COVID-19 and Children in Senegal: Epidemiological and Virological Insights
Ndongo Dia*, Moussa Moise Diagne, Gamou Fall, Mamadou Malado Jallow, Amary Fall, Mamadou Alioune Barry, Amadou Diallo, Oumar Faye, Marie Henriette Dior Dione, Ndeye Sakha Bop, Safietou Sankhe, Martin Faye, Idrissa Dieng, Mamadou Diop, Cheikh loucoubar, Christophe Peyreffite, Amadou Alpha Sall, Ousmane Faye
Pasteur Institute Dakar, Dakar, Senegal
*Corresponding author: Ndongo Dia, Virology Pole, Pasteur Institute Dakar, Dakar, Senegal
Received: 05 March 2022; Accepted: 14 March 2022; Published: 12 April 2022
Article Information
Citation: Ndongo Dia, Moussa Moise Diagne, Gamou Fall, Mamadou Malado Jallow, Amary Fall, Mamadou Alioune Barry, Amadou Diallo, Oumar Faye, Marie Henriette Dior Dione, Ndeye Sakha Bop, Safietou Sankhe, Martin Faye, Idrissa Dieng, Mamadou Diop, Cheikh loucoubar, Christophe Peyreffite, Amadou Alpha Sall, Ousmane Faye. COVID-19 and Children in Senegal: Epidemiological and Virological Insights. Archives of Clinical and Biomedical Research 6 (2022): 347-357.
View / Download Pdf Share at FacebookAbstract
Background: Children were less impacted by the COVID-19. Since its first case of COVID-19 on the 2nd of March 2020, Senegal has experienced 3 major epidemic waves with 1886 deaths reported. Here we aim to describe the epidemiology of SARS-COV-2 in Senegal, focusing on the pediatric population in order to appraise the specific virological and epidemic-ological characteristics of COVID-19 in this age stratum.
Methods: From February 2020 to September 2021, 158,076 suspected patients were tested for COVID-19 diagnostic. Demographical information, clinical details and history of exposure were collected from patients by the clinicians. SARS-CoV-2 diagnostic results (positive/negative), Ct values, are entered into an electronic master database (updated daily).
Results: Of these 12,535 (7.9%) were children under 15 years old. 1496 (11.9%) children were confirmed SARS-CoV-2 infected. Dakar was the most impacted region with 754 cases. SARS-CoV-2 positive cases per epidemic wave were distributed as follows: 846 (9.1%; 826/9287) in the 1st wave, 321 (15.8%; 321/ 2031) for the 2nd wave and 329 (27%; 329/1217) in the 3rd wave. We also noted lowest Ct values during the 3rd wave which coincides with the Delta variant emergence and dominance in Senegal. Indeed, the delta variant seems to have more resources for a more effective infection in children, including the very young children.
Conclusion: In addition to a high overall SARS-CoV-2 confirmation rate, this study specially showed that the pediatric population was much more affected during the 3rd epidemic wave with a higher prevalence and also higher viral loads in children.
Keywords
<p>COVID-19; Epidemiology</p>
Article Details
1. Introduction
In December 2019, a novel coronavirus designated as SARS- CoV-2 emerged in the city of Wuhan, China, and caused an outbreak of unusual viral pneumonia. Being highly transmissible, this novel coronavirus disease, also known as coronavirus disease 2019 (COVID-19), has spread fast all over the world [1, 2]. The new emerging virus has overwhelmingly surp-assed SARS and MERS in terms of both the number of infected people and the spatial range of epidemic areas [3]. On 11 March 2020, the WHO officially characterized the global COVID-19 outbreak as a pandemic [4]. As of 1 December 2021, the COVID-19 pandemic has generated millions of hospitaliza-tions and deaths worldwide (5 215 745 deaths, reported to WHO). Regarding ages, the overall finding was that children rarely develop a severe clinical presentation of SARS-COV2 infection and
are less prone to transmit the virus than adults [5-7].
For example in USA, the most COVID-19 impacted country, as of December 9 2021, among states reporting, children ranged from 1.7%-4.0% of their total cumulated hospitalizations, and 0.1%, and 0.00%-0.27% of all COVID-19 deaths, and 6 states reported zero child deaths [8]. Senegal declared its first case of COVID-19 on the 2nd of March 2020 [9], and since then the country has experienced 3 major epidemic waves with 1886 deaths reported since the beginning of the pandemic. The present retrospective analysis aims to describe the epidemiology of SARS-COV-2 in Senegal, focusing on the pediatric popul-ation in order to appraise the specific virological and epidemiological characteristics of COVID-19 in this age stratum.
2. Materials and Methods
As previously described [9], in Senegal, the COVID-19 surveillance is coordinated by the Ministry of Health (MoH). Nasopharyngeal swabs specimen were collected from symptomatic suspected case or contact persons and placed in universal viral transport medium (Becton Dickinson and company, Italy), stored at 4-8° C and transported to IPD within 24 hours of collection for SAR-SCoV-2-specific real-time RT-PCR detection. Samples were accompanied by a standardized investigation form collecting demographical information, clinical details and history of exposure (history of travel, or contact with a confirmed case) filled by the clinicians. All of these data as well as the SARS-CoV-2 diagnostic results (positive/negative), Ct values, are entered into an electronic master database (updated daily) established at IPD, the Teranga Database.
For our analyses in the present study, data (demogra-
phic, clinic, diagnostic results, Ct values etc…) of all pediatric cases (from 0 to 15 years age) were exacted from Teranga database. The R.4.1.1 tool was used to perform the analyses. Genomic surveillance of SARS-CoV-2 was also set up at IPD. Thus, on a regular basis, depending on the number of SARS-CoV-2 positive for each week, an amount of SARS-CoV-2 positive samples are subjected to an Illumina full genome sequencing in order to determine the genetic characteristics of circulating strains in each period (wild type, variants, variant type).
3. Results
In total, from February 2020 to September 2021, 158,076 suspected patients were tested for COVID-19 diagnostic. Of these 12,535 (7.9%) were children under 15 years old. In this pediatric group age ranged from 1 day to 15 years old, with a mean age of 8.35 years old and a median age of 9 years old. Children between 10-15 years old predominated with 38.8% (4865/12535) followed by children under 5 years old with 32.2% (4041/12535) (Table 1). Regarding SARS-CoV-2 infection, of the 12,535 children tested, 1496 (11.9%) were confirmed with the following distribution: 789 (52.7%) with 10-15 years old, 381 (25.4%) in the 5 to 10 years old, and 326 (21.8%) in the group under 5 years. Dakar, the capital city, was the most impacted with 754 positive cases (12.1%; 754/6236) reported in the four medical districts. The distribution of SARS-CoV-2 positive cases and prevalences per epidemic wave is as follows: 846 (9.1%; 826/9287) in the 1st wave, 321 (15.8%; 321/2031) for the 2nd wave and 329 (27%; 329/1217) in the 3rd wave.
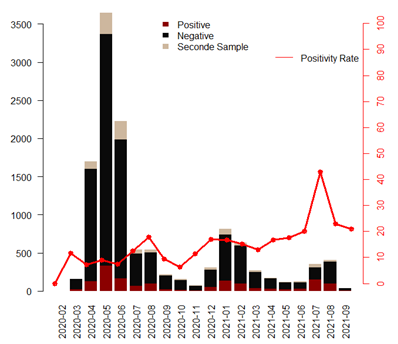
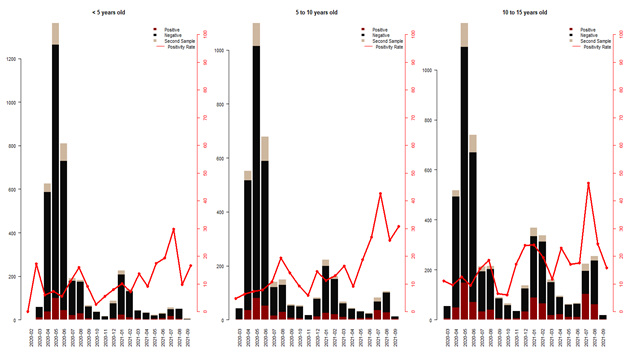
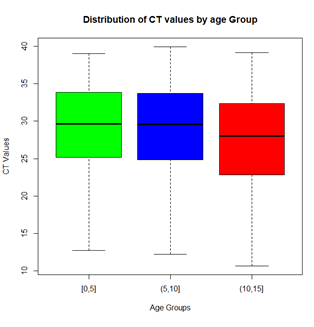
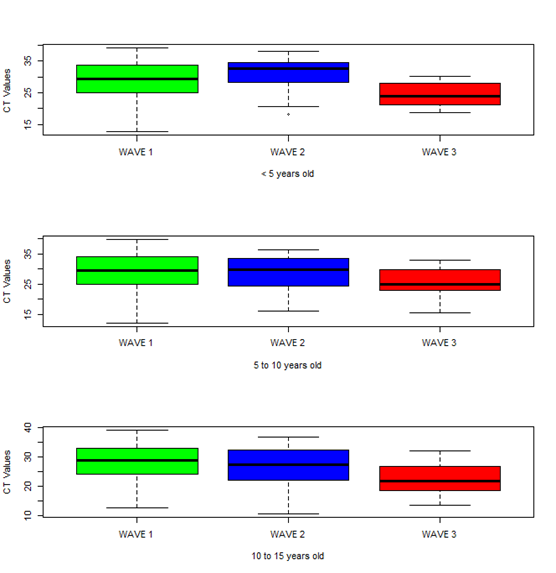
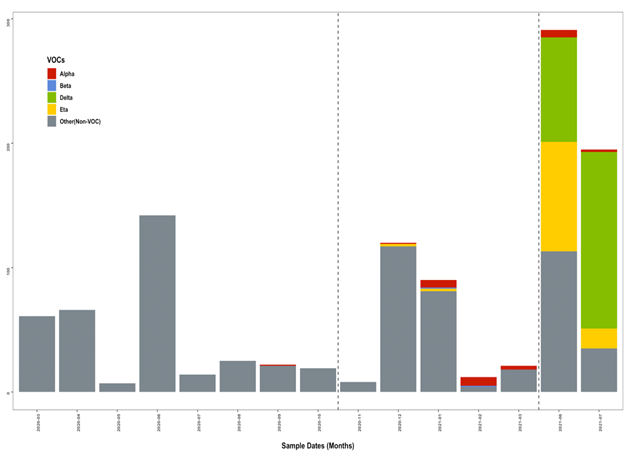
Figure 1 shows the dynamics of prevalence over the months since the beginning of the pandemic in Senegal. We can clearly see that even if the number of children having been sampled for suspected COVID is higher at the beginning of the epidemic, the SARS-CoV-2 positivity rates are much higher during the wave 3, with a peak in July-August 2021, at the height of the 3rd wave. This trend is still visible in the distribution of cases by wave and also by age group. And we see that the impact is slightly more important for children between 10 and 15 years old (Figure 2). We were also interested in Ct values which also reflect viral load in SARS-CoV-2 positive children. The bar plots in Figure 3 show lower Ct values in children aged 10 to 15 years with the bulk of the Ct values between 23 and 32. Children younger than 5 years of age had significantly highest Ct values bulk. We also compared the Ct values in each age group during the 3 epidemic waves (Figure 4). The results show a clear decrease in Ct values during the 3rd wave, and this is valid for all pediatric age groups. The results of genomic surveillance (Figure 5) show the emergence of the delta variant from the week 22 of the year 2021. This variant has progressively taken the lead over the other strains and was the most dominant during the 3rd wave in Senegal.
|
Characteristics |
Covid-19 Suspected cases |
Positive |
Negative |
2nd sample requested |
|
(N=12,535) |
(N=1,496) |
(N=10,014) |
(N=1,025) |
|
|
n(%) |
n(%) |
n(%) |
n(%) |
|
|
Age groups (years) |
||||
|
0 ≤ 5 |
4041 (32.24%) |
326 (22%) |
3403 (34%) |
312 (30%) |
|
5 - 10 |
3629 (28.95%) |
381 (25%) |
2914 (29%) |
334 (33%) |
|
10-1 5 |
4865 (38.81%) |
789 (53%) |
3697 (37%) |
379 (37%) |
|
More impacted district (with more than 50 cases Covid19) |
||||
|
Dakar Centre |
2117 (16.9%) |
284 (19%) |
1548 (15.4%) |
245 (23.9%) |
|
Dakar Nord |
1029 (8.2%) |
122 (8.1%) |
789 (7.9%) |
118 (11.5%) |
|
Dakar Sud |
1107 (8.8%) |
178 (11.9%) |
822 (8.2%) |
107 (10.4%) |
|
Dakar Ouest |
1983 (15.8%) |
330 (22.1%) |
1487 (14.8%) |
166 (16.2%) |
|
Guediawaye |
753 (6%) |
90 (6%) |
595 (5.9%) |
68 (6.6%) |
|
Touba |
542 (4.3%) |
12 (0.8%) |
476 (4.7%) |
54 (5.3%) |
|
Epidemic waves |
||||
|
Wave 1 |
9287 (74.1%) |
846 (9.1%) |
7691 (82.8%) |
750 (8.1%) |
|
Wave 2 |
2031 (16.2%) |
321 (15.8%) |
1543 (75.97%) |
167 (8.2%) |
|
Wave 3 |
1217 (9.7%) |
329 (27%) |
780 (64.1%) |
108 (8.9%) |
Table 1: COVID-19 suspected children, by age group, districts and epidemic wave in Senegal. Sars-CoV-2 positive and negative rates were calculated based on the number of suspected cases tested for each age group, district and wave.
4. Discussion
The present study describes the situation of COVID-19 focusing on epidemiological and virological aspects in the pediatric population in Senegal, a country that has already experienced three COVID-19 epidemic waves since the beginning of the pandemic. From February 2020 to September 2021, we recorded a SARS-CoV-2 confirmation rate of 11.9% in the tested pediatric group. This rate is much higher than that reported by UNICEF [10] where data collected in 106 countries showed a SARS-CoV-2 confirmation rate of less than 1 percent (0.9%) in children under the age of 15 years. However, a similar rate, 12.6% positivity rate for SARS-COV2 infection in tested children, was reported in Mexico city during the first 3 months of the pandemic [11]. And if we consider only Dakar, the capital city of Senegal, we are at a confirmation rate of 12.1%. In the United States that has subsequently emerged as the nation with the highest rates of infection, a confirmation rate of 17.2% was recorded in children during the period from 16 April 2020 and 9 December 2021 [12]. But globally, if we look at what has been reported in several other countries [13, 14], this SARS-CoV-2 confirmation rate in the pediatric population in Senegal still very high. Even in Australia, a country with the highest community rate testing, from 1 Jan 2020 to 15 Jun 2021 children contribute only for 4.6% of the known COVID-19 cases in the community, despite comprising 23% of the population tested for SARS-CoV-2 [15].
In our study we also noted that older children (10 to 15 years old group) represented more than the half of SARS-CoV-2 infection confirmations, which is consistent with many other studies. For instance, a cohort study in China reported attack rates of 2.3% for younger children (aged 0–5 years) and 5.4% for school children and adolescents (aged 6–17 years) [16]. In Singapore, a household study reported 1.3% SARS-CoV-2 infection in the age group 0–4 years old, 8.1% in the 5–9 years group, and 9.8% in the 10–16 years group [17]. A same study with a larger number of patients in Israel concluded that older children or adolescents (aged 5–17 years) were 61% less likely to be infected with SARS-CoV-2 than adults, and children of 0-4 years of age 47% lower than the risk for adults [18]. We also noted a significantly higher SARS-CoV-2 confirmation rate during the third wave, and this was observed in all pediatric age groups. This period coincided with the emergence of the variant delta in the world [19] and also its arrival in Senegal. So during this third wave, the B.1.617.2 (Delta) SARS-CoV-2 variant represented more than 90% of the SARS-CoV-2 strains sequenced in the framework of the genomic surveillance set up at IPD. Indeed, the B.1.617.2 has emerged with an increase in transmissibility [20], likely linked to the higher viral loads noted, shorter time to peak viral load and shorter incubation period [21, 22] compared to non-Delta SARS-CoV-2 strains. It was estimated that the Delta variant is approximately two times more transmissible than the previous variants [23] with a basic reproductive number (R0), of 4.04~5.0 [24]. And as shown by the results obtained in our study, this high transmiss-ibility did not spare children and this for all pediatric groups. In Indonesia a similar trend was reported in a study [25] with an infection rate in the < 18 years old group of 47.8% by the Delta variant and 14.1% by Non-Delta SARS-CoV-2 strains.
Higher viral loads were also recorded during the 3rd wave what is consistent with the characteristics of the delta variant, which tends to produce rapidly high viral loads in patients. These high loads were observed even in the < 5 years children. If it was speculated that SARS-CoV-2 is contained in the upper airways of children by the innate and adaptive immune system to prevent the virus progression to the lower airway [26], it seems that the children's ability to better contain the virus is less efficient with the Delta Variant. However the major limitation of our study is the lack of a well-documented clinical component. Indeed, deaths were reported during the 3rd epidemic wave, and it would be interesting to have hospital data in order to compare these 3 epidemic waves in terms of severe hospitalized cases and also in terms of deaths related to covid-19 in this group.
5. Conclusion
In this study we summarize the virological and epidemiological characteristics of COVID-19 in the pediatric population in Senegal since the first COVID-19 case. In addition to a high overall SARS-CoV-2 confirmation rate, this study specially showed that the pediatric population was much more affected during the 3rd epidemic wave with a higher prevalence and also higher viral loads in children. Indeed, the delta variant, which has been the main strain circulating in this period, seems to have more resources for a more effective infection in children, including the very young children. This situation has also resulted in more severe cases and deaths in this age stratum. At the time of writing, Senegal is experiencing its 4th epidemic wave with the Omicron variant as the majority strain. The first observations show, like the Delta variant, important pediatric cases, high viral loads but very few cases requiring hospitalization in both children and adults. A more detailed comparative analysis including hospital data will be made at the end of this Omicron episode.
Financial Support
None.
Acknowledgments
We would like to acknowledge the Ministry of Health for the COVID-19 surveillance coordination. We convey special thanks to the healthcare workers and also to the IPD COVID-19 diagnostic team for its unwavering investment.
Potential Conflicts of Interest
All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.References
- Wu J T, Leung K, Leung G M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 395 (2020): 689-697.
- David S Hui, Esam I Azhar, Tariq A Madani, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Intl. J. Infect. Dis. 91 (2020): 264-266.
- Fisher D, Heymann D. Q&A: the novel coronavirus outbreak causing COVID-19. BMC Med. 57 (2020).
- World Health Organization. Coronavirus disease 2019 (COVID-19). Situation report 51 (2020).
- Galow L, Haag L, Kahre E, et al. Lower household transmission rates of SARS-CoV-2 from children compared to adults. J Infect 83 (2021): e34-36.
- Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 Infection among Children and Adolescents Compared with Adults: A Systematic Review and Meta-Analysis. JAMA Pediatrics 175 (2021): 143-156.
- Zurl C, Eber E, Siegl A, et al. Low rate of SARSCoV-2 infections in symptomatic patients attending a pediatric emergency department. Front Pediatr 9 (2021): 637167.
- CDC COVID-19 Response Team. Coronavirus disease 2019 in children- United States, February 12-April 2, 2020. MMWR Morb MortalWkly Rep 69 (2020): 422-426.
- Dia N, Lakh NA, Diagne MM, et al. COVID-19 Outbreak, Senegal, 2020. Emerg Infect Dis. 26 (2020): 2772-2774.
- COVID-19 confirmed cases and deaths. Unicef (2022).
- Rivas-Ruiz R, Roy-Garcia IA, Ureña-Wong K, et al. Factors associated with death in children with COVID-19 in Mexico. Gac Med Mex. 156 (2021): 516-522.
- Children and COVID-19: State Data Report. A joint report from the American Academy of Pediatrics and the Children’s Hospital Association. Summary of publicly reported data from 49 states, NYC, DC, PR, and GU. Version (2021).
- Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA 323 (2020): 1335.
- Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatrics 8 (2020): e201346.
- COVID-19 weekly surveillance in NSW (2022).
- Li W, Zhang B, Lu J, et al. Characteristics of Household Transmission of COVID-19. Clin Infect Dis 71 (2020): 1943-1946.
- Yung CF, Kam KQ, Chong CY, et al. Household Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 from Adults to Children. J Pediatr 225 (2020): 249-251.
- Somekh E, Gleyzer A, Heller E, et al. The Role of Children in the Dynamics of Intra Family Coronavirus 2019 Spread in Densely Populated Area. Pediatr Infect Dis J 39 (2020): e202-e204.
- Yang W, Jeffrey S. COVID-19 Pandemic Dynamics in India and Impact of the SARS-CoV-2 Delta (B.1.617.2) Variant. MedRxiv (2021).
- Campbell F, Archer B, Laurenson-Schafer H, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill 26 (2021): 2100509.
- Ong SWX, Chiew CJ, Ang LW, et al. Clinical and Virological Features of SARS-CoV-2 Variants of Concern: A Retrospective Cohort Study Comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta) (2021).
- Li B, Deng A, Li K, et al. Viral infection and transmission in a large well-traced outbreak caused by the Delta SARS-CoV-2 variant (2021).
- Centers for Disease Control and Prevention. Delta Variant (2021).
- Tian D, Sun Y, Zhou J, et al. The Global Epidemic of the SARS-CoV-2 Delta Variant, Key Spike Mutations and Immune Escape. Front Immunol 12 (2021): 751778.
- Gunadi, Hakim MS, Wibawa H, et al. Is the Infection of the SARS-CoV-2 Delta Variant Associated With the Outcomes of COVID-19 Patients?. Front Med (Lausanne) 8 (2021): 780611.
- Matricardi PM, Negro RWD, Nisini R. The first, holistic immunological model of COVID-19: implications for prevention, diagnosis, and public health measures. Pediatr Allergy Immunol 31 (2020): 454-470.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 