Dihydrogen inhalation in the Management of Patients with Moderate Oxygen-Requiring COVID19: Towards an Innovative Therapy
Cordélia Salomez-Ihl1,2*, Joris Giai3, Mathieu Roustit3, François Boucher1, Philippe Cinquin1,2, Jean-Paul Brion4
1Université Grenoble Alpes, CNRS, UMR 5525, VetAgro Sup, Grenoble INP, CHU Grenoble Alpes, TIMC, UMR5525, 38000 Grenoble, France
2Université Grenoble Alpes, CHU Grenoble Alpes, Department of Pharmacy, 38000 Grenoble, France
3Université Grenoble Alpes, Inserm, CHU Grenoble Alpes, CIC1406, 38000 Grenoble, France
4CHU Grenoble Alpes, Department of Infectious and Tropical Diseases, 38000 Grenoble, France
*Corresponding author: Cordélia Salomez-Ihl, Université Grenoble Alpes, CNRS, UMR 5525, VetAgro Sup, Grenoble INP, CHU Grenoble Alpes, TIMC, UMR5525, 38000 Grenoble, France.
Received: 14 June 2024; Accepted: 20 June 2024; Published: 05 September 2024
Article Information
Citation: Cordélia Salomez-Ihl, Joris Giai, Mathieu Roustit, François Boucher, Philippe Cinquin, Jean-Paul Brion. Dihydrogen inhalation in the Management of Patients with Moderate Oxygen- Requiring COVID19: Towards an Innovative Therapy. Archives of Clinical and Biomedical Research. 8 (2024): 343-346.
View / Download Pdf Share at FacebookAbstract
The Coronavirus Disease 2019 (Covid-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has resulted in a substantial global health crisis, with millions of deaths reported since its initial discovery in China in November 2019. The global variability in immunization access underscores the critical necessity for ongoing research into therapeutic strategies.
This study explores the application of molecular dihydrogen (H2) inhalation as a potential adjuvant treatment for Covid-19. H2 therapy has shown promise in inhibiting inflammation-related intracellular signaling pathways, particularly when administered early in conjunction with nasal oxygen therapy.
Presented here are two cases from an ongoing phase one study evaluating the safety and Dose Limiting Toxicity (DLT) of H2 therapy delivered via a nasal cannula in addition to conventional oxygen therapy for Covid-19 patients requiring nasal oxygen (1 to 6 L/min). Tolerance was excellent in both cases, with no adverse events attributed to H2 reported.
Patient 1, a 56-year-old man, and Patient 2, a 59-year-old woman, exhibited positive responses to the H2 therapy, demonstrating improvements of O2 saturation and a decrease in C-Reactive Protein (CRP) levels. The gas mixture's safety and efficacy were supported by clinical and biological observations, aligning with existing literature on H2's anti-inflammatory effects.
This preliminary study suggests that inhaled H2, administered alongside oxygen therapy, may expedite the clinical improvement of pulmonary SARS-CoV-2 disease, potentially preventing Intensive Care Unit (ICU) transfers. The positive outcomes observed in these cases warrant further investigation in larger, controlled clinical trials.
Keywords
COVID 19; Anti-inflammatory Agents; Non-Steroidal; Inhalation
Article Details
1. Introduction
Coronavirus Disease 2019 (Covid-19), caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has led to millions of deaths since its discovery in China in November 2019 [1]. The primary manifestation is severe and potentially fatal lung damage, often associated with a significant release of cytokines (particularly interleukins IL-6, IL-8, and IL-10) due to macrophagic activation, primarily at the pulmonary level. Immunization access varies widely globally, emphasizing the crucial need for ongoing research into therapeutic strategies [2].
Hyperbaric molecular hydrogen (H2) inhalation was initially explored in the 1970s for potential cancer treatment, with the first atmospheric pressure experimental study conducted in 2007 on a rat model of cerebral infarction [3]. Subsequent research, such as that by Ito et al. [4], revealed that H2 inhibits inflammation-related intracellular signaling pathways independently of its anti-free radical effects.
Xie et al. [5] demonstrated that two 60-minute sessions of inhaling a gaseous mixture containing 2% H2 effectively limited multiple organ damage and reduced mortality in a mouse model of generalized inflammation [5]. Their research further revealed that H2 inhalation restored the PaO2 / FiO2 ratio in both a mouse model of sepsis induced by cecal ligation [6] and a model of lung damage induced by lipopolysaccharides [7]. Furthermore, although there is no reason to expect an anti-viral effect, H2 has been identified as a factor attenuating the considerable inflammatory stress exerted on the lung parenchyma during Covid-19 [8].
Early use in conjunction with nasal oxygen therapy has shown promise in preventing respiratory deterioration. Regarding administration, various methods have been explored, with the most prevalent being the ingestion of hydrogen-enriched water and the inhalation of a gaseous mixture. Due to hydrogen's high flammability (for concentrations exceeding 4.1% in the air), recent gas mixtures have typically maintained H2 levels between 2% and 4% [9,10].
Our ongoing phase one study assesses the safety and Dose Limiting Toxicity (DLT) of H2 therapy delivered via a nasal cannula in addition to conventional oxygen therapy for Covid-19 patients requiring nasal oxygen (1 to 6 L/min). We present two cases from this study demonstrating the tangible benefits of inhaled H2.
2. Case History or Examination
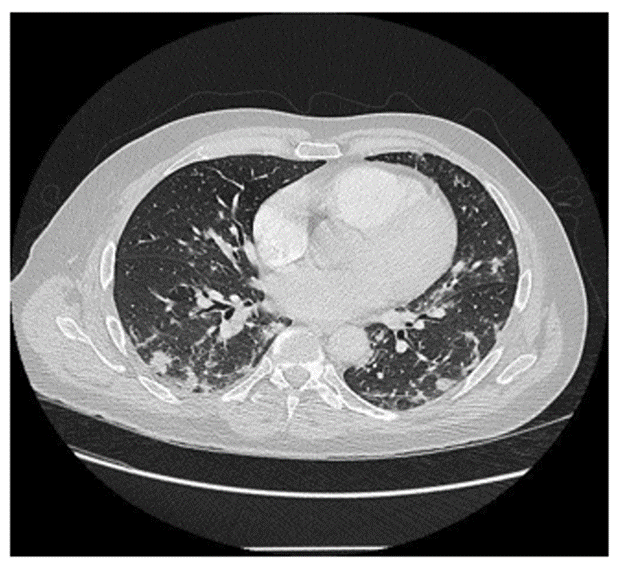
On September 03, 2021, a 56-year-old man was admitted in infectious diseases department for a moderate Covid-19 infection. He did not show previous diseases, was not a smoker and had a Body Mass Index of 29. He had no significant medical history. Symptoms had begun 10 days before admission. They consisted in fever (39°C), chills, anosmia, agueusia, asthenia and weight loss (5 kg). The first specific Polymerase Chain Reaction (PCR), done on 03/12/2021, was positive with English variant. C-Reactive Protein (CRP) test was high (185 mg/L), arterial gasometry showed before H2 therapy a significant hypoxemia with adjunction of 4L/min O2 by nasal canula (pO2: 8,41 kPa with normal values (NV): 9,50-13,30; pCO2: 4,98 kPa with NV: 4,70-6,10, pH: 7,44 with NV : 7,37-7,45 and bicarbonates : 24,5 mmol/L with normal values : 21,0-26,0). CT lung imaging is shown in Figure 1.
Concerning thoracic auscultation, crackles were found on both sides of the lung. The respiratory frequency was about 30 cycles per minute. Concerning chest X ray, performed on the day of admission, it showed multiple opacities, with infiltration confirmed by CT scan on the same day, which also highlighted extension of infiltrates to 50%, which suggests a severe pulmonary disease, as represented in Figure 2.
On April 6th 2021, a second patient was admitted to emergencies. She was 59 years old and had no specific medical history other than hypertension, treated with Lercanidipine. She was hemodynamically and respiratory stable, despite 89% saturation on room air, with a positive Covic-19 PCR. Symptoms had begun 8 days before admission She had respiratory alkalosis with mild hypoxemia (p02: 69.8mmHg; pC02:36.2mmHg; pH:7.46). She also had a biological inflammatory syndrome (CRP 69 mg/L), without hyperleukocytosis. D-Dimer were elevated at 1.02 mg/L. Troponin was 8 ng/L and BNP was 245 ng/L.
Concerning medical imaging, she had a typical Covid-19 infection, with extensive infiltration of lung parenchyma (25 - 50%), superinfection of both bases (figure 3), without additional pulmonary embolism (Figure 3).
3. Methods
An original medical delivery device has been designed by our team and has undergone a risk analysis by an independent organization. This device includes a flow regulator (a CE-marked medical device for clinical trials) allowing to guarantee a fixed flow of 1 L/min of a specific medical grade gas mixture (3,6% H2; 96.4% N2), manufactured and supplied by AIR PRODUCTS, packaged in B50 type cylinders. The gas mixture is combined with O2 from the oxygen outlet of the wall (adapted to the needs of the patient in accordance to standard of care).
Patient 1 and 2 were both included in H2 protocol for a three days treatment. They both received the usual standard of care during their hospitalization. They also required treatment with corticosteroids 0.6mg/kg for 5 days, then at half-dose for the following 5 days, combined with preventive anticoagulation. O2 flow for these two patients was between 3 and 4 L/min, to achieve a target of 95% of O2 saturation.
4. Results and Conclusion
Tolerance was excellent for both patients, with no adverse effect. Concerning patient 1, the clinical evolution during exposition was correct with improvement of hypoxemia and a decrease of CRP (33mg/L at the end of the 3 days of H2-therapy). Three months later the patient was examined for a control visit. He was well and did not show any respiratory symptom. Concerning patient 2, clinical evolution during exposition was correct with improvement of hypoxemia and a decrease of CRP (on 14th April, CRP returned to a normal value of 0 mg/L.) Hemoglobin was at 128 g/L, with no hyperleukocytosis, thrombocytosis was 591 Giga/L, neutrophils was 7.9 Giga/L. Given the good clinical and biological evolution, the patient was discharged on April 15, seven days after onset of H2-therapy.
5. Discussion
The clinical and biological observations of these clinical cases are consistent with the literature, considering that H2 has been described as able to reduce lung injury and thus to reduce the number of critically ill patients [11]. Another review of the literature has indicated that H2 may directly access lung tissue through respiratory activities, providing anti-inflammatory effects at various stages of the inflammatory response. This helps alleviate airway damage caused by the excessive activation of inflammatory cells and the substantial release of inflammatory factors [12]. Furthermore, in the context of Covid-19 related pulmonary injury, the activation of resident alveolar macrophages has resulted in the release of potent proinflammatory mediators and chemokines, fostering the accumulation of neutrophils and monocytes. Inhaled H2 exhibits a non-specific anti-inflammatory impact on macrophages, neutrophils, and lymphocytes, while also inhibiting the production of reactive oxygen species (ROS) [13].
When we initiated this research, the only established therapies demonstrating effectiveness were anti-inflammatory compounds like corticosteroids. Nevertheless, their application has not been without side effects. The use of H2 to address COVID-19 was initially proposed only in the Chinese recommendations [14,15]. Subsequently, a Chinese research team reported results in 2020 from an open-label clinical trial demonstrating efficacy. The trial involved administering a mixture comprising 67% H2 and 33% O2, showing statistically significant improvements in both clinical and biological parameters [16]. Our results seem to point in the same direction as this study, but our methodology does not involve use of very inflammable gas mixtures. Indeed, although our methodology does not allow us to test hypotheses of efficacy, clinicians were genuinely impressed by the positive evolution of all patients who had received hydrogen therapy. For example, at the same time as patient 1, a younger patient (54 years old, BMI 30) with no previous history and with positive PCR was admitted in infectious diseases department. He did not agree to be included in the study. He showed the same radio-clinical picture of SARS-CoV-2. He was admitted one day later in Intensive care for high flow nasal O2 (40L/min) delivered by OPTIFLOW™.
The absence of adverse events attributed to H2 is also consistent with the literature, since the tolerability of H2 has always been demonstrated in all clinical trials published in the literature.
These observations suggest a real efficacy of H2-therapy during 3 days combined treatment with oxygen to accelerate clinical improvement of pulmonary Covid-19 and notably to prevent ICU transfer.
We are currently preparing a phase II/III study to confirm this positive effect of inhaled H2 versus placebo on pulmonary inflammation during SARS-CoV-2.
Author’s contribution
Conceptualisation : J.Giai, M.Roustit, F.Boucher, P.Cinquin, JP Brion
Study procedures and analysis: C.Salomez-Ihl, J.Giai, M.Roustit, F.Boucher, P.Cinquin, JP Brion
Investigation: JP Brion
Writing – original draft preparation: C.Salomez-Ihl, F.Boucher, P.Cinquin, JP Brion
Writing – review and editing: all
Consent: Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy
References
- WHO, Global research on coronavirus disease https://Covid19.who.int/
- Ali HA, Hartner AM, Echeverria-Londono S, et al. Vaccine equity in low and middle income countries: a systematic review and meta-analysis. Int J Equity Health (2022).
- Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature Medicine (2007).
- Itoh T, Hamada N, Terazawa R, et al. Molecular hydrogen inhibits lipopolysaccharide/interferon γ-induced nitric oxide production through modulation of signal transduction in macrophages. Biochem Biophys Res Commun (2011).
- Xie K, Yu Y, Zhang Z, et al. Hydrogen gas improves survival rate and organ damage in zymosan-induced generalized inflammation model. Shock (2010).
- Xie K, Fu W, Xing W, et al. Combination therapy with molecular hydrogen and hyperoxia in a murine model of polymicrobial sepsis. Shock (2012).
- Xie K, Yu Y, Huang Y, et al. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock (2012).
- Russell G, Nenov A, Hancock JT. Oxy-hydrogen Gas: The Rationale Behind Its Use as a Novel and Sustainable Treatment for COVID-19 and Other Respiratory Diseases. European Medical Journal (2021).
- Alwazeer D, Liu FF, Wu XY, et al. Combating Oxidative Stress and Inflammation in COVID-19 by Molecular Hydrogen Therapy: Mechanisms and Perspectives. Oxid Med Cell Longev (2021).
- Gases-Explosion and Flammability Concentration Limits. Available online: https://www.engineeringtoolbox.com/explosive-concentration-limits-d_423.html (accessed on 12th January 2024)
- Yang F, Yue R, Luo X, et al. Hydrogen: A Potential New Adjuvant Therapy for COVID-19 Patients. Front Pharmacol (2020).
- Li Y, Wang Z, Lian N, et al. Molecular Hydrogen: A Promising Adjunctive Strategy for the Treatment of the COVID-19. Front Med (2021).
- Zhang H, Slutsky AS, Vincent JL. Oxygen free radicals in ARDS, septic shock and organ dysfunction. Intensive Care Medicine (2000).
- National Health Commission of the People’s Republic of China. New coronavirus pneumonia diagnosis and treatment guideline. 7th trial ed NHC: Bejing (2020).
- Chinese Center for Disease Control and Prevention. Protocol for prevention and control of COVID-19. 6th ed. CDCP: Bejing (2020).
- Guan WJ, Wei CH, Chen AL, et al. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis (2020).




 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 