Analysis of Molecular classification of Carcinoma Breast by Immunohisto chemistry in a series of core biopsied tissue at a Tertiary Care Hospital
Bidoura Naznin1*, Md. Mahabub Alam1, Tanny Tarafder2, Israt Yesmin3, Farzana Hafiz3, Sujit Mistry3 , Masud Rana3
1Department of Anatomic Pathology, Square Hospitals Limited, Dhaka, Bangladesh
3Department of Surgery, Square Hospitals Ltd, Dhaka, Bangladesh
4Department of Anatomic Pathology, Square Hospitals Limited, Dhaka, Bangladesh
*Corresponding Author: Dr. BidouraNaznin, Department of Anatomic Pathology, Square Hospitals Limited, Dhaka, Bangladesh
Received: 24 August 2025; Accepted: 02 September 2025; Published: 06 January 2026
Article Information
Citation: Bidoura Naznin, Mahabub Alam, Tanny Tarafder, Israt Yesmin, Farzana Hafiz, Sujit Mistry, Masud Rana. Analysis of Molecular classification of Carcinoma Breast by Immunohistochemistry in a series of core biopsied tissue at a Tertiary Care Hospital. Archives of Clinical and Biomedical Research. 10 (2026): 20-27.
View / Download Pdf Share at FacebookAbstract
Background: Breast cancer is a heterogeneous disease with diverse biological behavior and clinical outcomes. Molecular classification through immunohistochemistry (IHC) serves as a vital tool for predicting prognosis and tailoring therapy. This study aimed to evaluate the distribution of molecular subtypes by immunohistochemistry (IHC) in core biopsy samples in a Bangladeshi cohort and their correlation with clinicopathological parameters.
Methods: 124 invasive breast carcinoma patients who received core needle biopsy at a tertiary care institution (2023-2024) were included. IHC profiling of ER, PR, HER2, and Ki67 was performed, and the tumors were placed into Luminal A, Luminal B, HER2-enriched, and Triple-negative subtypes. Clinicopathological factors, including tumor grade, size, and nodal status, were also investigated. Statistical significance was assessed by chi-square tests (SPSS v26.0).
Results: Among 124 patients (median age: 50.2 ± 11.3 years), 46.8% of them had tumors <2 cm, and 80.6% had moderately differentiated (Grade 2) histology. Molecular subtyping revealed Luminal A as the most common subtype (46.0%), followed by Luminal B (26.6%), Triple-negative (17.7%), and HER2-enriched (9.7%). Hormone receptor expression was ER positive in 70.9% and PR positive in 62.0% of tumors, while HER2 overexpression was observed in 25.0%. The Ki67 proliferation index revealed low proliferative activity (<14%) in 51.6% of the tumors, intermediate (14-30%) in 30.6%, and high (>30%) in 17.8%. The upper outer quadrant was the most common location for the tumor (28.2%), and invasive ductal carcinoma represented 93.5% of all tumors. FNA-proved lymph node metastasis was detected in 9.7% of patients, most frequently BI-RADS category 5 lesions (41.9%).
Conclusion: MolecularSubtyping of breast cancer in core biopsies by IHC is a feasible stratification technique within resource-limited settings, which detects a significant predominance of hormone-sensitive Luminal A tumors. The high percentage of aggressive subsets (Triple-negative/HER2-enriched) calls for the generation of targeted treatments and extensive screening programs within Bangladesh. These findings are favorable for the inclusion of molecular diagnostics in clinical practice to optimize treatment planning.
Keywords
<p>Breast cancer; Molecular subtyping; Immunohistochemistry; Prognostic biomarkers</p>
Article Details
1. Introduction
Breast cancer remains one of the most significant global public health challenges and continues to be the leading cause of cancer-related mortality among women. Advances in our understanding of its complex biology have revealed remarkable heterogeneity, with substantial variability in morphological features, clinical behavior, and treatment responses [1]. This intrinsic heterogeneity necessitates refined classification systems that extend beyond conventional histopathologic evaluation to effectively inform clinical management. Traditionally, breast cancer staging has relied heavily on histomorphological parameters such as tumor grade, histologic subtype, and lymph node status [2]. Although these parameters have been instrumental, they alone do not capture the full biological diversity of breast carcinomas or reliably predict therapeutic outcomes. The introduction of molecular profiling techniques has significantly enhanced our ability to stratify breast cancers into biologically distinct subtypes, each with differing prognostic and therapeutic implications. Among these, immunohistochemistry (IHC) has emerged as a cost-effective, reproducible, and clinically applicable surrogate method for molecular subtyping in routine pathology practice [3]. By assessing the expression profiles of key biomarkers—estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and the proliferation marker Ki-67—breast cancers can be categorized into well-defined molecular subtypes: Luminal A, Luminal B, HER2-enriched, and Triple-negative/Basal-like [4-6].This molecular classification carries substantial therapeutic significance. Luminal subtypes (ER/PR-positive) typically exhibit favorable prognoses and heightened sensitivity to endocrine therapy [7]. In contrast, Luminal B tumors, often marked by elevated Ki-67 or HER2 co-expression, display more aggressive clinical behavior and generally require more intensive treatment strategies including chemotherapy [8]. HER2-enriched tumors respond dramatically to anti-HER2 targeted therapies, while Triple-negative breast cancers (TNBC), lacking expression of ER, PR, and HER2, present a major therapeutic challenge despite demonstrating initial chemosensitivity [9].The role of core needle biopsy has gained prominence as an initial diagnostic modality, offering sufficient tissue for both histopathologic assessment and IHC-based molecular analysis [10]. This approach facilitates comprehensive diagnostic evaluation at the pre-surgical stage, allowing for expedited treatment planning. Nevertheless, concerns persist regarding the concordance between biomarker status in core biopsies versus surgical specimens, highlighting the necessity of continuous validation of diagnostic accuracy.In this context, the present study aims to systematically analyze the molecular classification of breast carcinoma by IHC on core biopsy specimens. We seek to determine the distribution of molecular subtypes and explore their correlation with clinicopathological parameters. Beyond immediate diagnostic relevance, our findings may contribute valuable epidemiological insights into subtype prevalence in the Bangladeshi population, potentially uncovering unique genetic or environmental influences. The knowledge generated could inform public health strategies, optimize screening programs, and refine resource allocation for breast cancer management at both institutional and national levels. Through this systematic evaluation, we endeavor to bridge the gap between molecular advances and their real-world clinical application, thereby enhancing the quality and precision of breast cancer care in Bangladesh..
2. Methods
The study was conducted at Square Hospital anatomic pathology department between June 2023 and December 2024. The objective of this study was to analyze the molecular classification of breast carcinoma using immunohistochemistry (IHC) in a cohort of 124 patients who underwent core needle biopsy during the specified period. Inclusion criteria encompassed all patients with histopathologically confirmed breast carcinoma who underwent core needle biopsy at the study center. Demographic data, including age, were collected and categorized into age groups to facilitate an understanding of breast cancerincidence distribution. Clinical variables were also documented, such as lump size, family history of breast cancer, and parity status. Lump size was categorized based on measured tumor dimensions in centimeters, while family history data were collected to evaluate genetic predisposition. Parity status was recorded to explore any hormonal influence history on breast cancer development.
All patients underwent diagnostic imaging with ultrasonography (USG) to assess lymph node involvement, and lesions were categorized according to the Breast Imaging Reporting and Data System (BI-RADS) classification. Additionally, the anatomical position of the lump within the breast was noted. Histopathological evaluation was performed on core needle biopsy samples to classify the histological type of carcinoma, with a primary focus on identifying invasive ductal carcinoma (IDC) and other less common histological variants. Tumors were graded according to the Nottingham grading system, based on the degree of differentiation into well-differentiated, moderately differentiated, and poorly differentiated categories. Pathological features such as the presence of ductal carcinoma in situ (DCIS), lobular carcinoma in situ (LCIS), lymphovascular invasion (LVI), and microcalcifications were also assessed.
Immunohistochemical analysis was carried out to evaluate the expression of key biomarkers, including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), Ki67 proliferation index and E-cadherin for lobular subtyping also tested Based on the expression patterns of these biomarkers, tumors were classified into molecular subtypes, namely Luminal A, Luminal B, HER2-enriched, and Triple Negative. The molecular classification aimed to provide insights into the biological behavior of the tumors and their potential therapeutic responsiveness.
Data were analyzed using descriptive and inferential statistical methods. Descriptive statistics, including frequencies and percentages, were used to summarize categorical variables such as age group distribution, lump size categories, histological types, and biomarker status, whereas continuous variables were expressed as means and standard deviations. Relationships between categorical variables were assessed using the Chi-square test, with a p-value of less than 0.05 considered statistically significant.All statistical analyses were performed using SPSS software, version 26.0.
3. Results
The findings reveal that the majority of the patients were between the ages of 40-59 with 33.1% aged 40-49 and 27.4% aged 50-59, which reveals the preponderance of middle-aged people. The majority of the lumps were smaller than 2.0 cm (46.8%) and then between 2.0-2.9 cm (24.2%), which reveals early presentation in the majority of cases. A familial history of breast cancer was lacking in 73.4% of the patients, 9.7% had a positive general or specific history, and 15.3% were unknown. Malignancies in other organs were found only in 1.6% of the patients. The majority of the patients (59.7%) were multiparous, 32.2% were primiparous, and an insignificant proportion (8.1%) were nulliparous. This trend indicates the demographic and clinical trends generally identified with the onset of breast cancer in the population under study. (Table 1).
|
Variable |
Frequency (n) |
Percentage (%) |
|
Age Group |
||
|
20–29 years |
1 |
0.8% |
|
30–39 years |
19 |
15.3% |
|
40–49 years |
41 |
33.1% |
|
50–59 years |
34 |
27.4% |
|
60–69 years |
15 |
12.1% |
|
>70 years |
14 |
11.3% |
|
Lump Size |
||
|
< 2.0 cm |
16 |
12.9% |
|
2.0–2.9 cm |
35 |
28.2% |
|
3.0–3.9 cm |
12 |
9.7 |
|
4.0–4.9 cm |
5 |
4% |
|
>5.0 cm |
6 |
4.83% |
|
Unknown |
50 |
40.32% |
|
Family History of Breast Cancer |
||
|
None |
111 |
89.5% |
|
Present (mother/sister/maternal aunt/paternal aunt) |
13 |
10.4% |
|
Parity |
||
|
Nulliparous |
10 |
8.1% |
|
Primiparous |
40 |
32.2% |
|
Multiparous |
74 |
59.7% |
Table 1: Demographic and Clinical Characteristics (n=124).
Table 2 displays ultrasound results in 124 patients, emphasizing lymph node involvement and BI-RADS categorization. Almost half (48.4%) had nodes identified on ultrasound, but only 9.7% had FNA-proven metastasis, and 29.8% had unknown nodal status. For BI-RADS classification, the most common (41.9%) were Category 5 with high suspicion for malignancy, 21.8% were Category 4 (suspicious abnormality), and 2.4% were Category 3 (probably benign). Category 6 (conclusive evidence of malignancy) was 4.2%, and 29.8% were not classified. This outcome shows a majority of suspicious ultrasound patterns with a significant percentage of patients with high-risk findings. (Table 2).
|
Variable |
Frequency (n) |
Percentage (%) |
|
USG-detected Lymph Node |
||
|
Yes |
60 |
48.4 |
|
No |
15 |
12.1 |
|
FNA-proven metastasis |
12 |
9.7 |
|
Unknown |
37 |
29.8 |
|
total |
||
|
Category 3 |
3 |
2.4 |
|
Category 4 (4a, 4b, 4c) |
27 |
21.8 |
|
Category 5 |
52 |
41.9 |
|
Category 6 |
5 |
4.1 |
|
Unknown |
37 |
29.8 |
Table 2: Ultrasound Findings (n=124).
The position of the tumor lump was most frequently localized in the Upper Outer (UO) quadrant (28.2%), followed by the Upper Inner (UI) quadrant (16.9%) and subareolar region (8.1%). However, in over one-third of patients (34.7%), the specific lump position was not documented. Histologically, Invasive Ductal Carcinoma (IDC) was the predominant tumor type (93.5%), while other histological variants were rare, comprising small fractions of the study sample (Table 3).
|
Variable |
Frequency (n) |
Percentage (%) |
|
Position of Lump |
||
|
Upper Outer (UO) |
35 |
28.2 |
|
Upper Inner (UI) |
21 |
16.9 |
|
Lower Outer (LO) |
9 |
7.3 |
|
Lower Inner (LI) |
7 |
5.6 |
|
Subareolar |
10 |
8.1 |
|
Unknown |
42 |
33.9 |
|
Histological Type |
||
|
Invasive Ductal Carcinoma (IDC) |
116 |
93.5 |
|
Invasive Lobular Carcinoma |
3 |
2.4 |
|
Invasive Carcinoma with Micropapillary Features |
1 |
0.8 |
|
Mixed IDC + Mucinous Features |
1 |
0.8 |
|
Encapsulated Papillary Carcinoma |
1 |
0.8 |
|
Mixed Mucinous and DCIS |
2 |
1.7 |
Table 3: Tumor Site and Histological Type (n=124).
Tumor grading according to the Nottingham histologic grading system showed that the majority (80.6%) of tumors were moderately differentiated (Grade 2), while poorly differentiated tumors (Grade 3) accounted for 12.9%. Well-differentiated tumors (Grade 1) were rare, comprising only 6.5% of the sample (Table 4).
|
Grade |
Frequency (n) |
Percentage (%) |
|
Grade 1 (Well Differentiated) |
8 |
6.5 |
|
Grade 2 (Moderately Differentiated) |
100 |
80.6 |
|
Grade 3 (Poorly Differentiated) |
16 |
12.9 |
Table 4: Histologic Grade Distribution (n=124).
Pathological features were evaluated, and Ductal Carcinoma In Situ (DCIS) was present in 25.0% of cases, whereas Lobular Carcinoma In Situ (LCIS) was rare (2.4%). Lymphovascular invasion (LVI) was identified in 8.1% of tumors. Microcalcifications were observed in 12.1% of cases, while necrosis was noted in 19.4% of specimens (Table 5).
|
Variable |
Frequency (n) |
Percentage (%) |
|
DCIS |
||
|
Present |
31 |
25.0 |
|
Not identified |
93 |
75.0 |
|
LCIS |
||
|
Present |
3 |
2.4 |
|
Not identified |
121 |
97.6 |
|
Lymphovascular Invasion (LVI) |
||
|
Present |
10 |
8.1 |
|
Not identified |
114 |
91.9 |
|
Microcalcification |
||
|
Present |
15 |
12.1 |
|
Not identified |
109 |
87.9 |
|
Necrosis |
||
|
Present |
24 |
19.4 |
|
Not identified |
100 |
80.6 |
Table 5: Pathological Features and Invasion Indicators (n=124)
Table 6 presents an overall expression of immunohistochemical biomarker profiles in breast carcinoma instances. The majority (70.9%) of the instances were estrogen receptor (ER) positive, indicating the hormone-responsive character of this cancer type, while 25.8% were ER-negative and 3.2% had low positive expression. Progesterone receptor (PR), with only 62.0% showing positivity and 37.9% displaying negative expression. HER2 overexpression occurred in 25.0% of samples, with a further 7.3% equivocal, although confirmatory HER2 FISH was performed in just 2.4% of the series. The Ki67 proliferation index revealed that over half (51.6%) of tumors had low proliferative activity (<14%), as would be expected for the typically indolent carcinoma, and 30.6% had intermediate and 17.8% high proliferation. E-cadherin, a marker of lobular differentiation and cell adhesion, was tested in particular cases, with results for only 1.6% of patients (Table 6).
|
Biomarker |
Status |
Frequency (n) |
Percentage (%) |
|
ER Status |
Positive |
88 |
70.9 |
|
Negative |
32 |
25.8 |
|
|
Low Positive |
4 |
3.2 |
|
|
PR Status |
Positive |
77 |
62.0 |
|
Negative |
47 |
37.9 |
|
|
HER2 Status (IHC) |
Positive |
31 |
25.0 |
|
Equivocal |
9 |
7.3 |
|
|
Negative |
84 |
67.7 |
|
|
HER2 FISH Result |
Tested |
9 |
7.3 |
|
Not Tested |
121 |
97.6 |
|
|
Ki67 |
Low (<14%) |
64 |
51.6 |
|
Intermediate (14–30%) |
38 |
30.6 |
|
|
High (>30%) |
22 |
17.8 |
|
|
E-cadherin Status |
Available |
2 |
1.6 |
|
Not Available |
122 |
98.4 |
Table 6: Distribution of the study population based on Biomarker Status (n=124).
On molecular subtyping, Luminal A was the predominant subtype (45.9%), followed by Luminal B (26.6%). Triple Negative tumors constituted 17.7% of cases, while HER2-enriched tumors accounted for 9.7%, highlighting a substantial burden of aggressive subtypes within the population (Table 7).
|
Molecular Subtype |
Frequency (n) |
Percentage (%) |
|
Luminal A |
57 |
46% |
|
Luminal B |
33 |
26.6% |
|
Triple Negative |
22 |
17.7% |
|
HER2 Enriched |
12 |
9.7% |
Table 7: Molecular Subtyping (n=124)
Table 8 (A) represents the distribution of biomarker status (ER, PR, HER2, Ki67) by breast cancer molecular subtypes (Luminal A, Luminal B, HER2-enriched, triple-negative) in 124 patients and correlation coefficients (r-values). It shows rich ER/PR positivity in Luminal A, HER2 enrichment in Luminal B and HER2 subtypes, and elevated Ki67 in aggressive subtypes. The r-values measure the magnitude and direction of these correlations.Table 8 (B) interprets these correlations in clinical terms, ER/PR reliably predict hormonal responsiveness in Luminal A, but HER2 and Ki67 with more aggressive subtypes. r-values help assess biomarker-subtype correlations for diagnosis and treatment.
|
(A): Correlation Table Between Biomarker Status and Molecular Subtype (n = 124) |
||||||||
|
Biomarker Profile |
Luminal A (n=57) |
Luminal B (n=33) |
HER2 Enriched (n=12) |
Triple Negative (n=22) |
Total |
|||
|
ER Positive |
57 |
31 |
0 |
0 |
88 |
|||
|
ER Negative |
0 |
2 |
12 |
18 |
32 |
|||
|
r (ER vs Subtype) |
+0.82 |
+0.45 |
–0.68 |
–0.74 |
||||
|
PR Positive |
55 |
21 |
0 |
1 |
77 |
|||
|
PR Negative |
2 |
12 |
12 |
21 |
47 |
|||
|
r (PR vs Subtype) |
+0.79 |
+0.35 |
–0.63 |
–0.69 |
||||
|
HER2 Positive |
0 |
15 |
12 |
4 |
31 |
|||
|
HER2 Negative |
57 |
18 |
0 |
18 |
84 |
|||
|
r (HER2 vs Subtype) |
–0.32 |
+0.60 |
+0.88 |
–0.36 |
||||
|
Ki67 Low (<14%) |
46 |
15 |
1 |
2 |
64 |
|||
|
Ki67 Intermediate (14–30%) |
9 |
10 |
5 |
14 |
38 |
|||
|
Ki67 High (>30%) |
2 |
8 |
6 |
6 |
22 |
|||
|
r (Ki67 vs Subtype) |
–0.70 |
+0.52 |
+0.38 |
+0.41 |
||||
|
(B): Interpretation Table: Biomarker Correlation with Molecular Subtypes (n = 124) |
||||||||
|
Biomarker |
Subtype |
r-value |
Strength of Correlation |
Interpretation |
||||
|
ER |
Luminal A |
+0.82 |
Strong Positive |
Highly associated with Luminal A; indicates strong hormone responsiveness. |
||||
|
Luminal B |
+0.45 |
Moderate Positive |
Moderate ER positivity; overlaps with HER2 expression in some cases. |
|||||
|
HER2 Enriched |
–0.68 |
Moderate Negative |
Usually lacks ER; suggests non-hormonal HER2-driven behavior. |
|||||
|
Triple Negative |
–0.74 |
Strong Negative |
Strongly ER-negative; highly aggressive phenotype. |
|||||
|
PR |
Luminal A |
+0.79 |
Strong Positive |
Most Luminal A tumors are PR-positive, supporting hormonal regulation. |
||||
|
Luminal B |
+0.35 |
Mild Positive |
Some hormonal influence remains. |
|||||
|
HER2 Enriched |
–0.63 |
Moderate Negative |
PR rarely expressed. |
|||||
|
Triple Negative |
–0.69 |
Moderate to Strong Negative |
PR is largely absent; confirms hormone-independence. |
|||||
|
HER2 |
Luminal A |
–0.32 |
Weak Negative |
HER2 is rarely overexpressed in Luminal A. |
||||
|
Luminal B |
+0.60 |
Moderate to Strong Positive |
HER2 is often co-expressed with hormone receptors. |
|||||
Table 8: (A) Correlation Table Between Biomarker Status and Molecular Subtype (n = 124) (B): Interpretation Table: Biomarker Correlation with Molecular Subtypes (n = 124).
4. Discussion
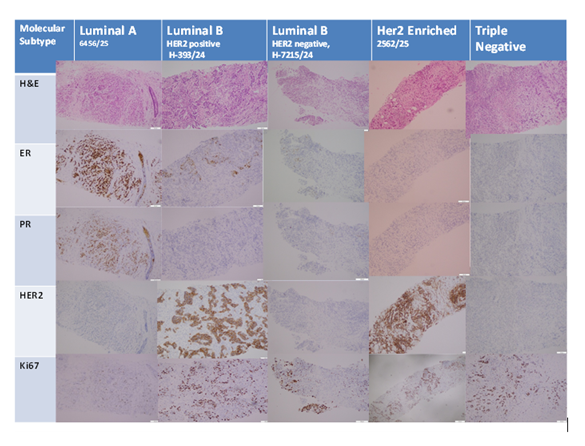
This study provides a comprehensive analysis of molecular typing of breast carcinoma by immunohistochemistry in core biopsy specimens from a Bangladeshi tertiary center, adding valuable epidemiological information with substantial clinical implications. The age distribution revealed that the majority of the patients were 40-49 years (33.1%), then 50-59 years (27.4%), and this was similar to the study of Zobair et al. [11] which revealed that midlife was the peak age of incidence for breast cancer in rural areas. This pattern of age is the typical finding among South Asians, wherein breast cancer occurs earlier in life compared to the West.Tumor size evaluation indicated that most of the patients had tumors ≥2 cm, which indicates concern about thelate detection. The late detection of tumors is due to the lack of awareness among the general population, and the unavailability of the screening test is also a factor. However, 40.32% of the patients had tumors of unknown size, which can indicate a lack of documentation in practice. Family history of breast cancer was documented for only 10.4% of the patients, much less than in Western nations but comparable to other developing countries [12]. The prevalence of multiparity (59.7%) fits the patterns of hormonal exposures common in breast cancer epidemiology in this region of the world [13].Imaging findings revealed ultrasound lymph node involvement in 48.4% of the patients and FNA-documented metastasis in 9.7%, in line with Kim and Jung's reporting [14]. BI-RADS category 5 lesions were most frequent (41.9%), consistent with Cedolini et al.'s diagnostic yields [15]. Anatomically, the Upper Outer Quadrant remained the most frequent tumor location (28.2%), consistent with prevailing anatomical traditions [16,17]. Histopathological analysis confirmed Invasive Ductal Carcinoma as the most prevalent subtype (93.5%), as found in international epidemiology trends [18]. Tumors were predominantly moderately differentiated (Grade 2: 80.6%), a percentage higher than in Rakha et al.'s [19] larger series. Synchronous DCIS was found in 25.0% of cases, in line and documented by Cedolini et al. [15], while LCIS remained rare (2.4%) as documented by Rattanasalee et al. [20]. Biomarker evaluation identified ER positivity in 70.9% of the cases, which agrees with the regional report by Gaffar et al. [13] and Mais et al. [21], albeit lower than that found internationally. PR positivity was identified in 62.0% of the cases and HER2 overexpression in 25.0%, which agrees with the regional and global reports [13,21]. Ki67 examination demonstrated low proliferative capacity (<14%) in 51.6% of the tumors, intermediate proliferation (14-30%) in 30.6%, and high proliferation (>30%) in 17.8%, and helped to discriminate between Luminal A and B subtypes as defined by Yan et al. [4] and Zhao et al. [5] .Molecular subtyping revealed Luminal A to be the most frequent subtype (46.0%), followed by Luminal B (26.6%), Triple Negative (17.7%), and HER2-enriched (9.7%). The pattern is closely akin to findings of Park et al. [12] and Su et al. [22], confirming Luminal A as the most prevalent subtype globally. Statistically significant biomarker-molecular subtype associations (p < 0.05) were as advised globally, with Luminal subtypes showing strong hormone receptor positivity and correct HER2 status patterns [23]. Correlation analysis detected significant correlations between biomarkers and molecular subtypes, with ER correlating most strongly with Luminal A (r = +0.82) and displaying significant negative correlations with aggressive subtypes. These findings underscore the critical function of immunohistochemistry in guiding diagnosis, prognosis, and treatment planning in resource-limited settings (Figure 1).
Photomicrograph
Luminal A (ER positive, PR positive, HER2 negative, Ki67 proliferative index low)
Luminal B HER2 positive (ER positive, PR negative, HER2 positive, Ki67 proliferative index high)
Luminal B HER2 negative (ER positive, PR negative, HER2 negative, Ki67 proliferative index high)
HER2 enriched (ER negative, PR negative, HER2 overexpressed,Ki67 index high)
Triple negative subtype (ER negative, PR negative, HER2 negative)
Limitations of The Study:
The study was conducted in a single hospital with a small sample size. So, the results may not represent the whole community.
5. Conclusion
In conclusion, this study provides a comprehensive analysis of the molecular classification of breast carcinoma using immunohistochemistry in core biopsy specimens from a Bangladeshi tertiary care hospital. The findings reveal that the majority of breast cancer cases were of the Luminal A subtype, with favorable hormonal receptor profiles, while Luminal B, Triple Negative, and HER2-enriched subtypes were less frequent but clinically significant. Molecular subtyping demonstrated strong concordance with biomarker expression profiles, highlighting the crucial role of immunohistochemistry in guiding diagnosis, prognostication, and therapeutic planning. These findings emphasize the need for expanded access to molecular diagnostics, tailored treatment strategies, and reinforced breast cancer screening programs in resource-limited settings like Bangladesh. Future studies incorporating genomic profiling and longitudinal follow-up are recommended to further refine risk stratification and therapeutic approaches in this population.
6. Future Recommendations
Future studies must incorporate large multi-institutional cohorts to enhance generalizability and validate these findings in other Bangladeshi populations. Integration of genomic profiling with immunohistochemistry would provide more complete molecular characterization. Survival outcome and treatment response patterns must be determined by longitudinal follow-up investigations. Further, deployment of standardized documentation protocols and greater availability of molecular diagnostic facilities across Bangladesh would improve clinical care quality.
Funding:
No funding sources
Conflict of Interest:
None declared
Ethical Approval:
The study was approved by the Institutional Ethics Committee
References
- Perou CM, Sørlie T, Eisen MB, et al. Molecular Portraits of Human Breast Tumours. Nature 406 (2000): 747-52.
- Bibi S, Biswas P, Tareq MMI, et al. Cordycepin and Its Structural Derivatives Effectively Suppress the High Expression of Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase in Breast Carcinomas: A Computational Drug Development Approach. Curr Med Chem (2025).
- Shovon MdHJ, Khan DA, Tareq MdMI, et al. A Comprehensive Assessment of VCAN Transcriptional Expression and Evaluation as an Effective Prognostic Biomarker Against Breast Cancer: An In Silico Study. Bull Natl Res Cent 47 (2023): 83.
- Zhao X, Yang X, Fu L, et al. Associations of Estrogen Receptor, Progesterone Receptor, Human Epidermal Growth Factor Receptor-2 and Ki-67 with Ultrasound Signs and Prognosis of Breast Cancer Patients. Cancer Manag Res 13 (2021): 4579-86.
- Yan J, Liu XL, Han LZ, et al. Relation Between Ki-67, ER, PR, Her2/neu, p21, EGFR, and TOP II-α Expression in Invasive Ductal Breast Cancer Patients and Correlations with Prognosis. Asian Pac J Cancer Prev 16 (2015): 823-9.
- Roy M, Fowler AM, Ulaner GA, et al. Molecular Classification of Breast Cancer. PET Clin 18 (2023): 441-58.
- Yu KD, Cai YW, Wu SY, et al. Estrogen Receptor-Low Breast Cancer: Biology Chaos and Treatment Paradox. Cancer Commun 41 (2021): 968-80.
- Dai X, Li T, Bai Z, et al. Breast Cancer Intrinsic Subtype Classification, Clinical Use and Future Trends. Am J Cancer Res 5 (2015): 2929-43.
- Lehmann BD, Bauer JA, Chen X, et al. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J Clin Invest 121 (2011): 2750-67.
- Houssami N, Ciatto S, Macaskill P, et al. Accuracy and Surgical Impact of Magnetic Resonance Imaging in Breast Cancer Staging: Systematic Review and Meta-Analysis in Detection of Multifocal and Multicentric Cancer. J Clin Oncol 26 (2008): 3248-58.
- Al Zobair AA, Jasim BI, Al Obeidy BF, et al. Prognostic Impact of Hormone and HER2 Status on the Prognosis of Breast Cancer in Mosul. Ann Trop Med Public Health (2020).
- Park S, Koo JS, Kim MS, et al. Characteristics and Outcomes According to Molecular Subtypes of Breast Cancer as Classified by a Panel of Four Biomarkers Using Immunohistochemistry. Breast 21 (2012): 50-7.
- Gaffar T, Baqui MN, Yasmin S, et al. Status of ER, PR, HER-2 and E-Cadherin Expression in Female Breast Cancer Patients from Bangladesh. SBV J Basic Clin Appl Health Sci 2 (2019): 61-4.
- Kim HR, Jung HK. Histopathology Findings of Non-Mass Cancers on Breast Ultrasound. Acta Radiol Open 7 (2018): 2058460118774957.
- Cedolini C, Bertozzi S, Londero AP, et al. Impact of the Presence and Quantity of Ductal Carcinoma In Situ Component on the Outcome of Invasive Breast Cancer. Int J Clin Exp Pathol 8 (2015): 13304-13.
- Wang YT, Chen ZJ, Zhang D, et al. Effect of the Primary Tumor Location on the Prognosis of Breast Invasive Ductal Carcinoma Patients Treated with Radical Mastectomy. Zhonghua Zhong Liu Za Zhi 41 (2019): 686-92.
- Ellsworth DL, Ellsworth RE, Love B, et al. Outer Breast Quadrants Demonstrate Increased Levels of Genomic Instability. Ann Surg Oncol 11 (2004): 861-8.
- Hofmeyer S, Pekár G, Gere M, et al. Comparison of the Subgross Distribution of the Lesions in Invasive Ductal and Lobular Carcinomas of the Breast: A Large-Format Histology Study. Int J Breast Cancer 2012 (2012): 436141.
- Rakha EA, El-Sayed ME, Lee AHS, et al. Prognostic Significance of Nottingham Histologic Grade in Invasive Breast Carcinoma. J Clin Oncol 26 (2008): 3153-8.
- Rattanasalee S, Chaiwun B, Sukhamwang N. The Prevalence of Lobular Carcinoma In Situ and Its Variants of Breast Cancer in Maharaj Nakorn Chiang Mai Hospital Over a 5-Year Period. Biomed Sci Clin Med 51 (2012): 111-7.
- Mais DD, Nazarullah AN, Guidi AJ, et al. Estrogen Receptor, Progesterone Receptor, and Human Epidermal Growth Factor Receptor 2 Expression Rates in Invasive Breast Carcinoma: A Study of 21 Institutions. Arch Pathol Lab Med 149 (2025): 8-13.
- Su Y, Zheng Y, Zheng W, et al. Distinct Distribution and Prognostic Significance of Molecular Subtypes of Breast Cancer in Chinese Women: A Population-Based Cohort Study. BMC Cancer 11 (2011): 292.
- Kakudji BK, Mwila PK, Burger JR, et al. Breast Cancer Molecular Subtypes and Receptor Status Among Women at Potchefstroom Hospital: A Cross-Sectional Study. Pan Afr Med J 38 (2021).


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 