Association of Triglyceride/HDL Cholesterol Ratio with CRP and Ferritin among Different Obesity Phenotypes of Non-diabetic Adult Bangladeshi
Waheed TB1*, Hoque MM2, Chowdhury SN1, Tasnim A1, Romana R3, Moyenullah M4, Alam A1
1Department of Biochemistry, Green Life Medical College and Hospital, Dhaka, Bangladesh
2Professor, Department of Biochemistry , International Medical College, Dhaka, Bangladesh
3Department of Biochemistry, Ahsania Mission Medical College, Dhaka, Bangladesh
4Department of Plastic Surgery, Dhaka Medical Hospital, Dhaka, Bangladesh
*Corresponding author: Dr. Tanha Waheed Brishti, Department of Biochemistry, Green Life Medical College and Hospital, Dhaka, Bangladesh.
Received: 16 August 2025; Accepted: 22 August 2025; Published: 29 August 2025
Article Information
Citation: Waheed TB, Hoque MM, Chowdhury SN, Tasnim A, Romana R, Moyenullah M, Alam A. Association of Triglyceride/HDL Cholesterol Ratio with CRP and Ferritin among Different Obesity Phenotypes of Non-diabetic Adult Bangladeshi. Archives of Clinical and Biomedical Research. 9 (2025): 342-347.
View / Download Pdf Share at FacebookAbstract
Introduction: Obesity is one of the major global problems now a days which is associated with insulin resistance and inflammation. Considering financial condition of Bangladesh, TG/HDL-c ratio can be used for assessment of IR. As CRP and ferritin usually respond to any type of inflammatory condition, they are expected to be raised in obesity. Early detection of association between TG/HDL-c ratio and inflammatory markers (CRP & ferritin) can help in prediction of severity of obesity induced health risks.
Materials and Methods: A cross sectional analytical study was conducted in the Department of Biochemistry, Bangladesh Medical University (BMU) with 512 samples. Fasting blood sample and 2 hours after 75gm glucose load blood sample were collected for lipid profile, CRP & ferritin. BMI was calculated. Obese individuals were classified into three phenotypes named as phenotype A (obese BMI ,non-obese WC), phenotype B (non-obese BMI, obese WC) and phenotype C (obese BMI,obese WC). TG/HDL-c ratio was calculated and serum ferritin and CRP were assessed of different obesity phenotypes. Then correlation between TG/HDL-c ratio and CRP as well as ferritin was done to see their association in different obesity phenotypes.
Results: TG/HDL-c ratio was significantly higher in phenotype B & C compared to phenotype A. Plasma CRP and ferritin level were found to be highest in phenotype C in comparison to phenotype A & B. Among phenotype C individuals, a positive correlation was found between TG/HDL-c ratio and serum ferritin but not with CRP and low positive correlation was found between CRP and ferritin.
Conclusion: A significant association was shown between TG/HDL-c ratio and serum ferritin among phenotype C. Plasma CRP showed no significant association with TG/HDL-c ratio among different obesity phenotypes.
Keywords
<p>TG/HDL-c ratio; Plasma CRP; Serum ferritin; Obesity phenotypes; Non-diabetic adult</p>
Article Details
Abbreviations: TG/HDL-c ratio: Triglyceride/High density lipoprotein cholesterol ratio; CRP: C reactive protein; BMI: Body mass index; WC: Waist circumference; IR: Insulin resistance; MetS: Metabolic syndrome
1. Introduction
Obesity is considered as one of the major health issues all over the world now a days. A recent survey of the World Obesity Federation (WOF) predicts that by 2030, approximately one billion people will be living with obesity worldwide which includes one in five women and one in seven men [1]. Previously, the focus has been on obesity in high income countries and undernutrition in low and middle income countries. But recent studies have shown that low and middle income countries are also facing increased prevalence of obesity [2]. Prevalence of obesity is increasing rapidly in South and Southeast Asia along with potentially serious consequences in local economies, healthcare systems, and quality of life. The recent Global Burden of Diseases 2019 estimated that obesity, high fasting plasma glucose and high body mass index are among the top 10 risk factors causing most death and disability in Bangladesh [3].
Obesity is associated with insulin resistance (IR) which is a core component of metabolic syndrome (MetS) [4]. Insulin resistance is a state in which the body’s sensitivity and response to insulin are reduced, resulting in the inability of insulin to appropriate transport glucose into cells, causing metabolic abnormalities such as hyperglycemia [5]. HOMA-IR is the gold standard method for assessment of IR. It is calculated from fasting insulin (U/mL) and fasting glucose (mmol/L) [6]. HOMA-IR is expensive method and many primary health care centers may not afford this. The triglyceride-to-high-density lipoprotein (TG/HDL) ratio has been proposed as a marker for assessment of IR because the metabolic processes involved in IR also lead to changes in lipid metabolism which are reflected in the levels of serum triglycerides and HDLcholesterol [7]. Moreover, Triglyceride (TG) and HDL-C are routine test and less expensive compared to insulin. For this reason, TG/HDL-C ratio have significant role in assessment of insulin resistance.
As CRP and ferritin usually respond to any inflammatory condition, they are expected to be raised in obesity. Hepatic secretion of CRP increases in obesity due to certain inflammatory mechanism [8]. Certain recent studies suggested that obesity induced chronic inflammatory reaction causes increased serum ferritin level which is not just because of an increased in iron stores [9].
According to WHO criteria for Asia-Pacific region (WHO, 2000) individuals with BMI ≥25.0kg/m2 are considered as generally obese and WC ≥90 cm (men) and ≥80cm (women) are considered as centrally obese. In this study obesity phenotypes are classified based on both BMI and WC. Early detection of association between TG/HDL-C ratio and inflammatory markers (CRP & ferritin) can help in prediction of severity of obesity induced health risks. For this purpose, our study aims to find out any association between inflammatory markers (CRP and Ferritin) and TG/HDL-c ratio in different phenotypes of obesity.
2. Materials and Methods
A cross sectional study was conducted in the Department of Biochemistry, Bangladesh Medical University (BMU) enrolling 512 study subjects. Participants included for the study were nondiabetic, either sex (25-65 years) having BMI ≥18.5 kg/m2. Individuals who had chronic diseases, cardiovascular diseases, malignancy, history of taking lipid lowering drugs, NSAID, steroids and pregnant women were excluded from the study groups. Subjects (non-obese and obese individuals) from the outpatient department of BMU, who match the inclusion and exclusion criteria were enrolled in the study by non-probability sampling technique BMI was calculated. Among them 149 study subjects were considered as nonobese comparison group (nonobese BMI,nonobese WC) and 363 subjects were obese. Fasting and 2 hours after 75gm glucose load blood sample was collected.. Obese individuals were classified into three phenotypes named as phenotype A (obese BMI, non-obese WC), phenotype B (non-obese BMI, obese WC) and phenotype C (obese BMI, obese WC). TG/HDL-C ratio was calculated from fasting lipid profile and serum ferritin and CRP were estimated in different obesity phenotypes. Then association between TG/HDL-C ratio and CRP as well as ferritin was done to see their association in different obesity phenotypes. Data were cleaned, entered and analyzed by Statistical Package for the Social Sciences (SPSS) software version 26.0. Spearman rank correlation test was done. According to data as needed to achieve level of significance. P-value ≤ 0.05 was considered statistically significant.
3. Results
Among total study subjects (512), majority were obese ( 363, 71%) and among obese subjects , obesity phenotype C was found to dominate. TG/HDL-C ratio was found to be significantly elevated in phenotype B and C, compared to phenotype A with statistically identical in phenotype B and C. Plasma CRP and ferritin level were found to be highest in phenotype C in comparison to phenotype A and B. A positive correlation was found between TG/HDL-C ratio and serum ferritin in phenotype C with very low positive correlation between CRP and ferritin.
Table 1 showed among total study subjects (512), majority were obese ( 363, 71%) and among obese subjects , obesity phenotype C was found to dominate.
|
Total subjects |
Non-obese group (reference group) |
Obese Group |
Total Obese |
||
|
Phenotype A |
Phenotype B |
Phenotype C |
|||
|
512 |
149 |
49 |
92 |
222 |
363 (71%) |
Table 1: Distribution of subjects with respect to obesity.
Table 2 showed the association of WC and BMI with non obese and obese phenotypes. BMI and WC were found significantly higher among phenotype A and phenotype B respectively. Both BMI and WC were found higher in phenotype C.
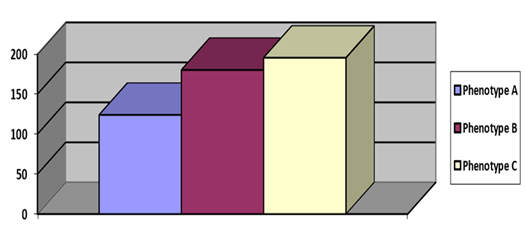
Figure 1 showed among the obesity phenotypes, TG/HDL-C ratio was significantly elevated in phenotype B and phenotype C, compared to phenotype A. Phenotype B and phenotype C found statistically identical with respect to TG/HDL-C ratio.
|
Variables |
Non obese |
Obese |
Chi-square |
P value |
||
|
WC |
|
Phenotype A |
Phenotype B |
Phenotype C |
|
|
|
Normal |
133 |
49 |
0 |
0 |
483.4 |
0 |
|
Abnormal |
0 |
0 |
92 |
222 |
||
|
BMI |
||||||
|
Normal |
133 |
0 |
92 |
0 |
488.1 |
0 |
|
Abnormal |
0 |
49 |
0 |
222 |
||
Table 2: Association of WC and BMI with non obese and obesity phenotypes.
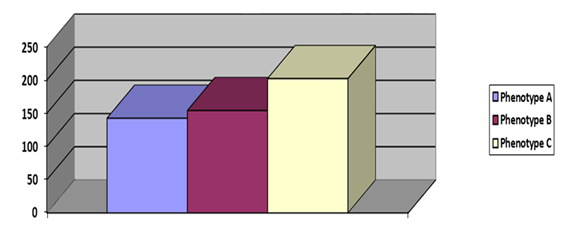
Figure 2 showed among the obesity phenotypes, plasma CRP and ferritin significantly elevated in phenotype C, compared to phenotype A and phenotype B. Phenotype A and phenotype B found statistically identical with respect to CRP and ferritin.
Table 3 showed a positive correlation between TG/HDL-C ratio and serum ferritin among phenotype C. A positive correlation was also found between CRP and ferritin in phenotype C individual.
|
Phenotype |
Parameters |
Correlation coefficient |
P value |
|
|
A |
TG/HDL-C ratio |
Ferritin |
0.162 |
0.265 |
|
CRP |
0.056 |
0.7 |
||
|
B |
TG/HDL-C ratio |
Ferritin |
0.17 |
0.104 |
|
CRP |
-0.031 |
0.769 |
||
|
C |
TG/HDL-C ratio |
Ferritin |
0.242 |
0 |
|
CRP |
0.033 |
0.624 |
||
|
CRP |
Ferritin |
0.142 |
0.035 |
|
|
Spearman rank correlation was done |
||||
Table 3: Association between TG/HDL-C ratio, CRP and ferritin among different obesity phenotypes.
4. Discussion
Prevalence of obesity is increasing rapidly in South and Southeast Asia along with potentially serious consequences in local economies, healthcare systems, and quality of life. The burden on economies and healthcare systems associated with the rise in obesity is difficult to overstate. Moreover, huge costs of treatment for obesity and associated diseases cause reduction in men's and women's productive time in the work force by 4–9 years across the countries within the Association of Southeast Asian Nations (ASEAN) [10]. Different metabolic risks are associated with different obesity phenotypes. Certain phenotypes are at higher risk than other phenotypes because of variation in insulin sensitivity/ resistance and various inflammatory markers.
In this study, among 512 total study subjects 363 (71%) were found obese possessing an obese BMI or an obese WC or both together. This indicates a very high proportion of obese individuals among our study subjects. This might be due to enrollment of subjects from hospital outpatient department (not from general population) where people with obesity related medical problems frequently attend. This study found significant association of obesity with BMI and WC. According to WHO criteria for Asia-Pacific region (WHO, 2000) individuals with BMI ≥25.0kg/m2 are considered as generally obese and WC ≥90 cm (men) and ≥80cm (women) are considered as centrally obese. That is why we classified different obesity phenotypes according to WHO (2000) criteria for Asia-Pacific region on the basis of BMI and WC [11].
The TG/HDL-c ratio has been proposed as a marker for assessment of IR because the metabolic processes involved in IR also lead to changes in lipid metabolism which are reflected in the levels of serum triglycerides and HDLcholesterol [7]. In this study, we observed TG/HDL-C ratio was significantly elevated in phenotype B and phenotype C, compared to phenotype A. Phenotype B and phenotype C found statistically identical with respect to TG/HDL-C ratio. Individuals of phenotype B contain high amount of visceral fat which indicates higher rate of lipolysis, increased FFA, increased hepatic production of TGs and VLDL as well as reduced clearance of serum triacylglycerol via peripheral lipolysis of triacylglycerol-rich lipoproteins. This ultimately increases the probability of lipid exchange via the action of cholesterol ester transfer protein. The cholesterol ester transfer protein pathway decreases cholesterol esters in HDL with simultaneous elevation of cholesterol ester in triacylglycerol-rich lipoprotein remnants. As a result TG/HDL-C ratio increased with higher proportion of visceral adiposity. These results are supported by Taksali et al. [12], who observed a significantly high TG level, decreased HDL-C levels and reduced insulin sensitivity in people with high proportion of visceral fat [12]. Tikkanen et al. [13], proposed that high percentage of slow twitch muscle fibers is associated with high serum HDL-C [13]. Obese individuals with high BMI usually have high lean body mass and skeletal muscle mass. Therefore, this group of people might have high serum HDL-C. In obesity phenotype C, since the individuals are obese with respect to WC as well as BMI; these people are expected to have high lean body mass as well as muscle mass with high serum HDL-C. Probably this is the possible explanation of TG/HDL-C ratio to be identical between phenotype B and C.
We found plasma CRP and ferritin significantly elevated in phenotype C, compared to phenotype A and phenotype B. Phenotype A and phenotype B found statistically identical with respect to both plasma CRP and serum ferritin. In subjects of phenotype B, the accumulation of visceral adipose tissue is responsible for the up-regulation of low-grade chronic inflammation and increases plasma CRP and ferritin. This finding was supported by Moscoso et al. who found positive correlation of CRP and ferritin with visceral adiposity [14]. Beyond the saturation point, subcutaneous adipose tissue cannot expand anymore and spillover fat to be deposited in undesirable non adipose tissue ectopic sites (eg: liver, pancreas etc). This ectopic fat depots are associated with adverse metabolic and inflammatory profile [11]. Individual of obesity phenotype A probably have ectopic fat depots because of which plasma CRP and ferritin of phenotype A did not differ from that of obesity phenotype B. Ferritin and CRP found to be highest in phenotype C in comparison to other phenotypes because of combined effect of both general and abdominal obesity. This finding was supported by Thompson et al. [15] and Holz et al. [16], who showed positive association of CRP and ferritin with subcutaneous adipose tissue and visceral adipose tissue [15,16].
In this study, we found a significant positive association of TG/HDL-c ratio with serum ferritin among phenotype C individuals. This finding was supported by Ellidag et al. who found that serum ferritin has positive correlation with triglyceride but negative correlation with HDL-c [17]. A significant positive association was also found between plasma CRP and serum ferritin in phenotype C. This finding was supported by Kerkadi and Rospleszcz who found positive correlation of serum ferritin and plasma CRP with visceral adiposity [18,19].
Our study has some limitations. The more accurate methods for measurements of body fat (eg. MRI , CT scan) could not be used to categorize the obesity phenotypes by differentiating the visceral fat from subcutaneous fat. At the same time, limited studies examined the association of TG/HDL-c ratio with CRP & ferritin after adequate adjustment of confounding factors. Thus, we found the real relationship between ferritin and TG/HDL-c ratio after a sufficient adjustment for confounders.
In short, a significant association was shown between TG/HDL-c ratio and serum ferritin among phenotype C. Plasma CRP showed no significant association with TG/HDL-c ratio among different obesity phenotypes.
Acknowledgements
I would like to express my sincere gratitude for the invaluable support and cooperation provided by my co-authors/colleagues, participants and the staff who contributed to this study.
Ethical Approval
The study was approved by the Institutional Review Board (IRB) of Bangladesh Medical University (BSMMU/2022/7184)
Author Contributions
Tanha Waheed Brishti: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Su- pervision, Validation, Visualization, Writing – original draft, Writing – review & editing
Mozammel Hoque: Investigation, Methodology, Project administration, Software, Validation, Writing – review & editing
Sanjeela Nahreen Chowdhury: Project administration, Software, Supervision, Visualization
Aniqa Tasnim: Project administration, Software, Visualization
Rimpi Romana: Project administration, Software, Visualization
Mohammad Moyenullah : Project administration, Supervision, sample collection
Azmeri Alam: Investigation, Project administration, Software, Supervision, Visualization
Funding: No funding sources
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Lobstein T, Brinsden H, Neveux M. World Obesity Atlas (2022). https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2022_WEB.pdf. Accessed March 2022.
- Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 395 (2020): 65-74.
- Collaborators GBDRF. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 (2020): 1223-49.
- Ahmed Bulbul, Rifat Sultana, Michael W. Greene. Adipose tissue and insulin resistance in obese. Biomedicine and Pharmacotherapy 137 (2021).
- Faerch K, Vaag A, Holst J, et al. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter 99 study. Diabetes Care 32 (2009): 439-44.
- Matthews Dr, Hosker Jp, Rudenski As, et al. Homeostasis Model Assessment: Insulin Resistance and Beta Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 28 (1985): 412-419.
- Nabipoorashrafi SA, Seyedi SA, Rabizadeh S, et al. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: a systematic review and meta-analysis. Nutrition, Metabolism, and Cardiovascular Diseases NMCD 32 (2022): 2677-88
- Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obesity Rev 14 (2013): 232-244.
- Zafon C, Lecube A, Simo R. Iron in obesity. An ancient micronutrient for a modern disease. Obes Rev 11 (2010): 322-328.
- Okunogbe A, Nugent R, Spencer G, et al. Economic impacts of over weight and obesity: current and future estimates for eight countries. BMJ Glob Health 6 (2021): e006351.
- Gyllenhammer LE, Alderete TL, Toledo-Corral CM, et al. Saturation of subcutaneous adipose tissue expansion and accumulation of ectopic fat associated with metabolic dysfunction during late and post-pubertal growth. International Journal of Obesity 40 (2016): 601-606.
- Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes 57 (2008): 367-371.
- Tikkanen HO, Härkönen M, Näveri H, et al. Relationship of skeletal muscle fiber type to serum high density lipoprotein cholesterol and apolipoprotein A-I levels. Atherosclerosis 90 (1991): 49-57.
- Hinojosa-Moscoso A, Motger-Albertí A, De la Calle-Vargas E, et al. The Longitudinal Changes in Subcutaneous Abdominal Tissue and Visceral Adipose Tissue Volumetries are Associated with Iron Status. International Journal of Molecular Sciences 24 (2023): 4750.
- Thompson AL, Koehler E, Herring AH, et al. Weight Gain Trajectories Associated with Elevated C-Reactive Protein Levels in Chinese Adults. J Am Heart Assoc 5 (2016): e003262.
- Holz T, Thorand B, Döring A, et al. Markers of Inflammation and Weight Change in Middle-Aged Adults: Results from the Prospective MONICA/KORA S3/F3 Study. Obesity 18 (2010): 2347-2353.
- Ellidag HY, Eren E, Akdag M, et al. The relationship between serum ferritin levels and serum lipids and HDL function with respect to age and gender. Ukr Biochem J 88 (2016): 76-86.
- Kerkadi A, Ali RM, Shehada AAH, et al. Association between central obesity indices and iron status indicators among Qatari adults. PLoS ONE 16 (2021): e0250759.
- Rospleszcz, S, Dermyshi D, Müller-Peltzer K, et al. Association of serum uric acid with visceral, subcutaneous and hepatic fat quantified by magnetic resonance imaging. Sci Rep 10 (2020): 442.
Related PubMed Articles
- Could serum uric acid to HDL cholesterol ratio predict sacroiliitis?
- The Triglyceride/HDL Ratio as a Non-Invasive Marker for Early-Stage NAFLD: A Retrospective Cross-Sectional Study of 2588 Patients.
- Trends in blood-based metabolic and cardiovascular risk profiles in men during treatment for testosterone deficiency: a longitudinal, retrospective cohort study.
- Association between estimated glucose disposal rate and metabolic dysfunction-associated steatotic liver disease and dyslipidemia in US adults: a cross-sectional study.
- Residual insulin secretion in long-standing type 1 diabetes.
- Prevalence and determinants of metabolic syndrome among adults living with HIV on first-line antiretroviral treatment in southern Ethiopia: a cross-sectional study.
- FOK1 and APA1 Gene Polymorphism Among Polycystic Ovary Syndrome: A Prospective Cohort Study.
- The association between visit-to-visit variability in risk factors and incident CVD: A Post-hoc analysis of the Multi-Ethnic Study of Atherosclerosis.
- Childhood exposure to polycyclic aromatic hydrocarbons (PAHs) and cardiometabolic indicators in childhood and adolescence: findings from a cohort study in rural Bangladesh.
- Elevated high-density lipoprotein triglycerides increase atherosclerotic risk.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 