immersive Low-Cost Virtual Reality Treatment for Phantom Limb Pain: Evidence from Two Cases. Front Neurol 9 (2018): 67.
- Hashim NA, Abd Razak NA, Shanmuganathan T, et al. On the use of virtual reality for individuals with upper limb loss: a systematic scoping review. Eur J Phys Rehabil Med 58 (2022): 612-620.
- Trevelyan EG, Turner WA, Robinson N. Acupuncture for the treatment of phantom limb pain in lower limb amputees: study protocol for a randomized controlled feasibility trial. Trials 16 (2015): 158.
- Johnson MI, Mulvey MR, Bagnall AM, et al. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Cochrane Database Syst Rev 8 (2015): CD007264.
- Horne CE, Engelke MK, Schreier A, et al. Effects of tactile desensitization on postoperative pain after amputation surgery. J Perianesth Nurs 33 (2018): 689-698.
- Limakatso K, Parker R. Treatment recommendations for phantom limb pain in people with amputations: An expert consensus Delphi study. PM R 13 (2021): 1216-1226.
- Moura VL, Faurot KR, Gaylord SA, et al. Mind-body interventions for treatment of phantom limb pain in persons with amputation. Am J Phys Med Rehabil 91 (2012): 701-714.
- Oakley DA, Whitman LG, Halligan PW. Hypnotic imagery as a treatment for phantom limb pain: two case reports and a review. Clin Rehabil 16 (2002): 368-377.
- Hogan WB, Anderson G, Kovoor M, et al. Phantom limb syndrome: Assessment of psychiatric and medical comorbidities associated with phantom pain in 44,028 below knee amputees. Injury 53 (2022): 3697-3701.
- De Jong R, Shysh AJ. Development of a multimodal analgesia protocol for perioperative acute pain management for lower limb amputation. Pain Res Manag 2018 (2018): 5237040.
- Williams ACC, Fisher E, Hearn L, et al. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 8 (2020): CD007407.
- Rosser BA, Fisher E, Janjua S, et al. Psychological therapies delivered remotely for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 8 (2023): CD013863.
- Eccleston C, Morley SJ, Williams AC. Psychological approaches to chronic pain management: evidence and challenges. Br J Anaesth 111 (2013): 59-63.
- Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 69 (2019): 3S-125S.e40.
- Thibaut A, Beaudart C, Maertens DE, et al. Impact of microprocessor prosthetic knee on mobility and quality of life in patients with lower limb amputation: a systematic review of the literature. Eur J Phys Rehabil Med 58 (2022): 452-461.
- Keszler MS, Heckman JT, Kaufman GE, et al. Advances in prosthetics and rehabilitation of individuals with limb loss. Phys Med Rehabil Clin N Am 30 (2019): 423-437.
- Petrini FM, Valle G, Bumbasirevic M, et al. Enhancing functional abilities and cognitive integration of the lower limb prosthesis. Sci Transl Med 11 (2019): eaav8939.
- Herr HM, Clites TR, Srinivasan S, et al. Reinventing extremity amputation in the era of functional limb restoration. Ann Surg 273 (2021): 269-279.
- Wang J, Zhang H, Li Y, et al. Comparative effects of interventions on phantom limb pain: A network meta-analysis. World Neurosurg 167 (2022): e888-e899.
- Zaheer A, Khan S, Malik R, et al. Effects of phantom exercises on pain, mobility, and quality of life among lower limb amputees: A randomized controlled trial. BMC Neurol 21 (2021): 424.
- Gauthier LV, Williams J, Rizzo JR, et al. Applying behavior change techniques to support client outcomes in outpatient neurorehabilitation: A clinicians guide. Arch Phys Med Rehabil 103 (2022): 1685-1693.
- Burger H, Marincek C. Return to work after lower limb amputation. Disabil Rehabil 23 (2001): 508-514.
- Üçeyler N, Sommer C, Rehm S, et al. Cortical binding potential of opioid receptors in patients with fibromyalgia syndrome and reduced systemic interleukin-4 levels: A pilot study. Front Neurosci 14 (2020): 512.
- Raspopovic S, Valle G, Ortiz-Catalan M. Personalized prosthetics with sensory feedback for functional restoration. Nat Mater 20 (2021): 991-1002.
- Nicora G, Rossi D, Bianchi A, et al. Systematic review of AI/ML applications in multi-domain robotic rehabilitation: Trends, gaps, and future directions. J Neuroeng Rehabil 22 (2025): 116.
- Ostler C. Meaningful outcome measurement following lower limb prosthetic rehabilitation. PhD Thesis. University of Southampton, School of Health Science, 2024.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 