Detection of Her 2/Neu Equivocal Immunochemistry by Fish in Breast Carcinoma – A Study from Single Tertiary Care Hospital
Naiding Momota1, Choudhury Biswadeep2, Duttagupta Sumita3, Deuri Biman4, Dasgupta Nivedita5* Deb Saurav6, Sharma Jyotika7
1Professor Department of Pathology, Silchar Medical College and Hospital, India
2Professor and Head Department of Biochemistry, Silchar Medical College and Hospital, India
3 Assistant Professor Department of Pathology, Silchar Medical College and Hospital, India
4Assistant Professor Department of Pathology, Silchar Medical College and Hospital, India
5 Scientist C, Multidisciplinary research Unit, Silchar Medical College and Hospital, India
6Project Technical Officer, ICMR Project under Multidisciplinary research Unit, Silchar Medical College and Hospital, India
7Scientist B, Multidisciplinary research Unit Silchar Medical College and Hospital, India
*Corresponding author: Dr Nivedita Dasgupta, Scientist C, Multidisciplinary research Unit, Silchar Medical College and Hospital, India
Received: 12 August 2023; Accepted: 21 August 2023; Published: 03 October 2023.
Article Information
Citation: Naiding Momota, Choudhury Biswadeep, Duttagupta Sumita, Deuri Biman, Dasgupta Nivedita, Deb Saurav, Sharma Jyotika. Detection of Her 2/Neu Equivocal Immunochemistry by Fish in Breast Carcinoma – A Study from Single Tertiary Care Hospital. Archives of Clinical and Biomedical Research. 7 (2023): 522-526.
View / Download Pdf Share at FacebookAbstract
Background: Both IHC and FISH were used for Human epidermal growth factor receptor-2 status testing but for confirmation of all equivocal specimens having +2 score (Her-2 status) in IHC, the FISH method is used. We report a prospective study to reflect the concordance between IHC and FISH in Her-2 status confirmation.
Methods: Sixty cases of breast carcinoma tissue samples tested by IHC and scored as 0, 1+, 2+, and 3+ and were further analyzed by FISH using a commercially available PathVysion HER-2 probe kit, and the FISH findings of equivocal samples were compared with IHC test results.
Results: A Total of sixty breast carcinoma patients' tissue sections were tested by the Immunohistochemistry method and out of that fifteen samples (9%) were found HER-2 neu positive with +3 Score and Forty samples (24%) were found negative with 0 to +1 score and five samples (3%) were found equivocal I.e., +2 score. The remaining 05 samples were tested by FISH for Her-2 Status confirmation. And all equivocal samples were found negative on the FISH assay.
Conclusion: The Immunohistochemistry test can be used to screen the HER-2 status, and after IHC assay FISH can be used as a confirmatory test for all Her-2 borderline cases. And only those equivocal cases or borderline cases with Her-2 status of IHC 2+ score should be confirmed with the FISH assay.
Keywords
<p>Her-2/neu; Breast carcinoma; Fluorescence in situ hybridization; Immunohistochemistry</p>
Article Details
1. Introduction
Fluorescence in situ hybridization (FISH) is a technique that is developed in the early stages of 1980s. Fluorescence in situ hybridization or the FISH method uses specific fluorescent DNA probes to target specific chromosomal locations within the nucleus, resulting emission of colored signals which is detected by a fluorescent microscope [1].
The conventional cytogenetic (CC) metaphase karyotype analysis when compared with fluorescence in situ hybridization or FISH which doesn't require the modern cell culturing technique. It can directly use the fresh or paraffin-embedded interphase nuclei of the tissue specimen for rapid evaluation. The applications of FISH broadened to include more severe genetic diseases like detection of HER2 amplification in breast carcinoma [2]. FISH acts a great role in detecting breast carcinoma. Breast cancer is a fairly heterogeneous malignancy that involves large numbers of genomic aberrations that are inherited or acquired during the initiation and progression of the disease. The standard protocol of FISH carried out on formalin-fixed paraffin-embedded (FFPE) tissue begins with a selection of the representative population of tumor cells and marks a section for Fluorescence in situ hybridization analysis on a Hematoxylin and Eosin (H&E) stained histopathological tissue specimen. A crucial issue at the preliminary analytical part is the percentage of tumor cells in the tissue specimen, since a low percentage may lead to an uninformative result of Fluorescence in situ hybridization (FISH) scoring and need to repeat the whole procedure, starting from the selection of a new or fresh formalin-fixed paraffin-embedded or FFPE section [3]. Her-2/neu (human epidermal growth factor receptor-2) protein is an active tyrosine kinase that plays a major role in normal cell growth and differentiation. It has been observed that Her-2/neu gene amplification occurs in 20-30% of breast cancer patients. Her-2/neu gene amplification leads to over expression on the cell surface. Its amplification indicates a poor prognosis, short survival time, and the existence of more aggressive phenotype of tumor cells. Her-2/neu over expressed breast cancer may be resistant to endocrine therapy and some chemotherapy. However, it is sensitive to Herceptin treatment and shows more reactive to the drug paclitaxel and anthracyclines [1]. At present both Immunohistochemistry and FISH methods or assays are best used for measuring Her-2/neu over expression for proper breast carcinoma detection. Generally, breast cancer is classified as high-risk carcinoma and low-risk carcinoma based on the patient's tumor size, shape, nodal status, and status of Estrogen receptor or ER. However a study conducted by Hu L et al observed that [1] 15% of patients with low-risk parameters (tumor size <1 cm, low grade, negative lymph node, and positive ER) have recurrent disease and usually die due to spread of cancer cells in other parts of the body (metastasis). Meanwhile, 15% of patients in the high-risk group (tumor size > 5 cm, high grade, positive lymph node-negative ER) have an unexpected favorable clinical outcome. These patients could receive mistreatment if only based on the histopathological findings. Therefore, in present days there is a need to establish advanced molecular laboratories and more accurate molecular testing schemes.
Aims and Objectives- This study was done to compare the results of Immunohistochemistry (IHC) and FISH methods for confirmation of HER-2 status in equivocal or borderline samples.
2. Materials and Methods
2.1 Inclusion criteria
All patients with histologically confirmed infiltrating ductal carcinoma of the breast were included in the study.
2.2 Exclusion criteria
Patients with inflammatory breast lesions, posttraumatic breast lesions, benign breast diseases and patients with breast cancer who received neoadjuvant chemotherapy were excluded from the study. In this retrospective study, we have done the immunohistochemistry (IHC) technique for evaluating the HER2 status or score. The paraffin-embedded tissue sections of breast carcinoma from 60 consecutive patients who had undergone surgery at the Department of Surgery, Silchar Medical College & Hospital (SMCH) between December 2020 - July 2022 were included in the study. Biopsy samples of Breast tissues were collected and processed by Immunohistochemistry (IHC) test, and all the equivocal samples which are having +2 Score in IHC are further processed by the FISH method and confirms an equivocal sample whether it is positive or negative for HER-2. Immunohistochemistry (IHC) of Her-2/neu onco-protein was performed on 4μm thick paraffin-embedded tissue sections placed on coated rosted slides. After deparaffinization and blocking of endogenous peroxidase, Her-2/neu immunostaining was performed using rabbit monoclonal antibody Her-2/neu (SP3) as primary antibody (Cell Marque, made in USA) at 1:100 dilution. The binding of the primary antibody was checked by CRFTM Anti–polyvalent HRP Polyvalent HRP Polymer (DAB) staining kit [4]. HER-2/neu scoring of IHC slides was done on light microscopy as per the guidelines of American Society of Clinical Oncology (ASCO). The immunostaining was read in a semi quantitative manner and graded as follows: 0, 1+, 2+, and 3+. Scores 0 and 1 were considered as HER-2/neu negative expressions while score 2 were considered as equivocal and score 3 were considered as positive expressions for HER- 2/neu [4,5]. Tissue sections that showed strong membrane staining of normal epithelia of the breast were rejected and IHC should be repeated. And IHC Scores of 2+ were considered as equivocal cases, which were further tested for Fluorescent in situ hybridization (FISH) method for Her-2 /neu amplification [4,6].
Fluorescence in situ hybridization (FISH) is now considered as the most accurate and predictive test for Her2/neu gene amplification and response to therapy in breast cancer [7]. The samples which are having IHC score of 2+ in HER2 gene amplification status were determined by using FISH or the fluorescence in situ hybridization method. FISH analysis was performed using the PathVysion HER-2 probe kit. In the kit there were two fluorescent-labeled probes: One is locus-specific identifier or LSI HER-2 specific for the HER-2 gene locus (17q11) and another one is chromosome enumeration probe or CEP 17 specific for the α satellite DNA sequence at the centromeric region of chromosome 17. Paraffin sections of 3-4 mm thickness using a microtome machine were cut and floated in a protein-free water bath at 400C, after that the sections were mounted on poly-L-Lysine coated slides and allowed to dry. The slides were kept in the hot air oven overnight at 560C. The slides were deparaffinized in xylene coplin jar at 370C or room temperature for 20 Minutes and dehydrated in 100 % ethanol for 15 Minutes at 370C or room temperature and the slides should be air dried. After drying the slides were treated with pretreatment solution like sodium thiocyanite is used and protease solution for 15 Minutes and were dehydrated with 70%, 80%, and 100% alcohol for 5 Minutes each and then air dried. The probe was denatured at 800C for 5 Minutes and applied to the slide and cover-slipped, and kept inside a humidified chamber for overnight incubation. After incubation post-hybridization washes were given with 0.4 percent 2xSCC (sodium saline citrate) 40 at room temperature or 370C. After removing the coverslips the slides were dipped in a post-hybridization buffer for 18 sec, then dried completely in dark and 10 μl DAPI was used and a coverslip is gently placed over the slide. The slides were screened under the florescent microscope using appropriate filters such as DAPI, FITC, TRITC dual, and triple bandpass filters. Signals were counted in at least 200 cells for both the HER-2/neu gene signals under oil immersion at 1000X magnification using recommended filters. Results were expressed as the ratio of HER-2/neu signal (orange) and the readings were read as follows: the expected ratio 1-1.8 indicates no gene amplification were considered as negative, a ratio of >2.2 as HER-2/neu gene amplification were considered as Positive, and a ratio between 1.8-2.2 were considered as equivocal cases [7].
2.3 Ethical consideration and clearance:
This study was conducted after obtaining the ethical approval from ethical committee of our institution vide letter No.SMC/13/3420 dated 11/03/2015.
Funding agency: This study is funded by DHR/ICMR.
3. Results
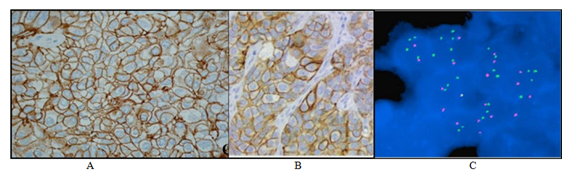
In our study (Table 1), 60 patients with breast carcinoma were taken and their tissue sections were tested by the IHC method, out of that 15 samples (9%) were found HER-2 neu positive (having +3 Score) and 40 samples (24%) were found negative (having 0 to +1 score) and 05 samples (3%) were found equivocal having +2 score as shown in figure 1. These 05 equivocal samples were tested by FISH for confirmation of the Her-2/ neu expression but all samples were found negative on the FISH method, Figure 1 is an image representation of FISH negative Specimen for Her-2/neu gene amplification, where total cell counted is30, Her-2/neu gene is 66, total CEP17 is 64, Her-2 gene mean per cell is 2.2, CEP17 mean per cell are 2.13 and Her-2 gene: CEP17 ratio is 1.03.
Table 1: Total patients’ IHC results.
|
Total patient |
60 |
|
IHC Positive |
15 (9%) |
|
IHC Negative |
40 (24%) |
|
IHC Equivocal |
05 (3%) |
4. Discussion
Fluorescence in situ hybridization commonly called FISH helps in proper diagnosis, prognosis and even predicting response to therapy in breast carcinoma. Using FISH, genetic changes that are linked to tumor response to certain therapies, can be identified. Our study indicated that the equivocal samples should be performed by the FISH method for confirmation of HER-2/neu status in all cases of breast tumors with a 2+ score by IHC. The FISH or fluorescence in situ hybridization test checks the DNA of cancer cells to find out extra copies of the HER-2/neu gene. This HER-2/neu gene is responsible to make a protein that is called as HER-2 or human epidermal growth factor receptor -2 that is used to attach to the surface of breast cells which further lead to breast carcinoma [8]. An Immunohistochemistry (IHC) technique is used to characterize intracellular proteins or antigens in various cell surfaces in all tissues. Individual markers or more often panels of various marker proteins can be used to characterize various tumor subtypes, confirm tissue of origin, distinguish metastatic from primary tumor and provide additional information which may be important for prognosis, predicting response to therapy, or evaluating residual tumor post-treatment [9]. The IHC test gives 0 to 3+ scores that measures the amount of HER2 receptor protein on the cell surface in a breast cancer tissue specimen. If the score is 0 to 1+, it's called HER2 negative. If the score is 2+, it's called a borderline or equivocal sample. A score of 3+ is called HER2 positive in the IHC method. The fluorescence in situ hybridization or FISH test is a more accurate process and helps to confirm the HER2 status in equivocal samples which are having +2 score in immunohistochemistry analysis. Studies by various authors [10,11, 12] suggests that fluorescence in situ hybridization (FISH) has higher accuracy rate than Immunohistochemistry (IHC) and it is a better prognostic indicator in the case of high-risk breast cancers. In clinical laboratories, HER-2/neu status is usually assessed in formalin-fixed and paraffin-embedded specimens using either IHC or FISH. Wang et al [10] achieved a high concordance rate of 98 percent with two FISH assays, one is INFORM (Ventana Medical Systems, Tucson, AZ) and another one is PathVysion (Vysis). All IHC negative cases and nearly all IHC low positive (1+) cases showed no gene amplification and having very low IHC scores, whereas most IHC were high positive (3+) and had more gene amplifications. However, the IHC equivocal or medium or borderline positive having 2+ scores those cases demonstrated significant discordance with the FISH method, i.e. some showed HER-2/neu gene amplification and others did not. Similar discordant results were seen in our 60 patients; 40 patients with a score of 0/1+ by IHC method and 15 patients with a score of 3+ by IHC method were not done for FISH for HER-2/neu gene amplification but 05 patients with a score of 2+ by IHC were done for FISH for HER-2/neu gene amplification and all were found FISH negative. In another study by Goud et al [4], comparing the FISH and IHC methods, Gould et al reported that FISH is increasingly considered to be the most accurate and predictive test for determining HER2 amplification and the response to the treatment of breast cancer. A total of 90 breast cancer tissue samples were used to analyze the FISH and IHC methods. So the HER2 status should be tested using FISH in all cases with IHC 2+ scores. And IHC 3+ results can be analyzed with FISH to differentiate chromosome 17 polysomy, which can be misinterpreted as HER2 over expression in when using the IHC method. In our study, we have 05 equivocal samples which are having +2 IHC scores that are found FISH negative and we did not perform the FISH test for our 15 samples with IHC +3 scores considering it as IHC positive. Based on the results of FISH tests, patients can be qualified for treatment with antibodies partially blocking HER-2 receptors, which inhibit tumor growth signals. However, many laboratories use IHC as the first method of choice. In a study by Timothy W. Jacobs et al [6] author suggested that the fluorescence in situ hybridization or FISH technique required more time than Immunohistochemistry or IHC although the routine use of FISH technique for examination of HER2 /neu status in breast cancer specimens is very expensive the choice of which method used is left for individual laboratories based on the economic consideration. In a study conducted by Negi et al and W. Jacobs et al [6,12], where all the samples were tested by both IHC and FISH, wherein 82 cases out of 90 cases of breast carcinoma which were tested by both FISH & IHC methods and out of that 26% of HER-2 /neu was amplified in FISH method and 23% were in IHC HER-2/ neu positive respectively which is in contrast with our study, where only IHC equivocal samples were analysed by FISH. In our study we did fluorescence in situ hybridization or FISH method for determination of HER-2 /neu status only for the equivocal samples which is having +2 IHC scores, i.e., we have tested 05 equivocal (+2 IHC Score) samples which shows FISH negative. Our results suggests that the equivocal samples of IHC can be done by FISH method for confirmation of HER-2/ neu status..
5. Conclusion:
Due to its sensitivity and specificity, FISH is regarded as the "gold standard test". Immunohistochemistry does not consistently discriminate chromosome 17 polysomy which might be misinterpreted as HER2 over expression, whereas FISH can discriminate chromosome 17 polysomy. HER-2/neu protein over expression in the absence of gene amplification occurs infrequently in breast cancer, early detection of HER2 status is very important for further patient management. So, this study shows that all equivocal samples having 2+ IHC Scores should be performed by the FISH method for confirmation as the FISH is a more accurate and predictive test for Her2/neu gene amplification in breast cancer detection.
Compliance with Ethical Standards:
This work is approved by institutional ethical committee.
Conflict of interest:
The authors have no conflict of interest.
Declaration:
Consent for publication:
All the authors have given their consent to publish the article.
Availibility of data and material:
All the data generated and analysed during this study are included in this published article.
Funding:
This work is funded by DHR (Department of Health Research).
Author’s contribution:
The authors MN, BC, BD, SDG contributed in analysing and interpreting the result. JS, SD contributed in performing the experiment, ND contributed in performing the experiment, analysing the data, writing the manuscript.
Acknowledgement:
This work is funded by DHR (Department of Health Research).
References:
- Hu L, Ru K, Li Zhang, et al. Fluorescence in situ hybridization (FISH): an increasingly demanded tool for biomarker research and personalized medicine. Biomarker Research 2 (2014): 1-13.
- Jiang H, Xue Y, Qinrong W, et al. The utility of fluorescence in situ hybridization analysis in diagnosing myelodysplastic syndromes is limited to cases with karyotype failure 36 (2012): 448-452.
- Chrzanoska NM, Kowalewski J, MA Lewandowska, et al. Use of Fluorescence in Situ Hybridization in diagnosis and tailored therapies in solid Tumors. Molecules 25 (2020): 1-21.
- Choudhury B, Naiding M, et al. Role of Hormone Receptors- Estrogen receptor, Progesterone receptor and Human Epidermal Growth Factor receptor 2/Neu (Her2/Neu) as a Prognostic and Therapeutic tool in Breast Cancer. International Archives of Biomedical and Clinical Research 7 (2021): 10-14.
- Chand P, Garg A, Singla V, et al. Evaluation of immunohistochemical profile of breast cancer for prognostics and therapeutic use. Nigerian Journal of Surgery 45 (2018): 100-106.
- Negi M, Kumar S, Soma D, et al. Profiling estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2/neu in breast carcinoma: Study of 111 consecutive cases. Journal of the Scientific Society 45 (2018): 13-16.
- Gould KI, Dayakar S, Vijayalaxmi K, et al. Evaluation of HER2/neu status in breast cancer specimens using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) assay. Indian Journal of Medical Research 135 (2012): 312-317.
- Nida I, Naveed I. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Molecular Biology International (2014): 1-9.
- Zaha DC. Significance of immunohistochemistry in breast cancer. World Journal of Clinical Oncology 5 (2014): 382-392.
- Wang S, Saboorian MH, E Frenkel, et al. Laboratory assessment of the status of Her-2/neu protein and oncogene in breast cancer specimens: comparison of immunohistochemistry assay with fluorescence in situ hybridization assays. Journal of Clinical Pathology 53 (2000): 374-381.
- Wesola M, Jelen M. A Comparison of IHC and FISH Cytogenetic Methods in the Evaluation of HER2 Status in Breast Cancer. Advances in Clinical and Experimental Medicine 24 (2015): 899-903.
- Jacobs W, Gown MA, H Yaziji, et al. Comparison of Fluorescence In Situ Hybridization and Immunohistochemistry for the Evaluation of HER-2/neu in Breast Cancer. Journal of Clinical Oncology 17 (1999): 1974-1982.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 