Differentiation Stages of Mesenchymal Stromal Cells Change the Osteogenic Induction Capacity of Extracellular Vesicles They Derived
Sharada Paudel1, Lumanti Manandhar2, Tyler Feltham3, Lew Schon4,5, Zijun Zhang5*
1Laboratory of Human Retrovirology and Immunoinformatics, Frederick National Laboratory for Cancer Research, Frederick, MD, USA
2Medstar Health Research Institute, Medstar Union Memorial Hospital, Baltimore, MD, USA
3Department of Emergency, Doctors Hospital, Columbus, OH, USA
4Institute for Foot and Ankle Reconstruction, Mercy Medical Center, Baltimore, MD, USA
5Center for Orthopaedic Innovation, Mercy Medical Center, Baltimore, MD, USA
*Corresponding Author: Zijun Zhang, Center for Orthopaedic Innovation, Mercy Medical Center, Baltimore, MD, USA.
Received: 02 September 2025; Accepted: 12 September 2025; Published: 23 September 2025
Article Information
Citation: Sharada Paudel, Lumanti Manandhar, Tyler Feltham, Lew Schon, Zijun Zhang. Differentiation Stages of Mesenchymal Stromal Cells Change the Osteogenic Induction Capacity of Extracellular Vesicles They Derived. Journal of Orthopedics and Sports Medicine. 7 (2025): 474-478.
View / Download Pdf Share at FacebookAbstract
This study investigated the osteogenic induction potential by extracellular vesicles (EVs) produced by undifferentiated and osteogenically differentiated bone marrow-derived MSCs (BM-MSCs) on adipose-tissue derived MSCs (AT-MSCs). Osteogenic differentiation of BM-MSCs was induced with osteogenic differentiation medium. EVs were isolated from osteogenically differentiated and undifferentiated BM-MSCs (designated as Ev-os and Ev, respectively). AT-MSCs were cultured with: 1) regular medium; 2) osteogenic differentiation medium; 3) osteogenic differentiation medium with Ev; 4) osteogenic differentiation medium with Ev-os. After three weeks of culture, AT-MSCs in regular medium showed no sign of osteogenic differentiation. There were sporadic spots of mineral deposition in the AT-MSCs cultured in osteogenic differentiation medium and osteogenic medium plus Ev. Culturing in osteogenic differentiation medium plus Ev-os, AT-MSCs increased matrix mineralization more than two-fold compared with those cultured in osteogenic medium plus Ev. In conclusion, EVs produced by osteogenically differentiated MSCs are more potent in inducing osteogenic differentiation of MSCs.
Keywords
<p>Mesenchymal stem/stromal cell; Extracellular vesicle; Osteogenic differentiation; Mineralization</p>
Article Details
1. Introduction
Mesenchymal stem/stromal cells (MSCs) leverage paracrine activity, including releasing extracellular vesicles (EVs) or exosomes, for tissue regeneration [1,2]. The particles of EVs, up to100 nm in diameter, are delimited by a lipid bilayer and contain biological active macromolecules. Upon endocytosis, the EV cargo, including proteins, nucleic acids, and glycolipids, can reprogram and change the recipient cell’s phenotype and function [3]. The content of the EVs resulted from a complex sorting process dictates the message of intercellular communication. Cell type and tissue origin of the donor cells influence the contents of the EVs. The EVs produced by MSCs derived from bone marrow, adipose tissue, and umbilical cord contained 341, 23, and 37 unique proteins, respectively [4]. EVs-directed intercellular communications may play a role in showing distinct regenerative features by the MSCs derived from different tissues. Other than tissue origins, the regenerative capacity of MSCs are shaped by their differentiation stages. Osteogenic MSCs undergo phenotypic transformation, along with a changed metabolomic profile: 31 metabolites increased in osteogenic MSCs intracellularly [5,6]. Since EV content is largely made up by cytosolic proteins and other molecules, osteogenic differentiation of MSCs could alter the regulatory signals carried by the EVs [7].
Bone marrow-derived MSCs (BM-MSCs) and adipose tissue-derived MSCs (AT-MSCs) have distinct osteogenic potentials [8,9]. While BM-MSCs are highly osteogenic, AT-MSCs are slow in osteogenic differentiation and relatively low in expression of osteogenic markers. This study factored in the differences of osteogenic differentiation between BM-MSCs and AT-MSCs, and the varied osteogenic induction capacity of the EVs produced by the MSCs at different differentiation stages. In this study, EVs were isolated from both osteogenically differentiated and undifferentiated BM-MSCs and applied to AT-MSCs to compare their effectiveness on osteogenic differentiation induction in vitro.
2. Materials and Methods
This study collected bone marrow and subcutaneous adipose tissue during surgery. The collection of tissue samples for research was approved by MedStar Health Institutional Review Broad (IRB; protocol # 2014-057). The IRB waived patient consent because this study “meets the criteria set forth in [45 CFR 46.101(b), Category (4)] and qualifies for exemption from the requirements of (45 CFR 46) federal regulation”. Bone marrow donors (n = 5) were between 46-68 years of age (average 56 years), including four females and one male. Two subcutaneous adipose tissue donors were a 45-year old female and a 64-year old male.
Isolation of MSCs: BM-MSCs were isolated using a gradient medium and plated as previously described [10]. AT-MSCs were isolated by digestion of adipose tissue in 0.1% collagenase, following a published protocol [11]. BM-MSCs and AT-MSCs were cultured in Dulbecco's Modified Eagle Medium (DMEM, Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS, Rocky Mountain Biologicals, Missoula, MT, USA) and 1% penicillin-streptomycin, at 37°C and 5% carbon dioxide in air. The medium was changed twice a week. The cells were passaged at 70% confluence and used at passages 3-5.
Osteogenic differentiation of BM-MSCs: Cells were seeded into T-175 culture flasks at a density of 1x104/cm2. Osteogenic differentiation medium consisted of DMEM, 10% FBS, 10 mM β-glycerophosphate, 100 nM dexamethasone, 50µg/ml L-ascorbic acid 2-phosphate. BM-MSCs were cultured in osteogenic differentiation medium or regular medium, as a differentiation control, for two weeks. Osteogenic differentiation of BM-MSCs was confirmed by positive Alizarin red staining in sampled flasks.
EV isolation and characterization: The osteogenic and control cultures of BM-MSCs were rinsed and applied with regular culture medium (20mL/T-175 flask), containing 10% EV-depleted FBS. Depletion of EVs from FBS was achieved by centrifugation at 100,000 x g for 18 hours. After 48 hours, the culture medium of osteogenically differentiated and undifferentiated BM-MSCs was collected and undergone sequential centrifugations at 300 x g for 10 minutes, 2,600 x g for 10 minutes and 10,000 x g for 30 minutes, for removal of cells, cellular debris and micro-vesicles, respectively. The supernatant was then centrifuged at 100,000 x g for 2 hours to harvest EVs. The resulted pellet was re-suspended with phosphate buffered saline (PBS), and re-pelleted by centrifugation at 100,000 x g for 2 hours. The isolated EVs were suspended in PBS and kept at 4°C. The yield of EVs was quantified by the amount of proteins, using Pierce™ BCA Protein Assay Kits (Thermo Fisher Scientific).
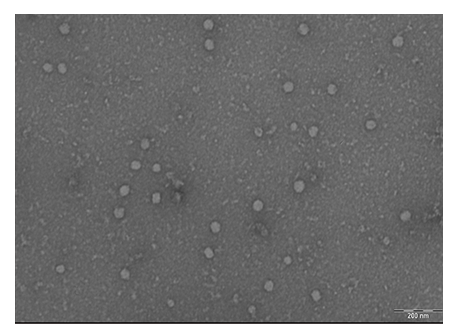
The size distribution of EVs was examined using a transmission electron microscope (TEM, 420, Royal Philips Electronics, The Netherlands). Briefly, EV samples were loaded onto carbon-coated copper grids and negatively stained with 1% uranyl acetate for measuring the diameter of EVs (11-50 EVs per imaging field). Western blots were performed to detect CD63 and flotillin-1, as markers of exosomes, in extracted EV protein samples.
Osteogenic differentiation of AT-MSCs with EVs: AT-MSCs were seeded in 24-well plates (1,000 cells/well). Four types of culture medium were separately applied: 1) regular medium (RM) based on DMEM and 10% EV-free FBS; 2) osteogenic differentiation medium (OM), as defined previously; 3) osteogenic differentiation medium with EVs produced by undifferentiated BM-MSCs (Ev; 5µg/ml); 4) osteogenic differentiation medium with EVs produced by osteogenically differentiated BM-MSCs (Ev-os; 5µg/ml). Each medium condition was set up in duplicate or triplicate on the same plate. The medium was changed twice a week. AT-MSCs cultures were ended at three weeks.
After fixed with 4% paraformaldehyde, the cultures were stained with 40 mM Alizarin Red S (ScienCell Research Laboratories, Carlsbad, CA, USA) for 20 minutes to detect mineral deposition. After evaluation under a microscope, the cultures were treated with 10% acetic acid for 30 minutes with shaking. Collected with a cell scraper, cell slurry in 10% acetic acid was vortexed and heated at 85°C for 10 minutes. After centrifugation, the supernatant was neutralized with 10% ammonium hydroxide and samples in triplicate were measured, along with Alizarin red standards, in a microplate reader at absorbance at 405 nm.
Statistical analysis: The amount of Alizarin red in different culture groups were analyzed with one-way ANOVA, followed by post hoc Tukey’s test, using MedCalc program (MedCalc Software Ltd., Ostend, Belgium). P < 0.05 was set as significant.
3. Results
Three T-175 flasks (6-15x106 cells, average 10.6x106 cells) of osteogenically differentiated or undifferentiated BM-MSCs were used for each batch of EV isolation. Each isolation produced 10.7-30.8 µg (average 21.2 µg) EVs. All EV samples were positive of CD63 and flotllin-1 (data not shown). TEM showed that the isolated EVs, both Ev and Ev-os, were uniformly spherical. The sizes of the EVs ranged between 17 and 52.6 nm (average 32.9 nm, Figure 1).
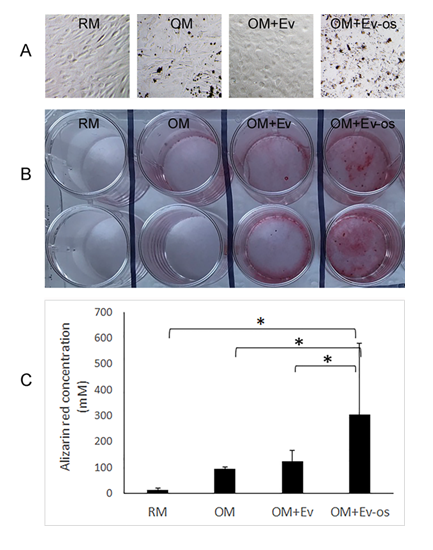
After three weeks of culture, dark mineral nodules appeared in the cultures of AT-MSCs in OM, and OM with Ev and Ev-os (Figure 2A). By Alizarin red staining, AT-MSCs in RM showed no mineral deposition (Figure 2B). There were sporadic spots of mineral deposition in the AT-MSCs cultured in OM and OM with Ev. Osteogenic differentiation was most abundant when AT-MSCs were cultured in OM with Ev-os. By colorimetric quantification, Alizarin red concentration was 12 mM in the AT-MSCs cultured with RM (Figure 2C). It increased to 95 mM when OM was applied, and further increased to 125 mM with the use of OM with Ev. Comparing with the RM group, these increases of Alizarin Red concentration, however, were not statistically significant. The greatest Alizarin red concentration among the four study groups was 304 mM, when OM with Ev-os was applied, which was significantly higher than the other three groups (p < 0.05).
Figure 2: A: Phase-contrast images of cultured AT-MSCs in RM, OM, OM with Ev and OM with Ev-os for three weeks. There are visible mineral nodules in the cultures using OM, and OM with Ev and Ev-os (Bar = 20 µm). B: Alizarin red staining of AT-MSCs after cultured for three weeks. Each culture condition is in duplicate. There is no sign of matrix mineralization in RM (Column 1). In OM, there are minimal but visible matrix mineralization (Column 2). Significant matrix mineralization is shown in the cultures using OM with Ev (Column 3). In the cultures using OM with Ev-os (Column 4), there is abundant mineral deposition. C: Quantification of Alizarin red in AT-MSCs cultured with different media. The concentration of Alizarin red in the cultures using OM with Ev-os is nearly three times of that in the cultures using OM with Ev (* indicates where p < 0.05).
4. Discussion
The tissue origins of MSCs were emphasized in this study. MSCs are isolated from a variety of tissues and the inherited tissue traits affect their differentiation potentials. The general consensus in the literature is that BM-MSCs possess greater osteogenic potential than AT-MSCs, in vitro and in vivo [8,9,12-14]. Additionally, the impurity or heterogeneity of MSCs resulted by the current isolation protocols means the inevitable presence of pre-differentiated progenitors in MSCs. It is not uncommon that BM-MSCs cultured in regular medium express osteogenic marker genes [9,15]. To avoid spontaneous osteogenesis, this study chose AT-MSCs over BM-MSCs as the target of osteogenic induction. AT-MSCs do not naturally mineralize extracellular matrix [8] and would unlikely produce false positive osteogenic differentiation results. Indeed, in this study, AT-MSCs showed no sign of osteogenic differentiation after cultured in RM for three weeks.
To make osteogenic EVs, BM-MSCs were preferred because of their great osteogenic potential. BM-MSCs were cultured in RM or OM and EVs prepared from both osteogenically differentiated and undifferentiated BM-MSCs. These EVs, did not differ in morphology and size. This is in line with the finding that MSCs in different states, such as naïve vs. inflammation-activated, produced similar sizes of EVs [16]. The amounts of EVs produced by osteogenic and undifferentiated BM-MSCs, according to the quantity of proteins, were also not significantly different.
By mismatching the tissue origins of MSCs used to produce EVs and as the target of osteogenic differentiation, this study aimed to harvest EVs that were strongly in osteogenic induction and induce osteogenic differentiation of MSCs that are not naturally osteogenic. Visual and colorimetrical Alizarin red staining showed that AT-MSCs differentiated into osteogenic lineage in OM. This set of data set up a baseline of the osteogenic effect of EVs produced by BM-MSCs on AT-MSCs. EVs produced by MSCs from multiple tissue sources, such as adipose tissue, bone marrow and synovium, promoted osteogenic differentiation of BM-MSCs in various degrees [17]. While the EVs produced by other MSCs might enhance osteogenic differentiation indirectly through promoting cell proliferation, EVs produced by BM-MSCs can directly regulate osteogenesis. Comparing to EVs produced by AT-MSCs, EVs of BM-MSCs were enriched Notch2 (about 2-fold), which regulates skeletal development [4]. In this study, EVs of undifferentiated BM-MSCs enhanced matrix mineralization by the relatively “less osteogenic” AT-MSCs though it was statistically unproven after quantifying Alizarin Red staining.
The EV content changes as BM-MSCs at different stages of differentiation and the current study was able to differentiate the difference in osteogenic differentiation of AT-MSCs. After osteogenic differentiation, the miRNA profile of EVs produced by BM-MSCs changed and miRNA in EVs is the main signals regulating MSC differentiation [9,18]. EVs isolated from BM-MSCs at an early stage of osteogenic differentiation (< 7 days) induced BM-MSCs expressing early osteogenic markers [19]. It was the EVs of MSCs at a later stage of osteogenic differentiation (15-21 days) increased calcium and phosphate deposition in the matrix of MSCs (unknown tissue origin) [20]. In this study, replacing Ex with Ex-os, which was produced by 14-day osteogenically differentiated BM-MSCs, nearly tripled the amount of mineral deposition by AT-MSCs, which are inferior to BM-MSCs in osteogenic differentiation [12]. This study design was able to measure the osteogenic capacity of EVs produced by BM-MSCs at different stages of differentiation and has the potential to be used as an in vitro model for EV and osteogenic evaluation.
Whether for biological characterization or clinical applications of isolated MSCs, it is essential to assess their differentiation potentials. To examine the potential of osteogenic differentiation, MSCs are often subjected to culture in a classic [21] or modified [22,23] chemically-defined osteogenic induction medium. These osteogenic differentiation in vitro models are simple to perform and have been widely used as standardized MSC characterization [11]. They, however, do not simulate osteogenic differentiation of MSCs in vivo and are missing the initial osteogenic signals of cell-cell communication. This study incorporated EVs into the osteogenic induction to build a more sophisticated environment for evaluation of the osteogenic potential of MSCs. EVs themselves have broad applications in regenerative medicine and are often required for assessment of induction capacity [24]. The model presented in this study, in practice, can be used for testing both osteogenic differentiation of MSCs and osteogenic induction of EVs.
In summary, this study presented a unique in vitro osteogenic model. The model incorporated EVs to simulating cell-cell communication during osteogenic differentiation. Furthermore, using “less-osteogenic” AT-MSCs as the target of osteogenic induction makes the model less likely interfered by spontaneous osteogenesis. The results of this study showed that this model was able to differentiate the osteogenic capacity of EVs produced by osteogenic and undifferentiated BM-MSCs. It is an osteogenic model that is easy to set up and can be used for measuring both the osteogenic capacity of biomaterials or reagents and the osteogenic potential of MSCs.
Acknowledgments:
A portion of the study was performed at the Orthobiologic Laboratory, Union Memorial Hospital. The authors wish to thank Integrated Imaging Center, Johns Hopkins University, for access of transmission electron microscopes.
References
- Gnecchi M, Danieli P, Malpasso G, et al. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol 1416 (2016): 123-146.
- Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther 7 (2016): 125.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 367 (2020): eaau6977.
- Wang ZG, He ZY, Liang S, et al. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther 11 (2020): 511.
- Kostina A, Lobov A, Semenova D, et al. Context-specific osteogenic potential of mesenchymal stem cells. Biomedicines 9 (2021): 1971.
- Surrati A, Evseev S, Jourdan F, et al. Osteogenic response of human mesenchymal stem cells analysed using combined intracellular and extracellular metabolomic monitoring. Cell Physiol Biochem 55 (2021): 311-326.
- Mathieu M, Martin-Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21 (2019): 9-17.
- Mohamed-Ahmed S, Fristad I, Lie SA, et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther 9 (2018): 168.
- Xu L, Liu Y, Sun Y, et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther 8 (2017): 275.
- Paudel S, Lee WH, Lee M, et al. Intravenous administration of multipotent stromal cells and bone allograft modification to enhance allograft healing. Regen Med 14 (2019): 199-211.
- Zhang Z, Paudel S, Feltham T, et al. Foot fat pad: characterization by mesenchymal stromal cells in rats. Anat Rec (Hoboken) 304 (2021): 1582-1591.
- Gholami Farashah MS, Mohammadi A, Javadi M, et al. Bone marrow mesenchymal stem cells' osteogenic potential: superiority or non-superiority to other sources of mesenchymal stem cells? Cell Tissue Bank 24 (2023): 663-681.
- Liao HT, Chen CT. Osteogenic potential: comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells 6 (2014): 288-295.
- Niemeyer P, Fechner K, Milz S, et al. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials 31 (2010): 3572-3579.
- Noel D, Caton D, Roche S, et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res 314 (2008): 1575-1584.
- Harting MT, Srivastava AK, Zhaorigetu S, et al. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells 36 (2018): 79-90.
- Li Q, Yu H, Sun M, et al. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater 125 (2021): 253-266.
- Shim J, Nam JW. The expression and functional roles of microRNAs in stem cell differentiation. BMB Rep 49 (2016): 3-10.
- Martins M, Ribeiro D, Martins A, et al. Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment. Stem Cell Reports 6 (2016): 284-291.
- Wang X, Omar O, Vazirisani F, et al. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS One 13 (2018): e0193059.
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 284 (1999): 143-147.
- Maegawa N, Kawamura K, Hirose M, et al. Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2). J Tissue Eng Regen Med 1 (2007): 306-313.
- Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater 7 (2011): 463-477.
- Luo Y, Li Z, Wang X, et al. Characteristics of culture-condition stimulated exosomes or their loaded hydrogels in comparison with other extracellular vesicles or MSC lysates. Front Bioeng Biotechnol 10 (2022): 1016833.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 