Clinical Response and Toxicities of Neo-Adjuvant Concurrent Chemoradiation with Tablet Capecitabine or Mayo-Clinic Regimen in locally advanced Rectal Cancer: A Comparative Study
Mukti Rani Datta1*, Nahid Naznin Rinky1, Dipok Saha1, Tanin Sultana2, Mohammad Ashraf-Us-Zaman Mahmud3, Sania Hossain4, A. K. M Shahidur Rahman5, Md. Waheed Akhtar3, Laila Siddika6, Fahmid-UZ-Zaman7, Aliya Shanaz1
1Department of Radiotherapy, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh
2Department of Radiotherapy, Sir Salimullah Medical College & Mitford Hospital (SSMC & MH), Mitford, Dhaka, Bangladesh
3Department of Radiation Oncology, National Institute of Cancer Research and Hospital (NICRH), Dhaka, Bangladesh
4Department of Cytopathology, National Institute of Cancer Research and Hospital (NICRH), Dhaka, Bangladesh
5Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
6Department of Laboratory Medicine, Sheikh Russel National Gastroliver Institute & Hospital, Mohakhali, Dhaka, Bangladesh
7Department of Surgery, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh
*Corresponding author: Dr. Mukti Rani Datta, Department of Radiotherapy, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh.
Received: 03November 2022; Accepted: 15November 2022; Published: 21November 2022
Article Information
Citation: Datta MR, Rinky NN, Saha D, Sultana T, Mahmud MAUZ, Hossain S, Rahman AKMS, Akhtar MW, Siddika L, Zaman FU, Shanaz A. Clinical Response and Toxicities of Neo- Adjuvant Concurrent Chemoradiation with Tablet Capecitabine or Mayo-Clinic Regimen in locally advanced Rectal Cancer: A Comparative Study. Archives of Clinical and Biomedical Research 6 (2022): 944-953.
View / Download Pdf Share at FacebookAbstract
Background: Neo-adjuvant Concurrent Chemoradiation (nCRT) with intravenous administration of 5-Fluorouracil/Leucovorin (Mayo-clinic regimen) or Tab. Capecitabine has been considered as standard protocol for locally advanced rectal cancer.
Objectives: To compare tumor response and toxicities of tablet Capecitabine or Mayo-clinic regimen along with External Beam Radiation Therapy (EBRT) in the treatment of locally advanced rectal cancer (stage IIC–IIIC). Methods: This quasi-experimental study was conducted at Department of Radiotherapy, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh from July 2019 to June 2020. A total of sixty (60) patients with locally advanced adenocarcinoma of the rectum (stage IIC–IIIC) were randomized into nCRT with tablet Capecitabine in Group A (Arm A) or Mayo-clinic regimen in Group B (Arm B) with thirty patients falling in each group. They were followed up for 3 months at a regular interval. Treatment response and toxicities were assessed and compared between the groups.
Results: The mean age of the patients in Arm A was 46.20 (SD±13.35) years and that was 41.80 (SD±12.55) years in Arm B (p= 0.193). Of them in Arm A; 19(63.33%) patients were male and 11(36.67%) were female and in Arm B; 17(56.67%) were male and 13(43.33%) were female. It was observed that, haematological toxicities, mucositis and proctitis were more common in the Mayo-clinic group. Gastrointestinal toxicities and the hand-foot syndrome were more common in the Caepacitabine group. There was a complete response rate of 33.33% in the Capecitabine group and 26.67% in the Mayoclinic group (p= 0.868).
Conclusion: There is no significant difference in tumor response and toxicities between Neo-adjuvant Concurrent Chemoradiation (nCRT) with ta
Keywords
<p>Capecitabine; Mayo-Clinic Regimen; Neo-adjuvant Concurrent Chemoradiation (nCRT); Rectal Cancer; Tumor Response; Toxicities</p>
Article Details
1. Introduction
Colorectal cancer (CRC) continues to be a major public health burden in developing countries [1]. CRC is the third most common cancer among adults of both sexes, according to GLOBOCAN 2020 data [2]. It is anticipated that there would be more than 2.2 million new cases of CRC and 1.1 million cancer deaths worldwide by 2030 [1, 3]. Though smoking and alcohol intake, a diet rich in fat and cholesterol, physical inactivity and other factors seem to be contributed to the development of CRC, the main aetiology involves multiple genes and three possible pathways: chromosomal instability, mismatch repair (MMR) and hypermethylation of the promoter of the MLH1 gene [3,4]. Mutations in genes such as APC, k-ras, and p53 are associated with chromosomal instability [4, 5]. Microsatellite instability (MSI) is a genetic hyper mutability, which is present in about 90% of Hereditary Non-polyposis Colorectal Cancer (HNPCC) that carry germline inactivation in DNA mismatch repair genes (transform growth factor-β receptor π, the proapoptotic BAX gene and β-catenin) [5]. Approximately 20% cases of CRC are associated with familial clustering and first-degree relatives of patients with colorectal adenoma or invasive colorectal tumor are at increased risk for colorectal cancer [3]. Therefore, it is recommended that all patients with colorectal cancer be queried regarding their family history and considered for risk assessment [3]. The predominant histopathological type of colorectal cancer is adenocarcinoma [6, 7]. Due to lack of adequate screening program, health care services, consciousness and infrastructural deficiencies majority of these patients come to the hospital with a locally advanced stage. Rectal cancer tends to recur locally in the pelvis and metastasize systemically; therefore, when approaching these patients, attention needs to be direct both at local and distant metastasis [8]. It was reported that preoperative chemoradiotherapy can reduce the tumor mass, block the tumor invasion, increase the tumor resection rate and anus retention rate, reduce iatrogenic dissemination during operation and reduce the local recurrence rate [5, 8]. The fluoropyrimidine 5-fluorouracil (5-FU) is an anti-metabolite drug that has been used concurrently with radiation because of its well-established potentiating effect [9]. Other drugs including- Oxaliplatin, Irinotecan, oral Fluoropyrimidines (Capecitabine) and Bevacizumab have recently been shown to be effective in the treatment of metastatic colorectal cancer [9]. These have now been incorporated into the testing of new strategies with neoadjuvant therapy [9]. According to the National Comprehensive Cancer Network (NCCN) guideline (Version 4.2020-May 21, 2020), there are 3 main modalities of treatment for locally advanced rectal cancer which are offered to the patients depending upon different situations [10]: (1) Neo-adjuvant Concurrent Chemoradiation (nCRT) followed by definitive surgery then systemic chemotherapy, (2) Short course radiation followed by definitive surgery then systemic chemotherapy and (3) Systemic chemotherapy followed by concurrent chemo-radiation then definitive surgery. Multiple techniques are used for providing radiation depending on the available facilities of the institution. Concurrent chemo-radiotherapy (CCRT) with a Mayo-clinic regimen is a widespread and well-established protocol for the treatment of locally advanced carcinoma rectum [11, 12]. However, concurrent chemo-radiotherapy (CCRT) with tablet Capecitabine is also a popular way to treat locally advanced carcinoma rectum, and a lot of studies had been conducted on this issue [9, 13]. Capecitabine is an oral fluoropyrimidine pro-drug that is readily absorbed in the gastrointestinal tract and mimics the efficacy of continuous infusion 5-FU while avoiding the risk of side effects and complications due to a central line for continuous venous infusion (CVI) for 5-FU [9, 13, 14]. In our study, we have chosen Tab. Capecitabine in one group and Mayo-clinic regimen (Inj. 5-FU and Inj. Leucovorin) in another group concurrently with Radiotherapy (RT). This study was aimed to compare these two chemotherapy regimens with radiation in terms of toxicity, clinical response and find out a more convenient modality of treatment.
2. Materials and Methods
This quasi-experimental study was conducted at Department of Radiotherapy, Dhaka Medical College Hospital (DMCH), Dhaka, Bangladesh from July 2019 to June 2020. This research protocol was approved by the ethical review committee of Dhaka Medical College, Dhaka, Bangladesh. A total of sixty (60) patients with histologically proven adenocarcinoma in locally advanced rectal cancer (stage IIC-IIIC) were enrolled according to the selection criteria by purposive sampling technique. Patients age between 18 to 75 years, histopathologically proven case of adenocarcinoma of the rectum with clinically stage IIC to IIIC and Karnosfsky performance status ³70 along with desirable laboratory parameters [Haemoglobin level should be >11gm/dl or >60%, total white blood cell (WBC) count >4000/mm3, total platelet count >1,00,000/mm3, serum bilirubin level <1 mg/dl, serum aspartate aminotransferase (AST) level <4 times the upper limit of normal, serum creatinine level <1.5 mg/dl] were included in this study. On the other hand; patients who were treated with radiotherapy/chemotherapy or definitive surgery, patients having distant metastasis, recurrent cases, existence of multiple malignancies (two or more), pregnant ladies and patients who were unwilling to participate in this study were excluded. Among total 60 study patients; 30 patients who received External Beam Radiotherapy (EBRT) 50 Gy in 25 daily fractions, 5 days in a week, concurrently with Tab. Capecitabine 825 mg/m2 twice daily (Day 1 to day 5)/week during radiotherapy were in one group (Arm A) and rest 30 patients who received EBRT 50 Gy in 25 daily fractions, 5 days in a week, concurrently with Mayo-clinic regimen (Inj. 5-FU 425 mg/m2/ day, day 1 to day 5 and Inj. LVR 20 mg/ m2/ day, day 1 to day 5) in the first week & fifth week of radiotherapy were in another group (Arm B).
2.1 Operational Definition
2.1.1 Locally advanced Rectal Cancer [15]: Locally advanced rectal cancer is characterized as tumors invading or extending close to the mesorectal fascia and/or the cancer has spread to adjacent lymph nodes, organ or tissues.
2.1.2 Mayo-clinic Regimen [12]: A chemotherapy combination used to treat colorectal cancer. It includes the drugs 5-Fluorouracil (5-FU) and leucovorin calcium.
2.1.3 5-Fluorouracil (5-FU) [11]: 5-Fluorouracil (5-FU) is a drug of an anti-metabolite group. It is a fluoropyrimidine analogue, which is a cell cycle-specific chemotherapy drug that acts in the “S” phase of the cell cycle. There are 3 active metabolites of 5-FU: Fluorodeoxyuridine monophosphate (FdUMP), Fluorodeoxyuridine triphosphate (FdUTP), and Fluorouridine triphosphate (FUTP). FUTP causes alterations in RNA processing and function, and FdUTP and FdUMP cause DNA damage; both of these processes affect RNA and DNA and cause cell death. Common toxicities of a fluoropyrimidine analogue include myelo-suppression, mucositis, diarrhea, hand-foot syndrome, cardiac symptoms, dry skin, photosensitivity, metallic taste in the mouth during intravenous bolus injection.
2.1.4 Capecitabine [14]: Capecitabine is a fluoropyrimidine carbamate with anti-neoplastic activity. Capecitabine itself is inactive and prodrug form of 5-FU. Activation to cytotoxic forms is a complex process that involves 3 successive enzymatic steps. Metabolized to 5'-deoxy-5-fluorocytidine (5'-DFCR) by the carboxylesterase enzyme than to 5'-deoxy-5-fluorouridine (5'-DFUR) by cytidine deaminase (that found in liver and tumour tissues). Subsequently converted to 5-FU by the enzyme thymidine phosphorylase (dThdPase) which is expressed in higher levels in tumor versus normal tissue. Then it acts as 5-FU. It is used in metastatic colorectal cancer, stage-III colon cancer. Common toxicities include diarrhea, hand-foot syndrome, nausea, vomiting, myelo-suppression, neurologic toxicity etc.
2.1.5 External Beam Radiation Therapy (EBRT) [16]: External beam radiation therapy (EBRT) is a form of radiotherapy which is commonly used in radiation oncology treatment. It provides a high dose radiation to destroy malignant cells and shrink tumors. In this procedure a machine directs external beams of radiation into cancerous areas inside patient’s body. This therapy is typically given daily, on an outpatient basis over the course of a number of weeks. As in our institution, the 2D technique is available, so EBRT with the 2D technique was applied.
2.1.6 Karnofsky Performance Status [17]: The Karnofsky Performance Status Scale (KPS) is widely used for a variety of purposes, including cancer response to chemotherapy and chronic disease assessment. It assesses a patient's functional abilities as well as the impact of treatments such as chemotherapy on their fundamental functional capacities.
2.2 Follow-up Schedule
Follow-up was done four times, 1st and 2nd follow-ups were done at 2nd and 5th week during treatment. Assessment of response was done at 4th week (3rd follow-up) after completion of concurrent chemo-radiotherapy (CCRT) and then after surgery (4th follow-up). Surgery had done six weeks after the completion of EBRT. Toxicities were assessed according to Radiation Therapy Oncology Group (RTOG) and Common Terminology Criteria for Adverse Events (CTCAE) guidelines. Symptoms and responses were assessed according to Response Evaluation in Solid Tumor (RECIST) criteria.
2.3 Data Analysis
Statistical analysis of the results was obtained by Statistical Package for Social Sciences (SPSS) for Windows version- 22. Continuous data were expressed as mean with standard deviation (±SD) and were compared by the Student “t” test. Categorical data were expressed as numbers with percentages and were compared by the Chi-squared test. A p value <0.05 was considered as statistically significant.
3. Results and Observations
This study was intended to compare the clinical response and toxicities of neo-adjuvant concurrent chemoradiation (nCRT) with Capecitabine (Arm A= 30 patients) or Mayo-clinic regimen (Arm B= 30 patients), in 60 patients having locally advanced rectal cancer (stage IIC – IIIC). The mean age of the patients in Arm A was 46.20 (SD±13.35) years and that was 41.80 (SD±12.55) years in Arm B (p= 0.193) (Table- 1).
|
Variable |
Arm- A, (n=30) |
Arm- B, (n=30) Mean±SD |
p- value |
|
Age (years) |
46.20±13.35 |
41.80±12.55 |
0.193ns |
Arm A= nCRT with Tab. Capecitabine, Arm B=nCRT with Inj.5-FU/ Inj.LVR, Data expressed as mean±SD, Unpaired student t-test was performed to compare between two groups, ns= not significant
Table 1: Comparison of age between two groups (N=60)
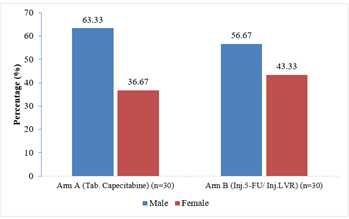
Of them in Arm A; 19(63.33%) patients were male and 11(36.67%) were female and in Arm B; 17(56.67%) patients were male and 13(43.33%) patients were female (Figure- 1).
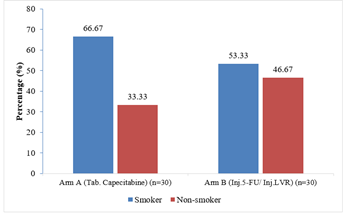
It was observed that out of total 60 study patients; 36 patients were smoker. In Arm A, 66.67% patients were smoked regularly. In Arm B, 53.33% patients were regular smoker (p= 0.292) (Figure- 2).
In this study, maximum patients (50.00% in Arm A and 46.67% in Arm B) were found in stage III B in each group. 7(23.33%) patients in Arm A and 8(26.67%) patients in Arm B were in stage III A. While, 4(13.33%) patients in Arm A were in stage IIC and IIIC, but 5(16.67%) patients and 3(10.00%) patients from Arm B were in these stages respectively. There was no significant difference in histopathological staging between the groups (p=0.949) (Table- 2).
|
Histopathological staging |
Arm- A, (n=30) |
Arm-B, (n=30) No. (%) |
p- value |
|
II C |
4(13.33) |
5(16.67) |
0.949 ns |
|
III A |
7(23.33) |
8(26.67) |
|
|
III B |
15(50) |
14(46.67) |
|
|
III C |
4(13.33) |
3(10.00) |
|
|
Total |
30(100) |
30(100) |
Arm A= nCRT with Tab. Capecitabine, Arm B=nCRT with Inj.5-FU/ Inj.LVR, Figures in the parentheses indicate the corresponding percentage, Chi-squared Test (x2) was done to analyze the data, ns= not significant
Table 2: Distribution of the patients by histopathological staging between two Arms (N=60)
It was found that most of the tumor was moderately differentiated; 66.67% and 73.33% in Arm A and Arm B respectively. 3 patients in Arm A and 4 patients in Arm B were histopathological graded as well differentiated adenocarcinoma. Poorly differentiated tumor was more common in Arm A (23.33%) than Arm B (13.33%). There was no significant difference in histopathological grading between the groups (p=0.59) (Table- 3).
|
Histopathological grading |
Arm- A, (n=30) |
Arm- B, (n=30) |
p- value |
|
Grade-1 Well differentiated |
3(10) |
4(13.33) |
0.59ns |
|
Grade-2 |
20(66.67) |
22(73.33) |
|
|
Grade-3 |
7(23.33) |
4(13.33) |
|
|
Total |
30(100) |
30(100) |
Arm A= nCRT with Tab. Capecitabine, Arm B= nCRT with Inj.5-FU/ Inj.LVR, Figures in the parentheses indicate the corresponding percentage, Chi-squared Test (x2) was done to analyze the data, ns= not significant
Table 3: Distribution of the patients by histopathological grading between two Arms (N=60)
A comparison of haematological toxicities at 1st follow-up is displaying in table- 4. Grade-1 leucopenia was noted among 6(20%) patients in Arm A and 8(26.67%) patients in Arm B. 2(6.67%) patients in Arm B showed Grade-2 leucopenia. Neutropenia was present among 6(20%) patients in Arm A and 7(23.34%) patients in Arm B. While 2(6.67%) patients in Arm B showed Grade-2 neutropenia. Grade-1 anemia was present among 5 and 6 patients in Arm A and Arm B respectively. Grade-1 thrombocytopenia was present among 3(10%) patients in Arm A and 5(16.67%) patients in Arm B. Only 1(3.33%) patient in Arm B experienced Grade-2 thrombocytopenia. There was no significant difference in haematological toxicities between the groups (p>0.05) (Table- 4). Haematological toxicities at 2nd follow-up are showing in Table- 4. Grade-1 leucopenia was found among same number of patients [7(23.33%)] in both Arms. 5(16.67%) patients in Arm B experienced Grade-2 leucopenia which was statistically significant (p= 0.047). Grade-1 neutropenia was noted among 7 patients in Arm A and 8 patients in Arm B, but 6 patients in Arm B experienced Grade-2 neutropenia. In this case, the p-value was statistically significant (0.040). Grade-1 anaemia was present in 5(16.67%) patients and 6(20%) patients from Arm A and Arm B respectively. Grade-1 thrombocytopenia was present in 5(16.67%) patients and 8(26.67%) patients from Arm A and Arm B respectively. 1(3.33%) patient in Arm A and 4(13.33%) patients in Arm B experienced Grade-2 thrombocytopenia respectively. But the p-value of this instance was not statistically significant (p>0.05) (Table- 4). Dissimilarities of haematological toxicities at 3rd follow-up are displaying in Table- 4. Grade-1 leucopenia was noted only 1(3.33%) patient in Arm A and 3(10%) patients in Arm B. Grade-1 neutropenia was present only 2(6.67%) patients in Arm B. Grade-1 anemia and thrombocytopenia were equally present only 1(3.33%) patient in Arm B at this follow up. No significant difference was seen in this occurrence (p>0.05) (Table- 4).
|
Haematological toxicities |
Arm- A, (n=30) |
Arm- B, (n=30) |
p- value |
|
No. (%) |
No. (%) |
||
|
At First follow up (at 2nd week) |
|||
|
Leucopenia |
|||
|
Grade-1 |
6(20) |
8(26.67) |
0.24 ns |
|
Grade-2 |
0(0) |
2(6.67) |
|
|
Neutropenia |
|||
|
Grade-1 |
6(20) |
7(23.34) |
0.215ns |
|
Grade-2 |
0(0) |
2(6.67) |
|
|
Anemia |
|||
|
Grade-1 |
5(16.67) |
6(20) |
0.38 ns |
|
Grade-2 |
0(0) |
1(3.33) |
|
|
Thrombocytopenia |
|||
|
Grade-1 |
3(10) |
5(16.67) |
0.45ns |
|
Grade-2 |
0(0) |
1(3.33) |
|
|
At second follow-up (at 5th week) |
|||
|
Leucopenia |
|||
|
Grade-1 |
7(23.33) |
7(23.33) |
0.047s |
|
Grade-2 |
0(0) |
5(16.67) |
|
|
Neutropenia |
|||
|
Grade-1 |
7(23.34) |
8(26.67) |
0.040s |
|
Grade-2 |
0(0) |
6(20) |
|
|
Anemia |
|||
|
Grade-1 |
5(16.67) |
6(20) |
0.224ns |
|
Grade-2 |
0(0) |
2(6.67) |
|
|
Thrombocytopenia |
|||
|
Grade-1 |
5(16.67) |
8(26.67) |
0.46ns |
|
Grade-2 |
1(3.33) |
4(13.33) |
|
|
At third follow-up (at 4th week after completion of CCRT) |
|||
|
Leucopenia |
|||
|
Grade-1 |
1(3.33) |
3(10) |
0.30ns |
|
Neutropenia |
|||
|
Grade-1 |
0(0) |
2(6.67) |
0.15ns |
|
Anemia |
|||
|
Grade-1 |
0(0) |
1(3.33) |
0.313ns |
|
Thrombocytopenia |
|||
|
Grade-1 |
0(0) |
1(3.34) |
0.313ns |
Arm A= nCRT with Tab. Capecitabine, Arm B= nCRT with Inj.5-FU/ Inj.LVR, Figures in the parentheses indicate the corresponding percentage, Chi-squared Test (x2) was done to analyze the data, s= significant, ns= not significant
Table 4: Comparison of haematological toxicities at different follow ups between two Arms (N= 60)
Comparison of gastrointestinal toxicities at 1st follow-up is presented in Table- 5. Among the study patients; 8(26.67%) patients in Arm A and 6(20%) patients in Arm B experienced Grade-1 nausea, while 12(40%) patients in Arm A and 8(26.67%) patients in Arm B showed Grade-1 vomiting. Grade-1 diarrhea was found among 15(50%) patients in Arm A and 8(26.67%) patients in Arm B respectively. Grade-2 diarrhea was found among 4(13.33) patients in Arm A. There was no significant difference between the groups (p>0.05) (Table- 5). Comparison of gastrointestinal toxicities at 2nd follow-up is presented in Table- 5. Among total study patients; 7(23.34%) patients in Arm A and 5(16.67%) patients in Arm B experienced Grade-1 nausea (p= 0.52). 8(26.67%) patients in Arm A and 6(20%) patients in Arm B experienced Grade-1 vomiting, while 7(23.34%) patients experienced Grade-2 vomiting in Arm A. In this case, the p-value was significant (p= 0.040). On the other hand; 14(46.67%) patients in Arm A and 8(26.67%) patients in Arm B presented Grade-1diarrhea, while 12(40%) patients in Arm B presented Grade-2 diarrhea. Here p-value was statistically significant (p= 0.017) (Table- 5). Comparison of gastrointestinal toxicities at 3rd follow-up is presented in Table- 5. Grade-1 nausea was present in only 2(6.67%) patients in Arm A. Grade-1 vomiting was present in only 3(10%) patients in Arm A. While 5(16.67%) patients in Arm A and 1(3.34%) patient in Arm B experienced Grade-1 diarrhea. There was no significant difference between the groups (p>0.05) (Table- 5).
|
Gastrointestinal toxicities |
Arm- A, (n=30) No. (%) |
Arm- B, (n=30) No. (%) |
p- value |
|
At First follow up (at 2nd week) |
|||
|
Nausea |
|||
|
Grade-1 |
8(26.67) |
6(20) |
0.54 ns |
|
Vomiting |
|||
|
Grade-1 |
12(40) |
8(26.67) |
0.27 ns |
|
Diarrhea |
|||
|
Grade-1 |
15(50) |
8(26.67) |
0.16 ns |
|
Grade-2 |
4(13.33) |
0(0) |
|
|
At second follow-up (at 5th week) |
|||
|
Nausea |
|||
|
Grade-1 |
7(23.34) |
5(16.67) |
0.52ns |
|
Vomiting |
|||
|
Grade-1 |
8(26.67) |
6(20) |
0.040s |
|
Grade-2 |
7(23.34) |
0(0) |
|
|
Diarrhea |
|||
|
Grade-1 |
14(46.67) |
8(26.67) |
0.017s |
|
Grade-2 |
12(40) |
0(0) |
|
|
At third follow-up (at 4th week after completion of CCRT) |
|||
|
Nausea |
|||
|
Grade-1 |
2(6.67) |
0(0) |
0.15ns |
|
Vomiting |
|||
|
Grade-1 |
3(10) |
0(0) |
0.076ns |
|
Diarrhea |
|||
|
Grade-1 |
5(16.67) |
1(3.34) |
0.085ns |
Arm A= nCRT with Tab. Capecitabine, Arm B= nCRT with Inj.5-FU/ Inj.LVR, Figures in the parentheses indicate the corresponding percentage, Chi-squared Test (x2) was done to analyze the data, s= significant ns= not significant
Table 5: Comparison of gastrointestinal toxicities at different follow-ups between two Arms (N= 60)
A comparison of the hand-foot syndrome among the study patients is presented in Table- 6. In 1st follow-up (at 2nd week) only 4(13.33%) patients from Arm A experienced Grade-1 toxicity (p= 0.04). In the 2nd follow-up (at 5th week) 5(16.67%) patients in Arm A and 2(6.67%) patients in Arm B experienced Grade-1 toxicity, while 13(43.34%) patients in Arm A experienced Grade-2 toxicity. In these 2 follow-ups, p-values were statistically significant in this instances (p= 0.04 and p= 0.042 respectively) (Table- 6). But at 3rd follow up only 2(6.67%) patients in Arm A experienced Grade- 1 toxicity, which was not significant between the groups (p= 0.15) (Table- 6).
|
Hand-foot syndrome |
Arm- A, (n=30) No. (%) |
Arm- B, (n=30) No. (%) |
p- value |
|
1st Follow-up (at 2nd week) |
|||
|
Grade-1 |
4(13.33) |
0(0) |
0.04s |
|
2nd Follow-up (at 5th week) |
|||
|
Grade-1 |
5(16.67) |
2(6.67) |
0.042s |
|
Grade-2 |
13(43.34) |
0(0) |
|
|
3rd Follow-up (after completion of CCRT) |
|||
|
Grade-1 |
2(6.67) |
0(0) |
0.15ns |
Arm A= nCRT with Tab. Capecitabine, Arm B= nCRT with Inj.5-FU/ Inj.LVR, Figures in the parentheses indicate the corresponding percentage, Chi-squared Test (x2) was done to analyze the data, s= significant, ns= not significant
Table 6: Comparison of hand-foot syndrome between two Arms (N= 60)
A comparison of other toxicities is presented in Table- 7. At 1st follow-up; 7(23.33%) patients in Arm A and 10(33.33%) patients in Arm B experienced Grade-1 oral mucositis (p= 0.39) (Table- 7). At this time; 6(20) patients in Arm A and 4(13.33%) patients in Arm B presented Grade-1 dermatitis (p= 0.56) (Table- 7). Same follow up; 12(40%) patients in Arm A and 14(46.67%) patients in Arm B experienced Grade-1 proctitis, but 5(16.67%) patients in Arm A and 7(23.33%) patients in Arm B experienced Grade-2 proctitis (p= 0.87) (Table- 7). While only 9(30%) patients in Arm A and 13(43.33%) patients in Arm B demonstrated Grade-1 genitourinary toxicity at this follow up (p= 0.28) (Table- 7). Comparison of other toxicities at 2nd follow-up is presented in Table- 7. At this follow up; 8(26.67%) patients in Arm A and 10(33.3%) patients in Arm B experienced Grade-1 oral mucositis, whereas only 6(20%) patients in Arm B experienced Grade-2 oral mucositis, (p=0.045) (Table- 7). At this time; 12(40%) patients in Arm A and 9(30%) patients in Arm B experienced Grade-1 dermatitis, while the same number of patients [3(10%)] experienced Grade-2 dermatitis in both arms (p= 0.076) (Table- 7). Grade-1 Proctitis was present in 14(46.67%) patients in Arm A and 17(56.67%) patients in Arm B, while Grade-2 proctitis was present among 9(30%) and 10(33.33%) patients in Arm A and Arm B respectively (p= 0.88) (Table- 7). On the other hand; 12(40%) patients in Arm A and 10(33.34%) patients in Arm B experienced Grade-1 genitourinary toxicity at this follow up (p= 0.59) (Table- 7). Comparison of other toxicities at 3rd follow-up of the study patients is displaying in Table- 7. At this time; Grade-1 oral mucositis was experienced only 3(10%) patients in Arm B. Only 3(10%) patients in Arm A and 2(6.67%) patients in Arm B experienced Grade-1 dermatitis. 2(6.67%) patients in Arm A and 3(10%) patients in Arm B experienced Grade-1 proctitis, while only 2(6.67%) patients in Arm A manifested Grade-1 genitourinary toxicity (p>0.05) (Table- 7).
|
Other toxicities |
Arm- A, (n=30) No. (%) |
Arm- B, (n=30) No. (%) |
p- value |
|
At 1st Follow-up (at 2nd week) |
|||
|
Oral Mucositis |
|||
|
Grade-1 |
7(23.33) |
10(33.33) |
0.39 ns |
|
Dermatitis |
|||
|
Grade-1 |
6(20) |
4(13.33) |
0.56 ns |
|
Proctitis |
|||
|
Grade-1 |
12(40) |
14(46.67) |
0.87 ns |
|
Grade-2 |
5(16.67) |
7(23.33) |
|
|
Genitourinary toxicity |
|||
|
Grade-1 |
9(30) |
13(43.33) |
0.28 ns |
|
At 2nd Follow-up (at 5th week) |
|||
|
Oral Mucositis |
|||
|
Grade-1 |
8(26.67) |
10(33.33) |
0.045s |
|
Grade-2 |
0(0) |
6(20) |
|
|
Dermatitis |
|||
|
Grade-1 |
12(40) |
9(30) |
0.076ns |
|
Grade-2 |
3(10) |
3(10) |
|
|
Proctitis |
|||
|
Grade-1 |
14(46.67) |
17(56.67) |
0.88ns |
|
Grade-2 |
9(30) |
10(33.34) |
|
|
Genitourinary toxicity |
|||
|
Grade-1 |
12(40) |
10(33.34) |
0.59ns |
|
At 3rd Follow-up (after completion of CCRT) |
|||
|
Oral mucositis |
|||
|
Grade-1 |
0(0) |
3(10) |
0.075ns |
|
Dermatitis |
|||
|
Grade-1 |
3(10) |
2(6.67) |
0.64ns |
|
Proctitis |
|||
|
Grade-1 |
2(6.67) |
3(10) |
0.64ns |
|
Genitourinary toxicity |
|||
|
Grade-1 |
2(6.67) |
0(0) |
0.15ns |
Arm A= nCRT with Tab. Capecitabine, Arm B= nCRT with Inj.5-FU/ Inj.LVR, Figures in the parentheses indicate the corresponding percentage, Chi-squared Test (x2) was done to analyze the data, ns= not significant
Table 7: Comparison of other toxicities between two Arms (N= 60)
Comparison of clinical responses that revealed by doing Magnetic Resonance Imaging (MRI), colonoscopy and per rectal digital examination at 4th week after the completion of CCRT is shown in the table- 8. At this time; 10(33.33%) patients from Arm A and 8(26.67%) patients from Arm B showed complete response. The equal number of patients [14(46.67%)] from Arm A and Arm B showed partial response. Stable disease existed in 3(10%) and 5(16.67%) patients in Arm A and Arm B respectively. The same number of patients [3(10%)] in both Arms experienced a progressive disease. There was no statistically significant differences was observed in this series (p= 0.868) (Table- 8).
|
Clinical Response |
Arm- A, (n=30) No. (%) |
Arm- B, (n=30) No. (%) |
p- value |
|
Complete Response |
10(33.33) |
8(26.67) |
0.868ns |
|
Partial Response |
14(46.67) |
14(46.67) |
|
|
Stable Diseases |
3(10) |
5(16.67) |
|
|
Progressive Diseases |
3(10) |
3(10) |
|
|
Total |
30(100) |
30(100) |
Arm A= nCRT with Tab. Capecitabine, Arm B=nCRT with Inj.5-FU/ Inj.LVR, Figures in the parentheses indicate the corresponding percentage, Chi-squared Test (x2) was done to analyze the data, ns= not significant
Table 8: Comparison of clinical response (at 4th week after the completion of CCRT) between two Arms (N=60)
Table- 9 depicts the surgical outcome of both Arms (Arm A and Arm B). Among the 60 patients; 22(73.34%) patients from Arm A became surgically operable and 20(66.67%) patients from Arm B became surgically operable. No significant difference was observed in this context (p= 0.57) (Table- 9).
|
Operability |
Arm- A (n=30) No. (%) |
Arm- B, (n=30) No. (%) |
p- value |
|
Operable Case |
22(73.34) |
20(66.67) |
0.57ns |
|
Inoperable Case |
8(26.67) |
10(33.34) |
|
|
Total |
30(100) |
30(100) |
Arm A= nCRT with Tab. Capecitabine, Arm B= nCRT with Inj.5-FU/ Inj.LVR, Figures in the parentheses indicate the corresponding percentage, Chi-squared Test (x2) was done to analyze the data, ns= not significant
Table 9: Comparison of operability between two Arms (N=60)
Table-10 disclosed the incidence of sphincter sparing surgery among the operable patients (n= 42). It was found that, 13(59.1%) patients out of 22 operable patients from Arm A were undergone sphincter spare surgery and 11(55%) patients out of 20 operable patients from Arm B had sphincter spare surgery (p= 0.79) (Table- 10).
|
Sphincter sparing Surgery |
Arm-A, (n=22) No. (%) |
Arm-B, (n=20) No. (%) |
p- value |
|
Yes |
13(59.1) |
11(55) |
0.79 ns |
|
No |
9(40.91) |
9(45) |
|
|
Total |
22(100) |
20(100) |
Arm A= nCRT with Tab. Capecitabine, Arm B= nCRT with Inj.5-FU/ Inj.LVR, Figures in the parentheses indicate the corresponding percentage, Chi-squared Test (x2) was done to analyze the data, ns= not significant
Table 10: Comparison of sphincter sparing surgery between two Arms (n=42)
4. Discussion
The current study was aimed to evaluate the therapeutic gain obtained either by neo-adjuvant concurrent chemoradiotherapy (nCRT) with Capecitabine or Mayo-clinic regimen for locally advanced rectal cancer. The study was conducted at the Department of Radiotherapy, Dhaka Medical College Hospital, Dhaka, Bangladesh. Histologically proven 60 patients with locally advanced rectal cancer (adenocarcinoma: stage IIC-IIIC) were enrolled accordingly. All patients got External Beam Radiotherapy (EBRT) to the pelvis a dose of 50 Gy in 25 fractions. Out of which 30 patients were in arm ‘A’ treated with concurrent Tab. Capecitabine 825 mg/m2 twice daily during treatment and 30 patients were in arm ‘B’ treated with concurrent Mayo-clinic regimen [Inj. 5-Fluorouracil (5-FU)/Inj. Leucovorin (LVR)] in the first and fifth week of radiotherapy. Mean(±SD) age of the patients in Arm A was 46.20 (±13.35) years and that was 41.80(±12.55) years in Arm B. A good number of patients we found in both Arms around 30 years. Age has a bigger impact on the incidence of colorectal cancer (CRC) than demographic factors [3]. It was reported that sporadic CRC increases dramatically above the age of 45 to 50 years, which was close to our study [3]. It is estimated that about 20%-30% of colorectal cancers are compatible with an inherited predisposition, independent of age and known syndromes [10]. In this study, majority of patients were male. The percentage of male patients in Arm A and Arm B was 63.33% and 56.67% respectively, whereas the percentage of female patients was 36.67% in Arm A and 43.33% in Arm B. The male to female ratio was 1.5:1 indicating a male predominance, which was consistent with previous reports [3, 5]. In Arm A, 66.67% of study patients and in Arm B 53.33% of study patients were smokers and they were mostly men, but there was no significant difference among the two arms (p=0.29). This result was supported by a similar previous study which indicates smoking is a known risk factor for rectal cancer [5]. Although we selected the locally advanced rectal cancer for our study, most of the patients in both arms were in stage III- B (15 patients in Arm A and 14 patients in Arm B). On the other hand, 7 patients in Arm A and 8 patients in Arm B were in stage III A. While, 4 patients in Arm A were in stage IIC and another 4 patients in Arm A were in stage IIIC, but 5 patients and 3 patients from Arm B were in these stages respectively. There was no statistical significant difference in the staging between the groups (p=0.949). Most of the tumor grade was found moderately differentiated; 66.67% and 73.33% in Arm A and Arm B respectively. Poorly differentiated was more common in Arm A (23.33%) than Arm B (13.33%). No significant difference in grading between the groups was observed (p=0.59). These findings were consistent with a couple of previous study [6, 7]. Regarding haematological toxicities, at 1st follow-up, Grade-1 leukopenia was present in 6(20%) patients in Arm A and 8(26.67%) patients in Arm B. In the 2nd follow-up approximately same number of patients showed Grade-1 toxicity, which was 7(23.33%) in both Arms. At the same follow-up, Grade-2 leukopenia presented by only 5 patients in Arm B (p= 0.047). Grade-1 neutropenia was present in 6(20.0%) patients in Arm A and 7(23.33%) patients in Arm B at 1st follow-up. At 2nd follow-up; 7(23.33%) patients from Arm A and 8(26.67%) patients from Arm B experienced Grade-1 neutropenia. Only 6(20%) patients from Arm B experienced Grade-2 neutropenia at the same follow-up (p= 0.040). We have found that some studies revealed Grade-3 or Grade-4 haematological toxicities were more prevalent in 5-FU/LVR group [18, 19]. In this study we also found that Grade-2 haematological toxicity was more common in Arm B. Regarding anaemia; 5(16.67%) patients from Arm A and 6(20%) patients from Arm B experienced Grade-1 anemia in both 1st and 2nd follow-up. Only 2 patients from Arm B experienced Grade-2 anemia during 2nd follow-up. But in the 3rd follow-up; haematological toxicities reduced markedly. Grade-1 leucopenia was found among 1(3.33%) patient and 3(10.00%) patients in Arm A and Arm B respectively, while Grade-1 neotropenia [2(6.67%) patients], anemia [1(3.33%) patient] and thrombocytopenia [1(3.33%) patient] were experienced only in Arm B. These findings were supported by previous studies [18, 19]. Analyzing gastrointestinal toxicity; it was observed that at 1st follow-up almost half of the patients in Arm A showed different gastrointestinal toxicities. We found, 8(26.67%) patients had Grade-1 nausea and 12(40%) patients had Grade-1 vomiting. 15(50%) patients from Arm A showed Grade-1 diarrhea and only 4(13.33%) patients from the same Arm showed Grade-2 diarrhea respectively. In Arm B all the three (3) gastrointestinal toxicities (nausea, vomiting, diarrhea) were comparatively low than Arm A (p>0.05) at 1st follow-up which done on 2nd week during treatment. At the 2nd follow-up done at 5th week during treatment, we found 7(23.34%) patients and 5(16.67%) patients had Grade- 1 nausea in Arm A and Arm B respectively (p= 0.52), while 8(26.67%) patients and 7(23.33%) patients from Arm A showed Grade-1 vomiting and Grade- 2 vomiting respectively, whereas 6(20%) patients from Arm B showed only Grade-1 vomiting (p= 0.040). In the case of diarrhea, it was observed that diarrhea became more frequent in Arm A; we found 14(46.67%) patients and 12(40%) patients had Grade-1 and Grade-2 diarrhea respectively but only 8(26.67%) patients from Arm B had Grade-1 diarrhea at the same follow-up (p= 0.017). In 3rd follow-up at 4th week after completion of CCRT, it was observed that nausea, vomiting and diarrhea became more frequent in Arm A (p>0.05). All gastrointestinal toxicities were in tolerable condition among the study patients and this finding was an agreement of a related previous study [20]. Next to gastrointestinal toxicity, hand-foot syndrome was more common in Arm A during all follow-ups. At 1st follow-up 4(13.33%) patients of Arm A experienced Grade-1 hand-foot syndrome, whereas at 2nd follow-up 5(16.67%) and 13(43.33%) patients from Arm A experienced Grade-1 and Grade-2 hand-foot syndrome respectively, but 2(6.67%) patients from Arm B experienced Grade-1 hand-foot syndrome at the same follow-up. On the other hand only 2(6.67%) patients from Arm A experienced Grade-1 hand-foot syndrome at the 3rd follow-up (p<0.05). In this context, Kim JS et al. reported that hand-foot syndrome occurred more frequently in the Capecitabine group that was relevant to our study [21]. Regarding other non-haematological toxicities, oral mucositis was more common in Arm B at all three follow-ups. At 2nd follow-up it was more prominent; where 10(33.33%) patients and 6(20%) patients from Arm B experienced Grade-1 and Grade-2 oral mucositis respectively, while 8(26.67%) patients from Arm A experienced Grade-1 oral mucositis at 2nd follow-up (p= 0.40). Grade-1 oral mucositis was experienced in only 3(10%) patients in Arm B at the 3rd follow-up (p= 0.07). Both proctitis and dermatitis were more prominent at 2nd follow-up; 14(46.67%) patients and 9(30%) patients from Arm A, 17(56.67%) patients and 10(33.34%) patients from Arm B experienced Grade-1 and Grade-2 proctitis respectively. On the other hand, 12(40%) patients from Arm A and 9(30%) patients from Arm B experienced Grade-1 dermatitis whereas the same number of patients [3(10%)] from both Arms experienced Grade-2 dermatitis at the same follow-up. At the 3rd follow-up; 3(10%) patients from Arm A and 2(6.67%) patients from Arm B experienced Grade-1 dermatitis, but 2(6.67%) patients from Arm A and 3(10%) patients from Arm B experienced Grade-1 proctitis. It was observed that Grade-1 genitourinary toxicities (frequency of urination, dysuria, haematuria etc.) developed in both Arms at 1st follow-up (p>0.05); 9(30%) patients in Arm A and 13(43.33%) patients in Arm B respectively. At 2nd follow-up, the same type of Grade-1 toxicities developed 12(40%) patients in Arm A and 10(33.33%) patients in Arm B (p>0.05). At 3rd follow-up these toxicities reduced noticeably; only 2(6.67%) patients from Arm A manifested Grade-1 genitourinary toxicity (p>0.05). These results were in line of a couple of previous study [22, 23]. At the 4th week after the completion of CCRT, Magnetic Resonance Imaging (MRI) was done in each study patients. With the help of this imaging study and also by doing per rectal digital examination and colonoscopy we found that, out of 60 patients 18(60%) patients had a complete response. An identical number [14(46.67%)] of patients in both Arms had a partial response. In this context, there was no significant difference in both Arms (p>0.05). Among the study patients, 22(73.33%) patients of Arm A and 20(66.67%) patients of Arm B became operable. Our aim was to preserve the sphincter in all study patients. Among the operable cases; 13(59.1%) patients from Arm A and 11(55%) patients from Arm B had sphincter sparing surgery, among others sphincter was not possible to spare. Singh K. et al. reported that among 5 patients who had sphincter-saving surgery 3 were in Cepacitabine Arm and 2 were in 5-FU Arm, which was close to our study [22]. This is confirmed by the results of many trials that CCRT with Capecitabine is as effective as the 5-FU/LVR in locally advanced rectal cancer [22, 23]. In this present study, patients treated with Cepacitabine were safe and showed an acceptable and manageable toxicity rate.
5. Conclusion
The current study concluded that, there is no significant difference between concurrent chemoradiation with tab. Capecitabine versus Mayo-clinic regimen. There is no difference in response rates between two regimens. There are minimal toxicities in Capecitabine group which are tolerable and manageable. So, concurrent chemoradiation with tab. Capecitabine seems to be an alternative option in the treatment of locally advanced rectal cancer both in terms of tumor response and toxicity.
Limitations of the Study
It was a single centre study with a relatively small sample size. As our institution has amenity of only conventional 2D (Two Dimension) technique so radiotherapy was given by 2D technique. Survival analysis was not done in this current study.
Recommendations
As Capecitabine is effective, less toxic and safe in short term loco-regional control, so large scale multi-centre studies with longer duration are needed to confirm the results of this study.
Conflicts of interest
The authors declared that they have no conflicts of interest regarding this publication.
References
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 66 (2017):683-691.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 71 (2021):209-249.
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. PrzGastroenterol 14 (2019): 89-103.
- Lizarbe MA, Calle-Espinosa J, Fernández-Lizarbe E,et al. Colorectal cancer: From the genetic model to posttranscriptional regulation by noncoding RNAs. BioMed research international (2017).
- Li Y, Wang J, Ma X, et al. A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. International journal of biological sciences 12 (2016):1022.
- Fleming M, Ravula S, Tatishchev SF, et al. Colorectal carcinoma: pathological aspect. J GastrointestOncol 3 (2012):153-173.
- Hamilton SR. Carcinoma of the colon and rectum. Pathology and genetics of tumours of the digestive system (2000):103-143.
- Ferrari L, Fichera A. Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterology Report 3 (2015):277-288.
- Fröbe A, MaricBrozic J, Soldic Z, et al. Neoadjuvant radiotherapy and chemotherapy in localy advanced rectal cancer: prospective, randomized phase III study, in colaboration with IAEA, ESTRO and UHC “Sestremilosrdnice. Rad Hrvatskeakademijeznanosti i umjetnosti. Medicinskeznanosti (2017):89-101.
- National comprehensive cancer network, rectal cancer (Version 4.2020).
- Ghafouri-Fard S, Abak A, TondroAnamag F, et al. 5-Fluorouracil: a narrative review on the role of regulatory mechanisms in driving resistance to this chemotherapeutic agent. Frontiers in Oncology(2021):1210.
- Tomiak A, Vincent M, Kocha W, et al. Standard dose (Mayo regimen) 5-fluorouracil and low dose folinic acid: prohibitive toxicity?. American journal of clinical oncology 23 (2000):94-98.
- Arnold D, Schmoll HJ. (Neo-) adjuvant treatments in colorectal cancer. Annals of oncology 16 (2005):ii133-40.
- Comella P. A review of the role of capecitabine in the treatment of colorectal cancer. Therapeutics and clinical risk management 3 (2007):421.
- de Wilt JH, Vermaas M, Ferenschild FT, et al. Management of locally advanced primary and recurrent rectal cancer. Clinics in colon and rectal surgery 20 (2007):255-264.
- Ling CC, Gerweck LE, Zaider M, et al. Dose-rate effects in external beam radiotherapy redux. Radiotherapy and Oncology 95 (2010):261-268.
- Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. Journal of Clinical Oncology 2 (1984):187-193.
- Zalcberg J, Kerr D, Seymour L, et al.Tomudex™ International Study Group. Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. European journal of cancer 34 (1998):1871-1875.
- Garg MB, Lincz LF, Adler K, et al. Predicting 5-fluorouracil toxicity in colorectal cancer patients from peripheral blood cell telomere length: a multivariate analysis. British journal of cancer 107 (2012):1525-1533.
- Fang Y, Sheng C, Ding F, et al. Adding Consolidation Capecitabine to Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Propensity-Matched Comparative Study. Frontiers in Surgery 8 (2021).
- Kim JS, Kim JS, Cho MJ, et al. Comparison of the efficacy of oral capecitabine versus bolus 5-FU in preoperative radiotherapy of locally advanced rectal cancer. Journal of Korean Medical Science 21 (2006):52-57.
- Singh K, Gupta MK, Seam RK, et al. A prospective randomized trial comparing capecitabine-based chemoradiotherapy with 5-FU-based chemoradiotherapy in neoadjuvant setting in locally advanced carcinoma rectum. Indian Journal of Cancer 54 (2017):347.
- Zhu J, Zeng W, Ge L, et al. Capecitabine versus 5-fluorouracil in neoadjuvant chemoradiotherapy of locally advanced rectal cancer: A meta-analysis. Medicine 98 (2019).


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 