Disorders of Magnesium Metabolism: Hypomagnesemia and Hypermagnesemia
Mohammad Tinawi*
Department of Internal Medicine and Nephrology, Nephrology Specialists, Munster, IN, USA
*Corresponding author: Mohammad Tinawi, Department of Internal Medicine and Nephrology, Nephrology
Specialists, P.C., 801 MacArthur Blvd., Ste. 400A, Munster, IN 46321, USA
Received: 26 May 2020; Accepted: 08 June 2020; Published: 11 June 2020
Article Information
Citation: Mohammad Tinawi. Disorders of Magnesium Metabolism: Hypomagnesemia and Hypermagnesemia. Archives of Clinical and Biomedical Research 4 (2020): 205-220.
View / Download Pdf Share at FacebookAbstract
Magnesium (Mg) is the second most abundant intracellular cation. It is a major factor in numerous cellular functions. Mg is a cofactor in hundreds of enzymatic reactions. Mg is essential for cellular energy production because it is a cofactor for adenosine triphosphate (ATP). Mg metabolism is linked to potassium (K) and calcium (Ca) metabolism. Hypomagnesemia is associated with several chronic diseases such as insulin resistance, hypertension (HTN) and osteoporosis. Severe hypermagnesemia is associated with significant toxicity. Mg can be easily replaced orally (PO) or intravenously (IV). Disorders of Mg metabolism are overlooked at times because Mg is not included in routine chemistry panels.
Keywords
<p>Hypomagnesemia, Hypermagnesemia, Electrolyte Disorders, Magnesium</p>
Article Details
1. Magnesium Homeostasis
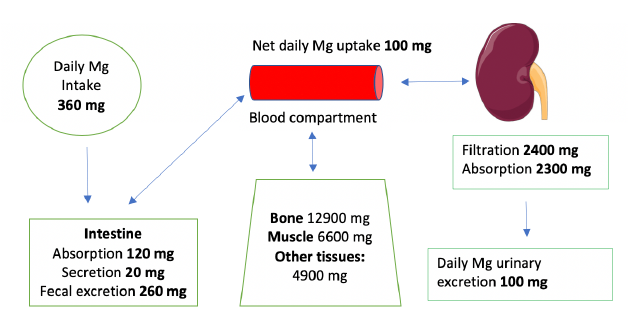
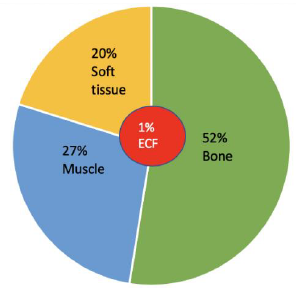
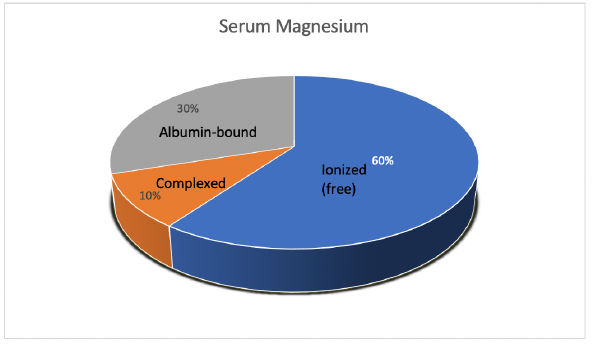
Mg is the fourth most abundant cation in the body and the second most abundant intracellular cation [1]. Normal serum Mg concentration is 1.7-2.6 mg/dl, this is equivalent to 1.4-2.2 mEq/l or 0.7-1.1 mmol/l. To convert from mmol/l to mEq/l, multiply by 2 which is the valence of Mg. To convert from mmol/l to mg/dl multiply by 24.3 (the atomic weight of Mg) and divide by 10. The intracellular Mg concentration is around 8-10 mmol/l. Free intracellular Mg is around 0.6-0.8 mmol/l. Most of intracellular Mg is bound to ATP and enzymes. The average daily intake of Mg is about 360 mg (15 mmol), 120 mg is absorbed in the intestines (mostly in the small intestines and to a lesser extent in the colon), Figure 1. About 20 mg is excreted with intestinal secretions. Therefore, net absorption is 100 mg which equals the amount excreted in the urine by the kidneys, the remaining 260 mg is excreted in the stool. About 52% of total body Mg is in the bone, 27% in muscles and 20% in non-muscular soft tissue [2], Figure 2. The extracellular fluid (ECF) contains only 1% of total body Mg. The serum contains only 0.3% of total body Mg, most of it is in the red blood cells (RBCs). It is important to know that Mg in the bone does not immediately equilibrate with serum Mg. Actually, that exchange takes place over several weeks. Any loss of Mg (for example due to diarrhea or diuretics) comes from the ECF, the kidneys adapt by lowering the amount excreted in the urine. The fractional excretion of Mg (FEMg) is 3%-5% in normal individuals. It can be lowered 10 times to < 0.5% with extrarenal hypomagnesemia [3]. Serum Mg may not reflect total body Mg [4], of which 60% of is free (ionized), 10% is complexed (bound to anions such as citrate, phosphate, bicarbonate or sulfate) and 30% is albumin-bound, Figure 3.
1.1 Magnesium absorption
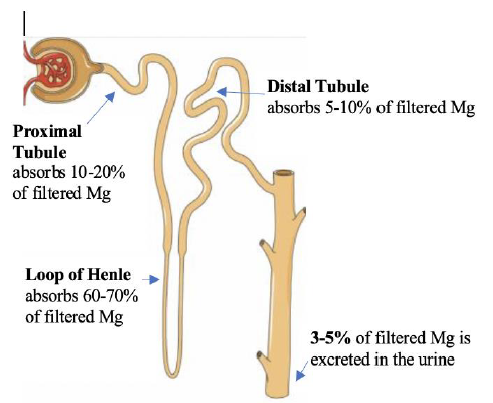
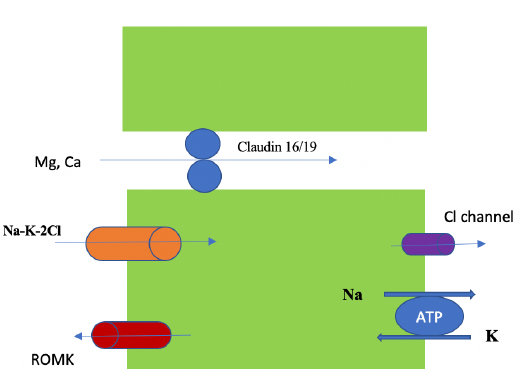
In the small intestine, Mg is absorbed paracellularly, mainly in the late jejunum and in the ileum [5]. In the colon, both transcellular (via TRPM 6 and 7) and paracellular absorption occur. TRPM (pronounced: trip M) channels are mammalian transient receptor potential melastatin non-selective cation channels [6]. Diarrhea fluid contains significant Mg (15 mEq/l), while vomitus contains only 1 mEq/l. In the kidney the proximal tubule (PT) reabsorbs 10-20% of filtered Mg via the paracellular route down a concentration gradient created by Na and water absorption [2]. Figure 4. The thick ascending limb (TAL) absorbs the majority of filtered Mg or about 60-70%, the absorption is paracellular (passive diffusion, not energy requiring). Claudin-16 interacts with claudin-19 (both are tight junction proteins) forming a cation-selective tight junction complex that facilitates Mg transport, Figure 5. The process is dependent on transepithelial voltage [7]. The distal collecting tubule (DCT) absorbs 5-10%, the process is active via the transcellular route and is dependent on membrane potential. The remainder 3-5% of filtered Mg is excreted in the urine. Most of Mg excretion occurs during the night following a circadian rhythm [8]. It is important to know that the kidney (not the intestines) acts as the main regulator of Mg metabolism by varying the amount excreted in the urine (in the TAL and DCT). The kidneys maintain normomagnesemia until glomerular filtration rate (GFR) is < 10-30 ml/min.
Hypermagnesemia is mainly seen in advanced chronic kidney disease (CKD) patients on exogenous Mg. Several factors affect Mg absorption in the TAL and DCT, Table 1. Serum Mg concertation is the most significant factor, hypomagnesemia increases Mg absorption in the TAL, while hypermagnesemia decreases Mg and Ca absorption in the TAL [3]. Hypercalcemia activates the calcium-sensing receptor (CaSR) on the basolateral membrane of the TAL and subsequently decreases Mg and Ca absorption in the TAL, resulting in hypermagnesuria and hypercalciuria. Both Mg and Ca bind to the CaSR in the parathyroid glands and the kidneys, however, each has a distinct binding site. Mg plays a role in parathyroid hormone (PTH) modulation [10]. Unlike calcium, there is no hormonal system that significantly affects Mg metabolism. Acute hypomagnesemia stimulates PTH secretion and hypermagnesemia suppresses PTH secretion via acting on the CaSR. It is important to note that profound hypomagnesemia suppresses (rather than stimulate) PTH secretion and increases PTH resistance in the bone leading to hypocalcemia [11]. Hypomagnesemia may result in recalcitrant hypokalemia [13]. This is because Mg inhibits ROMK channels; therefore, hypomagnesemia increases K secretion in the collecting duct [14].
Figure 1: Mg homeostasis [12]. Total Mg in an average 70 kg adult is about 24 g [8]. Image of kidney is courtesy of Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License. https://smart.servier.com
Figure 4: The Nephron. Courtesy of Servier Medical Art licensed under a Creative Commons Attribution 3.0 Unported License. https://smart.servier.com Labels added to illustrate Mg absorption in the nephron.
|
Factors that increase Mg absorption in TAL |
Factors that decrease Mg absorption in TAL |
|
· Hypomagnesemia · Metabolic alkalosis · Amiloride (acts at DCT) [9] |
· Hypermagnesemia · Metabolic acidosis · Loop diuretics · Thiazide diuretics (act at DCT) · Hypokalemia · Hypophosphatemia · Hypercalcemia |
Table 1: Factors affecting Mg absorption in TAL and DCT.
1.2 Magnesium and calcium
Mg acts as an inhibitor of Ca in the body. Mg antagonizes Ca-dependent acetylcholine release at neuromuscular junctions [15]. Mg is anti-apoptotic, while calcium is pro-apoptotic [16]. Serum level of Ca and Mg may not represent total body content. Both cations exist in 3 forms (free or ionized, albumin-bound, and complexed to anions). The body contains about 24 g of Mg [8] and about 1000 g of calcium. As mentioned above severe hypomagnesemia can lead to hypocalcemia due to PTH suppression and increased PTH skeletal resistance [17].
1.3 Magnesium and diet
It is recommended that adult males consume 400 to 420 mg of Mg daily [9]. For adult females the recommended amount of Mg is 310-320 mg daily, and it should be increased to 350-400 mg daily during pregnancy. The recommended amount of Mg during breastfeeding is 310-360 mg daily [18]. Mg is the central element in chlorophyll and plays a critical role in photosynthesis [19]. Mg rich foods include green vegetables, seeds (such as cashew, almond and brown rice) and nuts. Other good sources of Mg include cereals, baked potato with skin, oatmeal, yogurt, banana, black beans and peanut butter [18]. Processed and refined food is a poor source of Mg compared to unrefined food [20]. Increased consumption of processed food is resulting in decreased Mg intake especially in the Western world.
1.4 Definition of hypomagnesemia and hypermagnesemia
As mentioned above, normal serum Mg concentration is 1.7-2.6 mg/dl (1.4-2.2 mEq/l or 0.7-1.1 mmol/l). Hypomagnesemia is defined as serum Mg < 1.7 mg/dl (0.7 mmol/l). Significant symptoms and signs are seen when serum Mg is < 1.2 mg/dl (0.5 mmol/l) [21]. Hypermagnesemia is defined as serum Mg > 2.6 mg/dl (1.1 mmol/l). Significant symptoms and signs are seen when serum Mg exceeds 4.8 mg/dl (2 mmol/l) [22].
2. Prevalence of Magnesium Metabolism Disorders
Hypomagnesemia is seen in hospitalized and community dwelling subjects. Hypermagnesemia is less common than hypomagnesemia. A study in about 5000 community subjects aged 55 years or older (the Rotterdam Study) found hypomagnesemia (defined as Mg < 1.2 mg/dl) in 2.4% of individuals between 55-64 years and in about 1.8% in individuals aged 65 and older [23].
Hypomagnesemia in the same study was associated with increased adjusted mortality risk of 1.39 (1.06-1.81). A Japanese study measured serum Mg in 6252 inpatients [24]. Hypomagnesemia (Mg <1.5 mg/dl) was seen in 2.6% and hypermagnesemia (Mg >3.9 mg/dl) was seen in 0.8% of patients. Hypermagnesemic patients either had impaired renal function while on Mg-containing antacids or cathartics, or were women treated with Mg sulfate in the course of preeclampsia and eclampsia. Hypomagnesemia is common in postoperative intensive care patients. A study in 193 such patients found hypomagnesemia (Mg <1.5 mg/dl) in 61% [25].
3. Evaluation of Mg Status in the Body
Diagnosis of Mg metabolism disorders require a high index of suspicion because Mg in not included in routine chemistry panels. Serum Mg measurement is readily available, but it may not correlate with total body Mg. As emphasized above, the ECF contains only 1% of total body Mg. Most of serum Mg (only 0.3% of total body Mg) is in the RBCs; therefore, hemolysis of blood samples will lead to pseudohypermagnesemia [26]. Measurement of Mg in a 24 h urine is helpful in patients with hypomagnesemia [27]. 12 h collection may not be helpful due to the circadian excretion of Mg mentioned above. An elevated urine Mg indicates renal Mg wasting.
Normal urine Mg excretion is < 12-25 mg/ 24 h (0.5-1 mmol/ 24 h). In hypomagnesemia due to renal wasting of Mg, urine Mg is > 30 mg/ 24 h (1.25 mmol/ 24 h) [17]. Mg loading test or Mg retention test was proposed to identify patients with potential Mg deficiency despite normal serum Mg level. Mg is given IV or PO and then measured in the urine. If IV loading is chosen, subjects are usually given 7.5 g (30 mmol) of Mg sulfate over 8 h [28]. One study utilized 5 g of Mg lactate for oral loading test [29]. Patients who excrete > 60-70% are unlikely to have Mg deficiency. Mg loading test is rarely used because it has both false negatives (as in diabetics and alcoholics) and false positives (as in patients with CKD) [30].
4. Hypomagnesemia
4.1 Etiology
Hypomagnesemia can be due to dietary deficiency (malnutrition, total parenteral nutrition TPN), redistribution (hungry bone syndrome), gastrointestinal (GI) illness (chronic diarrhea, malabsorption), or renal wasting [31] (loop or thiazide diuretics, aminoglycosides, Bartter and Gitelman syndromes). See Table 2. Chronic treatment with proton pump inhibitors (PPI) is an important cause of hypomagnesemia [32]. The etiology is Mg loss is due to decreased intestinal absorption via downregulation of TRPM6 channels [12].
|
1.Dietary deficiency: malnutrition, TPN, chronic alcoholism. |
|
2.Gastrointestinal: chronic diarrhea, proton pump inhibitors (PPI), malabsorption, laxatives, short bowel syndrome, intestinal fistulas, nasogastric suction, primary infantile hypomagnesemia, patiromer (a potassium binder). |
|
3.Renal wasting: · Diuretics: loop and thiazide · Diuretic phase of acute tubular necrosis (ATN) and post-obstructive diuresis · Bartter syndrome and Gitelman syndrome · Aminoglycosides · Cyclosporine A and tacrolimus · Epidermal growth factor receptor [EGFR] inhibitors such as cetuximab · Cisplatin · Amphotericin B · Pentamidine · Foscarnet · Congenital or acquired tubular defects |
|
4.Redistribution: acute pancreatitis, hungry bone syndrome, refeeding syndrome, blood transfusions, insulin. |
|
5.Endocrine: primary and secondary hyperaldosteronism, hyperparathyroidism, hyperthyroidism, diabetes mellitus, syndrome of inappropriate anti-diuretic hormone secretion (SIADH). |
Table 2: Causes of hypomagnesemia [2], [4], [12], [17], [30].
4.1.1 Genetic hypomagnesemia: De Baaij et al. published an excellent review on this topic [2]. Genetic Mg disorders originate in the kidney, either in the TAL or DCT. Genetic GI causes of hypomagnesemia due to TRPM6 gene mutations are very rare. Examples of genetic hypomagnesemia in TAL include Bartter syndrome (type 1, 2, 3 and 4) and familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) type 1 and 2. Bartter syndrome [33] is characterized by Na wasting, hypokalemic metabolic alkalosis and high renin and aldosterone levels. The defective protein differs depending on the type (e.g. it is barttin in type 4 Bartter syndrome). FHHNC type 1 is due to defective claudin 16, while FHHNC type 2 is due to defective claudin 19. Both types are associated with nephrocalcinosis and renal failure. FHHNC type 2 is characterized by ocular abnormalities [12]. Examples of genetic hypomagnesemia in DCT include Gitelman syndrome (defective Na-Cl cotransporter or NCC), hypomagnesemia with secondary hypocalcemia [HSH] (due to defective TRPM6), and isolated recessive hypomagnesemia [IRH] (due to defective epidermal growth factor). Gitelman syndrome is characterized by hypokalemic metabolic alkalosis and hypocalciuria [34].
4.2 Symptoms and complications
The symptoms of hypomagnesemia depend of the severity of Mg deficiency and the rate of its decline [12]. As mentioned above, hypomagnesemia can result in hypokalemia and hypocalcemia [21]. Some patients have non-specific symptoms such as nausea, vomiting and anorexia. Neuromuscular symptoms in hypomagnesemia and hypocalcemia are similar and result from neuromuscular hyperexcitability. They include tetany, cramps, weakness, dysphagia and muscle fasciculations. Trousseau and Chvostek signs can be seen as well [4]. Neurological manifestations include agitation, psychosis, depression, tremors, vertigo and nystagmus. In severe cases, delirium, encephalopathy and seizures can be seen. Cardiac arrhythmias should prompt emergency treatment and include ventricular arrhythmias, torsade de points and supraventricular tachycardia [17]. Hypomagnesemia enhances sensitivity to digoxin toxicity [21]. Hypomagnesemia has been associated with numerous chronic complications including osteoporosis, migraines, increased insulin resistance, HTN, asthma, and atherosclerosis [12, 21].
4.3 Diagnosis
The following principles should be kept in mind when approaching a patient with hypomagnesemia:
- The diagnosis of hypomagnesemia requires a high index of suspicion because Mg is not included in routine chemistry panels. K and Ca are routinely ordered when evaluating
- Serum Mg measurement is readily available in laboratories. This simple test should be ordered in patients with electrolyte disorders especially recalcitrant hypokalemia and hypocalcemia, and in the clinical settings specified above (see Table 2). Serum Mg should also be ordered in patients presenting with the above-mentioned symptoms, signs or complications.
- Mg loading test is rarely done.
- Fractional excretion of Mg (FEMg) is helpful in differentiating renal wasting from GI wasting of Mg. In renal wasting of Mg, FEMg is > 4%, while in GI wasting of Mg it is < 2% [21]. In normal individual FEMg is 3%-5%.
- A 24 h urine collection for Mg may also help in differentiating renal from extra-renal (GI) Mg wasting. Urine Mg is > 30 mg/ 24 h (1.25 mmol/ 24 h) in renal Mg wasting [17].
- As in other electrolyte disorders, medications review is critical.
- An electrocardiogram (ECG) is ordered if cardiac arrhythmias are suspected.
- If genetic hypomagnesemia is suspected, a specialist consultation is required. Genetic testing is needed to ascertain the diagnosis.
4.4 Treatment
Serum Mg does not reflect total body Mg. Patients with low normal Mg are supplemented with Mg in certain clinical settings to alleviate their symptoms and avoid complications. Examples include patients with hypokalemia, hypocalcemia and cardiac arrhythmias. The underlying cause of hypomagnesemia should be addressed. Dietary counseling to increase Mg intake is helpful. Many clinicians add spironolactone or amiloride to a loop or a thiazide diuretic to mitigate hypokalemia and hypomagnesemia [35]. Patients who can take oral medications are given Mg orally unless their hypomagnesemia is severe or is associated with severe complications such as cardiac arrhythmias or seizures. There are several oral Mg formulations available in capsules (cap) or tablets (tab). Some formulations are available over-the-counter (OTC). See Table 3. It is noteworthy that Mg citrate and Mg hydroxide are used as laxatives. It is critical to consult the manufacturer prescribing information for each product because the amount of elemental Mg varies. Note that the table below provides general information only. Diarrhea, nausea and vomiting are the most common adverse reactions of oral Mg supplements.
|
Mg formulation |
Dosage forms |
Elemental Mg content |
Typical dose |
|
Mg Oxide |
Cap: 250 mg, 400 mg, 500 mg Tab: 241.3 mg, 250 mg, 253 mg, 500 mg |
60% is elemental Mg. e.g. 400 mg cap contains 240 mg (~20 mEq or 10 mmol) |
1-4 tab or cap/day divided in 1-4 doses Bioavailability: 4% |
|
Mg Gluconate (available OTC) |
Tab: 500 mg |
5.4% elemental Mg. e.g. 500 mg tab contains 27 mg (2.25 mEq or 1.12 mmol) |
1-2 tab/day divided in 1-2 doses Bioavailability: Human data not available |
|
Mg Chloride (also available OTC in combination with Ca carbonate) |
Extended release tab: 535 mg Mg content varies in OTC products |
12% elemental Mg. Each extended release tab contains 64 mg elemental Mg |
2 tab/day Bioavailability: 12% |
|
Mg Lactate (available OTC) |
Ex: Mag-Tab SR ® |
12% elemental Mg. Each tablet contains 84 mg of elemental Mg |
2-4 tab in 2 doses Bioavailability: 12% |
Table 3: Oral Mg formulations [9], [36], [37].
Persistent hypomagnesemia in hospitalized patients require IV Mg because oral replacement is limited by diarrhea and poor bioavailability. Mg chloride and Mg lactate have a bioavailability of 12% (this is the fraction absorbed of the dose given), while Mg oxide has a bioavailability of only 4% [9, 36]. Mg sulfate is utilized for parenteral replacement (IV and intramuscular IM) [37]. Parenteral Mg replacement is indicated in patients who cannot tolerate oral Mg supplements, and in patients with severe Mg deficiency especially in the presence of severe complications such as cardiac arrhythmias. Mg sulfate is available as a 10% solution (1 g/10 ml) for IV use. IM Mg sulfate is available as a 50% solution (1 g/2 ml). 1 g of Mg sulfate contains 98.4 mg of elemental Mg (8.12 mEq or 4.06 mmol). A typical dose in a stable patient is 1-2 g IV over 30-60 minutes. A higher dose such as 2-4 g IV over 4-12 h is appropriate in patients with serum Mg < 1 mg/dl (0.4 mmol/l).
Serum Mg should be monitored, and the dose is repeated until normomagnesemia is achieved. In unstable patients such as those with seizures or cardiac arrhythmias 1-2 g is given IV over 15 minutes, this is followed by a Mg sulfate drip at 3-20 mg/min. For example, 10 g of Mg sulfate in 1 L of 5% dextrose in water infused over 24 h, would provide 6.94 mg of Mg sulfate/min. Mg sulfate drip is discontinued once normomagnesemia is achieved. Mg sulfate is the drug of choice to prevent seizures in women with preeclampsia [38]. High doses of Mg sulfate are utilized, e.g. 6 g IV over 15-20 minutes followed by a continuous infusion at 1-2 g/h [37]. This will result in hypermagnesemia. Target Mg level is not well defined [39], and a wide range is reported in clinical studies (4.8-8.4 mg/dl or 2-3.5 mmol/l). This level of hypermagnesemia rarely results in toxicity in women with preeclampsia unless there is a concomitant acute kidney injury or CKD. It is important to note that a review of data on this topic showed that a lower Mg target (< 4.8 mg/dl or < 2 mmol/l) may be adequate for seizures prevention [39]. Mg sulfate is relatively contraindicated in patients with myasthenia gravis because Mg inhibit the release of acetylcholine [40].
5. Hypermagnesemia
5.1 Etiology
Hypermagnesemia is less common than hypomagnesemia. It is seen in patients with advanced CKD (GFR is < 10-30 ml/min) with concomitant intake of Mg salts as in some laxatives (Mg citrate, Mg hydroxide) or antacids (aluminum-magnesium hydroxide). Therefore, significant hypermagnesemia is unlikely even in patients with end-stage renal disease (ESRD) without exogenous Mg intake. Hypermagnesemia is more common in the elderly due to increased prevalence of CKD. Serum Mg should be routinely monitored in dialysis patients who take Mg carbonate as a phosphate binder. Note that sodium picosulfate/magnesium citrate is used as a bowel preparation agent. As above hypermagnesemia is intentionally induced in patients with preeclampsia to prevent seizures. Hyperreflexia may signal impending eclampsia and mandate immediate initiation of IV Mg sulfate [41]. Some clinicians use normalization of hyperreflexia in women with preeclampsia as an indication of adequate seizure prophylaxis with Mg sulfate [42]. Other causes of hypermagnesemia include milk-alkali syndrome, hemolysis, tumor lysis syndrome, rhabdomyolysis, diabetic ketoacidosis, lithium therapy, bone metastases, familial hypocalciuric hypercalcemia and Addison’s disease [22]. The main ingredient in Epsom salt is Mg sulfate. Epsom salt has an elemental Mg content of 10% [37] and has a bioavailability of 4% [9]. It should not be used as a laxative without physician consultation [43]. It should not be ingested for weight loss or detoxification.
5.2 Symptoms and complications
The symptoms, signs and complications of hypermagnesemia are summarized in table 4. Note that both severe hypermagnesemia and severe hyperkalemia can induce cardiac arrhythmias [44, 45].
|
Serum Mg levels |
Clinical manifestations |
|
2.7-5.0 mg/dl (1.1-2.0 mmol/l) |
Usually no symptoms |
|
5.1-7.0 mg/dl (2.1-2.9 mmol/l) |
Mild symptoms: nausea, vomiting, dizziness, weakness, drowsiness, diminished deep tendon reflexes. |
|
7.1-12 mg/dl (3.0-5.0 mmol/l) |
Urinary retention, lethargy, ileus, flushing, sleepiness, blurred vision, confusion, loss of deep tendon reflexes |
|
>12 mg/dl (> 5.0 mmol/l) |
Flaccid paralysis, respiratory depression, apnea, low BP, bradycardia, complete heart block. Symptoms can progress to coma and cardiopulmonary arrest |
Table 4: Clinical manifestations of hypermagnesemia [2], [12], [22].
5.3 Diagnosis
Hypermagnesemia is diagnosed by measuring serum Mg in the appropriate clinical setting such as patients with advanced CKD who are on Mg salts. There is no role for urine Mg measurement in hypermagnesemia. As mentioned above, most of serum Mg is in the RBCs. Hemolysis in the sample will lead to pseudohypermagnesemia and pseudohyperkalemia due to release of Mg and K from the RBCs [44]. ECG should be obtained in severe hypermagnesemia or if cardiac arrhythmias are suspected. Basic chemistry panel is needed to evaluate renal function and other electrolytes including K, Ca and phosphorus.
5.4 Treatment
All sources of exogenous Mg should be discontinued. If GFR is > 60 ml/min and hypermagnesemia is mild with no or minimal symptoms, simple observation is adequate. Mg elimination half-life is about 20.2 hours [46], therefore, Mg is rechecked on a daily basis. Patients with severe hypermagnesemia (serum Mg 7.1-12 mg/dl or 3.0-5.0 mmol/l) are monitored in a telemetry unit. These patients require treatment with calcium gluconate or chloride. Calcium antagonizes the effect of Mg on the myocardium and neuromuscular junction [22]. Calcium is utilized in a similar fashion in hyperkalemia associated with ECG changes [44]. 1 g of calcium gluconate is given over 5-10 minutes. The dose can be repeated after 5 minutes if no improvement in ECG. To augment Mg excretion, IV normal saline is given along with an IV loop diuretic such as furosemide or bumetanide. The rate of normal saline and the dose of loop diuretics vary according to renal function and volume status. Hemodialysis (HD) is utilized if the above measures fail especially in patients with advanced CKD exhibiting severe symptoms. HD removes Mg efficiently [47], a 3-4 h treatment reduces Mg by 30%-50% [22]. HD should not be done on a low calcium bath in order to avoid precipitating hypocalcemia. A calcium bath > 2.5 should be used. Patients receiving Mg sulfate infusion for seizure prophylaxis in preeclampsia require close monitoring. The infusion is stopped and IV calcium gluconate (or chloride) is given, if urine output declines (<25 ml/h), deep tendon reflexes become absent or if hypoventilation is observed (respiratory rate < 12 per minute). Frequent serum Mg measurements is required.
- Clinical Vignettes
- A 52 year-old-man presents with nausea, dizziness, weakness and muscle cramps. His past medical history is significant for HTN and gastroesophageal reflux disease (GERD). His medication regimen includes amlodipine 5 mg daily for HTN and pantoprazole 40 mg daily for GERD. He has been taking both medications for 7 years. A chemistry panel was obtained and was significant for: K 3.3 mEq/l, Mg 1 mg/dl, Ca 7.7 mg/dl, albumin 4.1 g/dl. What is the etiology of his symptoms?
Answer: He has hypomagnesemia due to chronic use of pantoprazole. PPI lead to GI Mg loss due to decreased absorption via downregulation of TRPM6 channels (12). Hypomagnesemia leads to hypokalemia (13) and hypocalcemia.
2.A 35-year-old woman G1P0 was diagnosed with severe preeclampsia and noted to have hyperreflexia on exam. She is immediately started on Mg sulfate IV for seizure prophylaxis. She was given a loading dose of 6 g IV over 30 minutes, followed by a continuous infusion of 3 g per hour. 5 hours later she became lethargic, unable to urinate and her deep tendon reflexes were absent. What should be done next?
Answer: The patient is showing signs of Mg toxicity. Mg sulfate was discontinued, and she was started on 0.9 normal saline at 125 ml/h, 1 g of calcium gluconate was given IV over 5 minutes, her ECG was unremarkable. Serum Mg level came back at 7.4 mg/dl. Mg sulfate is the drug of choice for seizure prophylaxis in preeclampsia (38). The latter can be achieved with lower Mg sulfate doses without inducing Mg toxicity (39).
3.A 56-year-old man with a known history of ESRD was brought to the emergency department due to lethargy, confusion, and blurred vision. He has been on HD for 5 years. His family reported that he has been using a variety of OTC medications for severe constipation. Neurological exam was remarkable for absent deep tendon reflexes. How would you manage
this patient?
Answer: The patient has been ingesting large doses of milk of magnesia (MOM). The active ingredient in MOM is Mg hydroxide (37). This patient with ESRD developed Mg toxicity. His serum Mg level was 8.3 mg/dl. ECG showed no significant abnormalities. He was given 0.9 normal saline and 1 g of calcium gluconate. Since he is already on HD and a dialysis access is readily available, he was emergently dialyzed. After a 4-h session his serum Mg was 4.8 mg/dl. He was instructed to avoid Mg containing laxative and antacids and to consult with his nephrologist prior to starting any new medication.
- A 69-year-old man with chronic systolic heart failure (ejection fraction is 20%) presents with serum Mg of 1.4 mg/dl. He is on lisinopril 40 mg daily, furosemide 40 mg twice daily, and carvedilol 25 mg twice daily. His BP control is optimal, serum creatinine is stable at 0.8 mg/dl, serum K+is 3.4 mEq/l. What is the best approach to his hypokalemia and hypomagnesemia?
Answer: Discontinuation or dose reduction of furosemide may lead to fluid overload. Supplementation with oral K and Mg salts would significantly increase the number and frequency of his medications. Given his diagnosis of chronic systolic congestive heart failure with low ejection fraction, spironolactone 25 mg daily was started to mitigate both hypokalemia and hypomagnesemia resulting from his loop diuretic. Spironolactone decreases morbidity and mortality in patients with severe heart failure (48).
- A 60-year-old woman with a known history of stage 4 CKD secondary to diabetic nephropathy presents with nausea, dizziness, muscle cramps and fasciculations. Her medications include glimepiride 4 mg daily, atorvastatin 40 mg daily, lisinopril 40 mg daily, furosemide 40 mg daily and patiromer 8.4 daily. Patiromer (a potassium binder) was started due to hyperkalemia resulting from the increase in lisinopril dose. What should be done next?
Answer: A chemistry panel was ordered, creatinine 3.1 mg/dl, K 4.9 mEq/l, Mg 1 mg/dl. Her symptoms are due to hypomagnesemia. Patiromer binds both K and Mg (49). In this patient with stage 4 CKD, the use of patiromer allowed her to continue the use of lisinopril at an optimal dose. Furosemide aggravated her hypomagnesemia. In this case patiromer was continued and the patient was supplemented with Mg oxide 400 mg PO twice daily.
- A 7-year-old girl presents for evaluation of CKD. She is complaining of muscle cramps and fasciculations, polyuria and polydipsia. Her past medical history is significant for recurrent urinary tract infections and severe myopia. Physical exam revealed that her growth is in the 40th percentile. She is noted to have corneal calcifications. Laboratory evaluation was remarkable for microhematuria, serum creatinine 1 mg/dl , serum Mg 1.2 mg/dl, serum Ca 9 mg/dl, fractional excretion of Mg was elevated at 15%, urine calcium was elevated at 12 mg/kg/24 h (normal < 4 mg/kg/24 h). Intact parathyroid hormone level was elevated at 131 pg/ml (normal: 10-65 pg/ml). Renal ultrasound revealed nephrocalcinosis. What is the diagnosis?
Answer: This presentation is consistent with familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) type 2. Ocular abnormalities are seen only in type 2 FHHNC. This rare genetic disorder is due to a mutation in the tight junction protein claudin 19. A detailed family history is paramount, including inquiring about consanguineous parents (50).This patient exhibited many of the characteristic features of FHHNC type 2 (51). Genetic testing is required to ascertain the diagnosis.
7. Conclusion
- Diagnosis of Mg metabolic disorders require a high index of suspicion because Mg is not included in routine chemistry panels.
- Hypomagnesemia and hypermagnesemia present with a variety of non-specific manifestations that overlap with other electrolyte disorders especially those of potassium and calcium.
- Hypomagnesemia is an important cause of recalcitrant hypokalemia and hypocalcemia.
- Hypermagnesemia is less common than hypomagnesemia and is seen mainly in patients with advanced chronic kidney disease on exogenous Mg.
- Mg can be replaced easily via the oral or intravenous route.
- Severe hypermagnesemia is a serious electrolyte disorder that require emergency treatment with IV calcium, IV fluids and in some cases hemodialysis.
Conflicts of Interest
The author declares no conflict of interest.
References
- Gröber U, Schmidt J, Kisters K. Magnesium in Prevention and Therapy. Nutrients 7 (2015): 8199-8226.
- De Baaij J, Hoenderop J, Bindels R. Magnesium in Man: Implications for Health and Disease. Physiol Rev 95 (2015): 1-46.
- Al-Ghamdi S, Cameron E, Sutton R. Magnesium deficiency: pathophysiologic and clinical overview. Am J Kidney Dis 24 (1994): 737-752.
- Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J 5 (2012): (Suppl 1) i3-i14.
- Romani A. Cellular Magnesium Homeostasis. Arch Biochem Biophys 512 (2011): 1-23.
- Zholos A. Pharmacology of transient receptor potential melastatin channels in the vasculature. Br J Pharmacol 159 (2010): 1559-1571.
- Hou J, Renigunta A, Konrad M, et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118 (2008): 619-628.
- Fox C, Ramsoomair D. Magnesium: its proven and potential clinical significance. South Med J 94 (2001): 1195-1201.
- Ahmed F, Mohammed A. Magnesium: The Forgotten Electrolyte-A Review on Hypomagnesemia. Med Sci 7 (2019): 56.
- Kumar R, Thompson J. The Regulation of Parathyroid Hormone Secretion and Synthesis. J Am Soc Nephrol 22 (2011): 216-224.
- Blaine J, Chonchol M, Levi M. Renal Control of Calcium, Phosphate, and Magnesium Homeostasis. Clin J Am Soc Nephrol 10 (2015): 1257-1272.
- Al Alawi A, Majoni S, Falhammar H. Magnesium and Human Health: Perspectives and Research Directions. Int J Endocrinol (2018).
- Tinawi M. Hypokalemia: A Practical Approach to Diagnosis and Treatment. Arch Clin Biomed Res 4 (2020): 48-66.
- Huang C, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 18 (2007): 2649-2652.
- Levine B, Coburn J. Magesium, the Mimic/Antagonist of Calcium. N Engl J Med 310 (1984): 1253-1255.
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4 (2003): 552-565.
- Martin KJ, González E, Slatopolsky E. Clinical Consequences and Management of Hypomagnesemia. J Am Soc Nephrol 20 (2009): 2291-2295.
- Magnesium: Fact Sheet for Health Professionals [Internet]. NIH, Office of Dietary Supplements (2020).
- Tränkner M, Tavakol E, Jákli B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol Plant 163 (2018): 414-431.
- DiNicolantonio J, O’Keefe J, Wilson W. Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. Open Hear 5 (2018): e000668.
- Pham P-C, Pham P-A, Pham S V, et al. Hypomagnesemia: a clinical perspective. Int J Nephrol Renov Dis 7 (2014): 219-230.
- Cascella M, Vaqar S. Hypermagnesemia. [Updated 2020 Jan 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2020).
- Liamis G, Rodenburg E, Hofman A, et al. Electrolyte Disorders in Community Subjects: Prevalence and Risk Factors. Am J Med 126 (2013): 256-263.
- Hashizume N, Mori M. An analysis of hypermagnesemia and hypomagnesemia. Jpn J Med 29 (1990): 368-372.
- Chernow B, Bamberger S, Stoiko M, et al. Hypomagnesemia in patients in postoperative intensive care. Chest 95 (1989): 391-397.
- Elin R. Assessment of magnesium status for diagnosis and therapy. Magnes Res 23 (2010): 194-198.
- Elin R. Magnesium metabolism in health and disease. Dis Mon 34 (1998): 161-218.
- Gullestad L, Midtvedt K, Dolva L, et al. The Magnesium Loading Test: Reference Values in Healthy Subjects. Scand J Clin Lab Invest 54 (1994): 23-31.
- Vizinová H, Bártek J, Jirka Z, et al. The Oral Magnesium Loading Test for Detecting Possible Magnesium Deficiency. Cas Lek Ces 132 (1993): 587-589.
- Karosanidze T. Magnesium - So underappreciated. Pract Gastroenterol 38 (2014): 29-34.
- Classen H, Gröber U, Kisters K. Drug-induced magnesium deficiency. Med Monatsschr Pharm 35 (2012): 274-280.
- Srinutta T, Chewcharat A, Takkavatakarn K, et al. Proton pump inhibitors and hypomagnesemia A meta-analysis of observational studies. Medicine 98 (2019): e17788.
- Calò L, Punzi L, Semplicini A. Hypomagnesemia and chondrocalcinosis in Bartter’s and Gitelman’s syndrome: review of the pathogenetic mechanisms. Am J Nephrol 20 (2000): 347-350.
- Blanchard A, Bockenhauer D, Bolignano D, et al. Gitelman syndrome: consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 91 (2017): 24-33.
- Gao X, Peng L, Adhikari C, et al. Spironolactone Reduced Arrhythmia and Maintained Magnesium Homeostasis in Patients With Congestive Heart Failure. J Card Fail 13 (2007): 170-177.
- Firoz M, Graber M. Bioavailability of US Commercial Magnesium Preparations. Magnes Res 14 (2001): 257-262.
- Guerrera M, Volpe S, James J. Therapeutic Uses of Magnesium. Am Fam Physician 80 (2009): 157-162.
- Tinawi M. Hypertension in Pregnancy. Arch Intern Med Res 2020 3 (1): 10-17.
- Okusanya B, Oladapo O, Long Q, et al. Clinical Pharmacokinetic Properties of Magnesium Sulphate in Women With Pre-Eclampsia and Eclampsia. Br J Obstet Gynaecol 123 (2016): 356-366.
- Lake A, Al Khabbaz A. Severe Preeclampsia in the Setting of Myasthenia Gravis. Case Rep Obstet Gynecol (2017).
- Girard B, Beucher G, Muris C, et al. Magnesium Sulphate and Severe Preeclampsia: Its Use in Current Practice. J Gynecol Obs Biol Reprod 34 (2005): 17-22.
- Chao A. The Patellar Reflex in Preeclamptic Women With Subtherapeutic and Therapeutic Serum Magnesium Levels. J Reprod Med 35 (1990): 678-681.
- Shoaib Khan M, Zahid S, Ishaq M. Fatal Hypermagnesemia: an acute ingestion of Epsom Salt in a patient with normal renal function. Casp J Intern Med 9 (2018): 413-415.
- Tinawi M. Diagnosis and Management of Hyperkalemia. Arch Clin Biomed Res 4 (2020): 153-168.
- Jhang W, Lee Y, Kim Y, et al. Severe hypermagnesemia presenting with abnormal electrocardiographic findings similar to those of hyperkalemia in a child undergoing peritoneal dialysis. Korean J Pediatr 56 (2013): 308-311.
- Taber F, Tan L, Chao C, et al. Pharmacokinetics of ionized versus total magnesium in subjects with preterm labor and preeclampsia. Am J Obs Gynecol 186 (2002): 1017-1021.
- Faridi A, Weisberg L. Acid-Base, Electrolyte, and Metabolic Abnormalities. In: Critical Care Medicine (Third Edition) (2008): 1203-1244.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341 (1999): 709-717.
- Palmer BF. Potassium Binders for Hyperkalemia in Chronic Kidney Diseased-Diet, Renin-Angiotensin-Aldosterone System Inhibitor Therapy, and Hemodialysis. Mayo Clin Proc 95 (2020): 339-354.
- Al-Shibli A, Konrad M, Altay W, et al. Familial Hypomagnesemia With Hypercalciuria and Nephrocalcinosis (FHHNC): Report of Three Cases With a Novel Mutation in CLDN19 Gene. Saudi J Kidney Dis Transplant 24 (2013): 338-344.
- Weber S, Schneider L, Peters M, et al. Novel paracellin-1 Mutations in 25 Families With Familial Hypomagnesemia With Hypercalciuria and Nephrocalcinosis. J Am Soc Nephrol 12 (2001): 1872-1881.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 