Effect of Actovegin® Versus Cortisone on PMAâ€Induced Inflammation on Human Cells
Franz-Xaver Reichl1*, Christof Hogg1, Fangfang Liu1, Markus Schwarz2, Daniel Teupser2, Reinhard Hickel1, Wilhelm Bloch3, Helmut Schweikl4, Peter Thomas5, Burkhard Summer5
1Department of Conservative Dentistry and Periodontology, University Hospital, LMU Munich, Goethestr. 70,80336 Munich, Germany
2Institute for Laboratory Medicine, University Hospital, LMU Munich, Munich, Germany
3Molecular and Cellular Sport Medicine, German Sport University, Cologne, Germany
4Department of Conservative Dentistry and Periodontology, University Hospital, Regensburg, Germany
5Department of Dermatology and Allergy, University Hospital, LMU Munich, Munich, Germany
*Corresponding Author: Franz-Xaver Reichl, Department of Conservative Dentistry and Periodontology, University Hospital, LMU Munich, Goethestr. 70,80336 Munich, Germany.
Received: 29 November 2022; Accepted: 06 December 2022; Published: 13 December 2022
Article Information
Citation:
Reichl FX, Högg C, Liu F, Schwarz M, Teupser D, Hickel1 R, Bloch W, Schweikl H, Thomas P, Summer B. Effect of Actovegin® Versus Cortisone on PMAâ€Induced Inflammation on Human Cells. Journal of Orthopedics and Sports Medicine 4 (2022): 317-326.
View / Download Pdf Share at FacebookAbstract
Purpose: The effect of Actovegin® versus Cortisone was investigated on PMA induced human Peripheral Blood Mononuclear Cells (PBMCs).
Methods: PBMCs (1 × 10 cells/ml) from ten blood donors (5 f, 5 m; 45–55 years) were grown in medium and exposed to Actovegin® or Cortisone in the presence or absence of PMA. Supernatants were collected to assess the concentration of cytokines/substances: IL-6, TNF-α, IL-8, IL-1 beta, and IL-10. The Reactive Oxygen Species (ROS) were assessed by a ROSGloTM H2O2 assay.
Results: Stimulation of cells with PMA (1 μg/ml) (without Actovegin® or Cortisone) significantly (p<0.05) increased the secretions of IL-6, TNF-α, IL-8, IL-1 beta, and IL-10 from PBMCs, compared to controls (without PMA). Addition of Actovegin® (20 μg/ml) or Cortisone (0.7 and 7 μg/ ml) plus PMA significantly decreased the secretion of TNF-α, compared to controls (without Actovegin® or Cortisone). Addition of Actovegin® (1 and 20 μg/ml) plus PMA significantly decreased the secretion of IL- 8, compared to controls (without Actovegin®). However, addition of Cortisone (0.7 and 7 μg/ml) plus PMA did not influence the secretion of IL-8, compared to controls (without Cortisone). Addition of Actovegin® (20 μg/ml) plus PMA significantly decreased the secretion of IL-1Beta, compared to controls (without Actovegin®). Addition of Cortisone (0.7 μg/ml) plus PMA increased the secretion of IL-1Beta, compared to controls (without Cortisone). Addition of Actovegin® (20 μg/ml) plus PMA significantly increased the secretion of IL-10, compared to controls (without Actovegin). However, addition of Cortisone (0.7 and 7 μg/ml) plus PMA did not influence the secretion of IL-10, compared to controls (without Cortisone). Addition of Actovegin®
Keywords
<p>Sports; Inflammation; PMA; Human PBMCs; IL-6; TNF-α; IL-8; IL1-beta; IL-10; ROS</p>
Article Details
Abbreviations:
MRI- Magnetic Resonance Imaging; NADPH- Nicotinamide Adenine Dinucleotide Phosphate; NLRP3- Nucleotide-Binding Oligomerization Domain-Like Receptor Containing-Pyrin Domain 3; PAMP- Pathogen-Associated Molecular Pattern; PBMCs- Human Peripheral Blood Mononuclear Cells; PKC- Protein Kinase C; PMA- Phorbol 12-Myristate 13-Acetate; ROS- Reactive Oxygen Species; TLR4- Toll-like Receptor 4
WADA- World Anti-Doping Agency
1. Introduction
Cortisone is a corticosteroid and it has been used as a medical drug for the past 70 years in the treatment of various musculoskeletal conditions. This includes its use for joint pain such as inflammation reactions, rheumatoid arthritis, osteoarthritis, and many others. There are numerous review articles to corticosteroids (cortisone) including the history, the effectiveness, and adverse effects in the treatment of joint pain [1,2]. The current evidence would suggest that the use of corticosteroids provides moderate evidence for short-term pain reduction and improvement in function. There are multiple potential adverse effects, such as toxic damage to articular cartilage, as well as numerous systemic side effects, including increase in blood glucose levels, a reduction in immune function and an increased risk of infections [1,2].
Actovegin® is also a medical drug obtained from natural calf blood. Over 60 years, many medical indications are treated by Actovegin®, e.g., acute stroke [3,4] or postpartum hemorrhage (intravenous infusion) [5], skin ulcers (topical medication) [6], and long bone fractures (intraarterial infusion) [7], malfunction of the blood circulation in the brain and trophic disturbances (e.g., ischemic insult, craniocerebral injury) [8], impairment of peripheral blood circulation (e.g., angiopathy and ulcus cruris) [7,9,10], wound healing issues (e.g., torpid wounds, decubitus) [7,11-13] and mucosal lesions after radiation [7,14-17].
Muscle injury incidence varies from 30 to 55%; therefore, it is one of the most common sports-related injuries [18-20]. Twelve percent of all muscle injuries are hamstring injuries, which are 2.5 times more frequent than, for example, quadriceps injuries [21,22]. It has been shown that muscle healing can be promoted by administration of anti-inflammatory drugs [23]. However, anti-inflammatory drugs also can have an adverse effect on the entire healing process [24]. Moreover, a recent systematic review illustrates the potential myotoxicity of local anesthetics and non-steroidal anti-inflammatory drug injection, while there is no evidence that Actovegin® has such a side effect [25]. A variety of treatments such as growth factor injection therapy is still very experimental and has shown initial results in some pilot studies; however, due to their performance enhancing and anabolic properties, they are prohibited by the World Anti-Doping Agency (WADA) [26].
In 1990 Pfister and Koller [27] first described intramuscular injection of Actovegin® as treatment of muscle injuries in a partially blinded case control study with 102 patients [27]. Their study showed a reduction in recovery time in a treatment group of 5.5 weeks, compared to 8.3 weeks for the control group [27]. However, in this study, the diagnosis of specific muscle injuries was only based on clinical findings and was not graded according to imaging, e.g., Magnetic Resonance Imaging (MRI). Furthermore, Actovegin® was mixed with anesthetics before injection resulting in pharmacodynamic and pharmacokinetic alterations [27].
In vivo and in vitro studies suggest that Actovegin® contains some active components, although they were not identified [6,28-30]. In a previous in vitro study, an enhancement of the mitochondrial oxidative phosphorylation was registered in permeabilized human muscle fibers (obtained from overweight and untrained subjects) acutely exposed to Actovegin® [31]. Hitherto, the effect of stand-alone Actovegin® therapy in muscle precursor cells highly relevant in skeletal muscle regeneration was not investigated in vivo and/or in vitro studies. To investigate effects of various substances/solutions on muscle precursor cell proliferation, optimal experimental conditions are represented by C2C12 muscle cells [30]. In our recent study, the effect of a stand-alone Actovegin® addition on the proliferation of C2C12 muscle cells was described, and Actovegin® increased the proliferation of muscle cells [32]. Furthermore, in this study the ingredients of Actovegin® were identified and the active substances on muscle proliferation were discussed in detail [32].
There is much media attention and there are many anecdotal beliefs regarding Actovegin® injection therapy. In the lay press, controversial discussions between proponents and opponents have been published in recent years regarding the use of Actovegin® in high performance athletes. In our recent study a risk assessment was given and it could be demonstrated that Actovegin® may not be classified as a doping agent [32]. Furthermore, some clinical studies for Actovegin® confirm its safety [28,33,34]. The effect of anti-inflammatory drugs on muscle regeneration is controversial discussed. It was described that anti-inflammatory drugs can improve muscle regeneration by reducing degeneration and inflammation [23]. However, in other studies, it was described that anti-inflammatory drugs are not conducive to the healing process [24,35].
Human Mononuclear Cells of the Peripheral Blood (PBMCs) are a useful tool to investigate anti-inflammatory effects of substances or antigens, as these immune cells of the peripheral blood actively participate in the healing processes after inflammation [36,37].
In the present study the effects of Actovegin® versus Cortisone were investigated on inflammation reactions on human PBMCs. Our hypothesis was that Actovegin® and Cortisone have anti-inflammatory effects on human cells.
2. Materials and Methods
2.1 Cell culture and cell exposure
Stimulation assays were performed according to Summer et al. [36], with the optimizations reported by Stander et al. [38]. Heparinized blood was taken from anonymized healthy blood donors (five females, five males, 45 − 55 years, non-smokers, no drug administration, no medication). After isolation of Peripheral Blood Mononuclear Cells (PBMCs) by density centrifugation, PBMCs of each blood donor were separately cultivated with Phorbol 12-Myristate 13-Acetate (PMA) (1 μg/ml, Sigma-Aldrich, Munich, Germany) with or without Actovegin® or Cortisone in different concentrations in quadruplicate. Cells (1 × 106 cells/ml) were grown in RPMI 1640 medium in 96-well plates at 37°C for 24 h. Actovegin® (200 mg/5 ml; Lot-No. 10946788; Takeda Austria GmbH, Linz, Austria) was directly diluted in cell culture medium (1 and 20 μg/ml) or to Cortisone (0.7 and 7 μg/ml), exactly as described in a recent investigation on muscle cell proliferation [32]. Cell cultures were exposed to these Actovegin® or Cortisone concentrations in the presence or absence of PMA (1 μg/ml) for 24 h. After the exposure, culture supernatants were collected for cytokine/substance analysis.
The ten blood donors were healthy individuals with normal blood cell counts with 1500–3000 lymphocytes/μl blood and 280–500 monocytes/μl blood. As for the healing process after inflammation all blood cells support the healing process we wanted to simulate a quite physiological situation with all mononuclear blood cells as already described in our previous study [39].
2.2 Cytokine assays
The amount of IL-6, TNF-α, IL-8, IL-1 beta, and IL-10 was assessed by a multiplex cytometric bead assay according to the manufacturer's protocol (BD, Biosciences, Heidelberg, Germany) in a FACS Canto flow cytometer.
2.3 ROS assessment
The Reactive Oxygen Species (ROS) were assessed in an identical experimental assay by ROS-GloTM H2O2 Assay (Promega, Mannheim, Germany) according to the manufacturer protocol.
2.4 Statistical analyses
Individual data from independent experiments were now summarized as medians (25–75% quartiles). Statistically significant differences between mean values were calculated using now the one-way ANOVA-Test followed by Games Howell post hoc test (SPSS Statistics 23, IBM, Armonk, NY, USA). The level of statistical significance was set to p < 0.05.
3. Results
Stimulation of cells with PMA (1 µg/ml)(without Actovegin® or Cortisone) significantly (p<0.05) increased the secretions of IL-6, TNF-α, IL-8, IL-1 beta, and IL-10 from PBMCs, compared to controls (without PMA) (Table 1).
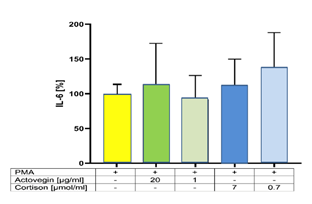
Addition of Actovegin® (1 and 20 μg/ml) or Cortisone (0.7 and 7 μg/ml) plus PMA did not influence the secretion of IL-6, compared to controls (without Actovegin® or Cortisone) (Figure 1).
|
IL-6 |
TNF alpha |
IL-8 |
IL-1 beta |
IL-10 |
ROS |
|
|
Medium |
0.0 pg/ml |
0.0 pg/ml |
1319 pg/ml ± 1000 |
0.0 pg/ml |
0.0 pg/ml |
212160 rlu ± 2345 |
|
PMA |
783 pg/ml ± 124 |
678 pg/ml ± 39 |
11968 pg/ml ± 2322 |
755 pg/ml ± 358 |
1.2 pg/ml ± 1.0 |
1096560 rlu ± 4521 |
Table 1: Cytokine and ROS formation of PBMC after stimulation with PMA for 24h (without Actovegin® or Cortisone) (mean ± sem, n=10). rlu = relative light unit (luminescence).
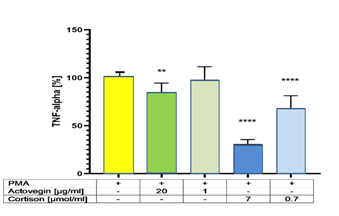
Addition of Actovegin® (20 μg/ml) or Cortisone (0.7 and 7 μg/ml) plus PMA significantly (p<0.05) decreased the secretion of TNF-α, compared to controls (without Actovegin® or Cortisone) (Figure 2).
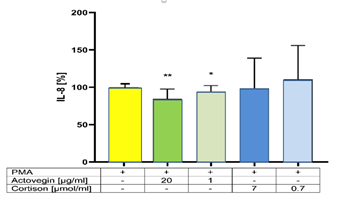
Addition of Actovegin® (1 and 20 μg/ml) plus PMA significantly (p<0.05) decreased the secretion of IL-8, compared to controls (without Actovegin®). However, addition of Cortisone (0.7 and 7 μg/ml) plus PMA did not influence the secretion of IL-8, compared to controls (without Cortisone) (Figure 3).
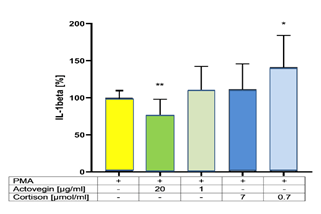
Addition of Actovegin® (20 μg/ml) plus PMA significantly (p<0.05) decreased the secretion of IL-1Beta, compared to controls (without Actovegin®). Addition of Cortisone (0.7 μg/ml) plus PMA significantly (p<0.05) increased the secretion of IL-1Beta, compared to controls (without Cortisone) (Figure 4).
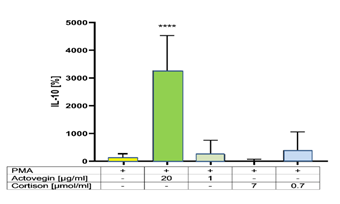
Addition of Actovegin® (20 μg/ml) plus PMA significantly (p<0.05) increased the secretion of IL-10, compared to controls (without Actovegin). However addition of Cortisone (0.7 and 7 μg/ml) plus PMA did not influence the secretion of IL-10, compared to controls (without Cortisone) (Figure 5).
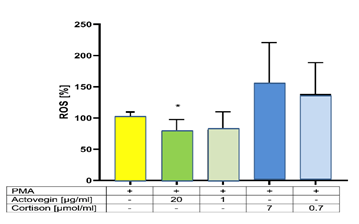
Addition of Actovegin® (20 μg/ml) plus PMA significantly (p<0.05) decreased the ROS formation, compared to controls (without Actovegin®). However addition of Cortisone (0.7 and 7 μg/ml) plus PMA did not influence the ROS formation, compared to controls (without Cortisone) (Figure 6).
4. Discussion
Until today, the effect of anti-inflammatory drugs on muscle healing after injury is controversially discussed: Anti-inflammatory drugs can improve muscle regeneration by reducing degeneration and inflammation [23], in contrast, it is described that anti-inflammatory drugs are not conducive to the healing process [24,35].
PBMCs are a useful tool to investigate inflammation reactions. PBMCs (i.e., lymphocytes and monocytes) play a central role in muscle repair and regeneration during the inflammation that follows muscle injury. Therefore, we used in our model the PBMCs as described in previous study [40]. Here, we study the effect of Actovegin® versus Cortisone in Phorbol 12-Myristate 13-Acetate (PMA)-induced inflammation on human PBMCs.
PMA is a potent tumor promoter via activation of the signal transduction enzyme Protein Kinase C (PKC) [41-43]. The PKC pathway is critically involved in function and development of B cells, as well [44]. PMA is routinely used as an inducer for endogenous superoxide production, as well, since it has been demonstrated to induce superoxide as the major ROS [45,46]. ROS are generated by NADPH oxidase in a process called the respiratory burst. This key enzyme catalyzes the generation of superoxide and hydrogen peroxide using electron provided by the hexose monophosphate shunt [47-49]. NADPH oxidase of phagocytic cells can also be activated by protein kinase C agonists such as PMA [50].
PMA initiates acute inflammatory responses in mammals that are characteristic for the host reaction to tissue injury and/or infection [51].
Tissue response following injury or surgical trauma involves participation of blood-derived components, cytokines and growth factors. PMA induces high cytokine responses in PBMCs after 24 h stimulation including proinflammatory cytokines as IL-1beta, IL-6, IL-8 and TNF-α but also anti-inflammatory cytokines as for example IL-10 and is, therefore, widely used as a suitable positive control in in vitro PBMCs stimulation experiments, where high cytokine production is expected and where freshly isolated PBMCs are used [52,53].
Wu et al. [54] described IL-1 beta, IL-6, IL-8, IL-10 or TNF-α as important inflammatory cytokines/substances. In the present study the effects of Actovegin® versus Cortisone on the release of these cytokines/substances were investigated in PMA-stimulated human PBMCs. The PMA-induced increase in the secretion of the pro-inflammatory cytokine IL-1 beta was dose dependently inhibited by addition of Actovegin® but dose dependently increased by Cortisone. Whereas the secretion of the pro-inflammatory TNF-α was dose-dependently decreased with both, Actovegin® and Cortisone. However, the secretion of the anti-inflammatory cytokine IL-10 was significantly increased by addition only with Actovegin® but not with Cortisone. Oxidative damage plays a key role in inflammatory reactions and can induce several injuries (e.g., septic shock, atherosclerose) induced by PMA which is known to enhance the formation of Reactive Oxygen Species (ROS) [46,55]. PMA can induce the activation of TLR4 in the cell wall, which can induce ROS, e.g., superoxide [46]. In the present study a dose dependent decrease of ROS was observed after addition with Actovegin®, but not with Cortisone.
Two questions are arising from our results:
- what is the mechanism of Actovegin’s® effect of ROS formation?
- why is there a significant effect of Actovegin® on IL-1beta, IL-8, and IL-10 production but not with cortisone?
4.1 Ad 1 (ROS formation)
Possible explanations are that Actovegin® may support the antioxidative systems in the cells consisting of enzymatic and non-enzymatic antioxidants. Enzymes like catalase and substances such as glutathione as the major non-enzymatic antioxidant reduce oxidative stress by decreasing the levels of Reactive Oxygen Species (ROS). Concentrations of ROS are crucial in the formation of inflammatory cytokines such as IL-1beta and IL-6 as well [56]. In our recent study high levels of cystathionine in Actovegin® were detected, compared to the human adult serum/plasma [32]. Cystathionine is a precursor of cysteine synthesis which in turn is a component of the tripeptide glutathione. Both cysteine and glutathione contain sulfhydryl-groups and can, therefore, effectively act as antioxidants [57]. Thus, an antioxidative effect of Actovegin® may be at least in part explained by the high availability of cystathionine.
Another antioxidative system represents the enzyme catalase (oxidoreductase). Human catalase is a peroxisomal enzyme. It is implicated in inflammation, ethanol metabolism, apoptosis, aging and cancer [58]. It is a common enzyme found in nearly all living organisms predominantly in the liver, kidney and erythrocytes [58]. It is a very important enzyme in protecting the cell from oxidative damage by Reactive Oxygen Species (ROS). Catalase has one of the highest turnover numbers, each second one molecule can convert millions of hydrogen peroxide molecules to water and oxygen [58]. Superoxide is also biologically toxic and is employed by the immune system to kill invading microorganisms. Superoxide can be converted in cells into hydrogen peroxide which can further be catalyzed by catalase. Catalase is a tetramer of four polypeptide chains, each over 500 amino acids long, with four iron-containing heme groups that allow the enzyme to react with the ROS. Most intersubunit contacts are confined to the amino-terminal arms and the wrapping domains. The amino-terminal domain becomes almost completely buried between neighboring subunits in the tetramer. There are numerous salt bridges at the interfaces between monomers, mostly involving glutamic acid, asparagine acid, and arginine [59,60]. In our recent study high levels of glutamic acid, asparagine acid, and arginine in Actovegin® were detected, compared to the human adult serum/plasma [32]. The antioxidative effect of Actovegin® may be explained by the high availability of these Actovegin® ingredients. Therefore, the observed antioxidative effect of Actovegin® may be better understandable.
4.2 Ad 2 (differential effects after Actovegin® or Cortisone addition)
PMA has been shown to exert its well-known effect on monocyte differentiation via caspase-1 activation [61]. Ribeiro and colleagues [62] could further demonstrate that activated Protein Kinase C (PKC) further activates mTORC1/ S6K pathway [62]. This observation is important for the interpretation of our results, since PMA is a potent activator of PKC [63]. PMA is known to promote tumor outgrowth through activation of serine/threonine-specific protein kinases. These serine/threonine-specific protein kinases appear to be responsible for the abnormal phosphorylations of CD23 protein in healthy B cells, as well [64]. Antagonism of this mechanism might be relevant for the effect of Actovegin® as well.
In this study a significant decrease was found only for the pro-inflammatory cytokines IL1-beta and IL-8 after addition of Actovegin® with PMA, compared to the release without Actovegin®, but not with addition of cortisone. In contrast the addition of cortisone even increased the IL-1beta formation, compared to Actovegin® addition.
L1-beta is a key inflammatory mediator driving the host response to infection, injury, and disease. IL1-beta driven inflammation has often disastrous consequences, and thus represents a therapeutic target [65]. Caspase 1 is activated by recruitment to a molecular platform called an inflammasome [66] and caspase 1 is considered to belong to the inflammatory group [67]. Inhibition of caspase 1 would be anti-inflammatory by preserving cell viability and, therefore, limiting the release of Pathogen-Associated Molecular Pattern (PAMPs), consequently resulting in less inflammation [68]. Inhibition or deletion of caspase 1 improves, e.g., outcome after myocardial infarction [69-71]. Studies describing clinical use of anti-IL-1 therapies focus almost on the use of biologicals such as IL-1Ra (anakinra) or anti-IL-1b antibodies such as canakinumab and other substances [72] but were not successful [65].
ICEberg is a protein that inhibits generation of IL-1beta by interacting with caspase-1 [73]. ICEberg is induced in human cells by pro-inflammatory stimuli, suggesting that it may be part of a negative feedback loop. Consistent with this, enforced retroviral expression of ICEberg inhibits IL-1beta generation [54]. The distribution of surface charge is complementary to the homologous prodomain of caspase-1, suggesting that charge–charge interactions mediate binding of ICEberg to the prodomain of caspase-1 [74]. Humke et al. [74] detected in ICEberg in main domains (helix 1–6) the following aminoacids: Arginine (Arg), Lysine (Lys), Glutamic acid (Glu) and Asparagine acid (Asp). The surface of ICEberg contains three highly charged patches [74]. In our recent study it could be demonstrated that Actovegin® contains many physiological substances in significantly higher concentrations, compared to human adult serum [32]. The ICEberg aminoacids Arg, Lys, Glu, and Asp were found with 2-, 4-, 14-, and 14-fold higher concentrations, compared to human adult serum [32]. For the intact ICEberg synthesis and ICEberg function, these aminoacids are necessary and must be also available in the cells. The significantly decreased IL-1beta release, after Actovegin® application may be explained by the successful synthesis of the enzyme ICEberg, which is only possible by availability of these relevant aminoacids. Then ICEberg may powerfully inhibit caspase 1 and may, therefore, result in an anti-inflammatory effect.
For an intact antioxidative and/or anti-inflammatory system with many proteins, not only amino acids are necessary, for the anabolic and catabolic pathways just like energy (e.g., ATP) and important inorganic substances (e.g., potassium, chloride, sodium, phosphate) are also necessary. ATP may be formed from increased availability and uptake of glucose. In the recent Actovegin® analysis for glucose a fourfold higher level, and for potassium, chloride, sodium, and phosphate up to tenfold higher levels were detected, compared to the corresponding substance levels in the adult human physiological serum/plasma [32]. Therefore, the observed anti-inflammatory effect of Actovegin® may be explained by the high availability of these Actovegin® ingredients in cells.
It is to note that TNF-α and IL-6 are also pro-inflammatory cytokines. However, the addition of Actovegin® or cortisone with PMA did not lead to a decrease of the release of IL-6. Therefore, IL-6 does not contribute to the reduction of inflammatory reactions with Actovegin® or with Cortisone.
The addition of Cortisone decreased the secretion of TNF-α, compared to the Actovegin® addition. Only in this case cortisone was superior to Actovegin®. Why cortisone is superior to Actovegin® only in TNF-α inhibition has to be clarified in further studies. Cortisone is a powerful drug but it also has many side effects as described above, especially with prolonged use [1,2].
IL-10 is an anti-inflammatory cytokine. The addition of Actovegin® (20 µg/ml) with PMA did lead to an increase of the IL-10 release; therefore, IL-10 significantly contributes to the reduction of inflammatory reactions with Actovegin®, but not with cortisone. Why? High contents of following amino acids were found in the IL-10 protein structure: glutamic acid, asparagin acid, leucine, glycine, isoleucine, alanine, lysine, valine, tyrosine, histidine, methionine, threonine, arginin, and histidine. In our recent study it could be demonstrated that Actovegin® contains these physiological amino acids in up to 14-fold higher concentrations, compared to human adult serum [32]. For an optimal IL-10 synthesis and function, these aminoacids are necessary and must be also available in the cells. The significantly increased IL-10 release, after Actovegin® application (and not by cortisone) may be explained by the successful synthesis of the IL-10, which is only possible by availability of these relevant aminoacids. Then IL-10 may be powerful synthethized and may therefore, result in a higher anti-inflammatory effect, compared to cortisone.
Our hypothesis is confirmed. Both, Actovegin® and Cortisone exert an anti-inflammatory effect, by dose-dependently diminishing the PMA-induced release of the pro-inflammatory TNF-α. However, a dose-dependent diminish of the PMA-induced release of the pro-inflammatory IL-8, IL-1Beta and ROS was observed only for Actovegin® but not for Cortisone - likewise an increase of the release of the anti-inflammatory IL-10 was found only for Actovegin® but not for Cortisone.
It is mentioned that the transferability of in vitro results to the human physiological situation is limited but it is to note that this study has also a new direct relation to inflammations in sports medicine. Actovegin® is not only used in the above mentioned scopes of application and in skeletal muscle, but also as anti-inflammatory medication in skeletal muscle and in tendinopathies, e.g., on the patellar and achilles tendon [3-17,32]. Actovegin® is used as peritendinous injection (not intra-tendinous). Clinical experience indicates that inflammatory response and adhesions in the peritendinous tissue can be reduced with several injections of Actovegin® [75]. The present study was conducted to analyse if Actovegin® has an anti-inflammatory effect, compared to Cortisone. The data support the clinical therapeutic findings and can help to explain how Actovegin® may work as a therapeutic agent when it is injected into inflamed tissue, e.g., around the patellar tendon, the achilles tendon or other locations that are mechanically inflamed and therefore could be an alternative biocompatible drug for these treatments, compared to cortisone.
5. Conclusion
Both, Actovegin® and Cortisone exert an anti-inflammatory effect, by dose-dependently diminishing the PMA-induced release of the pro-inflammatory TNF-α. However, a dose-dependent decrease of the pro-inflammatory IL-8, IL-1Beta and ROS and an increase of the anti-inflammatory IL-10 were observed only for Actovegin® but not for Cortisone. These findings may help to explain the positive effects of Actovegin® on inflammation injuries, compared to Cortisone. Therefore, Actovegin® may also contribute to the reduction of inflammation reactions.
Acknowledgements
The authors would like to thank Mr. Stefan Schulz for his technical support.
Author contributions
Conceptualization, FXR, CH, BS, HS; Methodology, FL, BS, MS, HS; Data analysis and interpretation, BS, FXR, CH, MS; Manuscript preparation, FXR, CH, MS, BS; Review/editing, DT, RH, WB, PT, CH; All authors read and approved the final manuscript for submission.
Funding
This work received no funding from any grant or organization.
Data availability
All data generated or analysed during this study are included in this published article.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee (LMU Munich, Project Nr: 19-331 KB) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human and animal participants
This article does not contain any studies with animals performed by any of the authors.
References
- Burns CM. The History of Cortisone Discovery and Development. Rheum Dis Clin North Am 42 (2016): 1-14.
- Stone S, Malanga GA, Capella T. Corticosteroids: Review of the History, the Effectiveness, and Adverse Effects in the Treatment of Joint Pain Physician 24 (2021): S233-246.
- Boiarinov GA, Mukhina IV, Penknovich AA, et al. Mechanisms of actovegin effect on the central nervous system during postischemic period. Biull Eksp Biol Med 126 (1998): 395-398.
- Derev’yannykh EA, Bel’skaya GN, Knoll EA, et al. Experience in the use of Actovegin in the treatment of patients with cognitive disorders in the acute period of stroke. Neurosci Behav Physiol 38 (2008): 873-875.
- Appiah AK. Treatment of severe primary postpartum hemor- rhage with a deproteinized hemodialysate. Int J Gynaecol Obstet 76 (2002): 75-76.
- Biland L, Hurlimann F, Goor W, et al. Treatment of venous ulcers. A multi-center randomized double-blind study. Vasa 14 (1985): 383-389.
- Buchmayer F, Pleiner J, Elmlinger MW, et al. Actovegin (R): a biological drug for more than 5 decades. Wien Med Wochenschr 161 (2011): 80-88.
- Somogyi E, Sotonyi P, Nemes A. The effects of a deproteinized blood extract on the myocardial changes developing during experimentally induced intermittent hypoxia. Arzneimittelforschung 29 (1979): 1376-1381.
- Chanh PH, Chanh AP, Basile JP, et al. Car- diovascular activity of a deproteinized blood extract. Arzneimit- telforschung 30 (1980): 1874-1877.
- Lanner G, Argyropoulos. Pharmacological effect of Solcoseryl on the metabolism of the brain Animal experiments and clinical research. Wien Med Wochenschr 125 (1975): 681-685
- Mochida H, Kikuchi T, Tanaka H, et al. Influence of Actovegin® containing infusion solutions on wound healing and function of the intestinal tract in rats. Pharmacol Ther 17 (1989): 789-797.
- Neinhardt J. Extra- und intraorale Wundheilung. Dissertation/ PhD Thesis, Universitats- und Poliklinik fur Zahn-, Mund-, und Kieferkrankheiten Wurzburg (1967).
- Schonwald D, Sixt B, Machicao F, et al. Enhanced proliferation of coronary endothelial cells in response to growth factors is synergized by hemodialysate compounds in vitro. Res Exp Med (Berl) 191 (1991): 259-272
- Basu SK, Srinivasan MN, Chuttani K, et al. Evaluation of some radioprotectors by the survival study of rats exposed to lethal dose of wholebody gamma radiation. J Radiat Res 26 (1985): 395-403.
- Bauer D, Locker A. The radioprotective effect of solcoseryl. Experientia 30 (1974): 643.
- Beetz A, Machicao F, Ried C, et al. Radiopro- tective effects of a protein-free hemodialysate in human epider- mis. Skin Pharmacol 9 (1996): 197-202.
- Spessotto P, Dri P, Baschong W, et al. Effect of a protein-free dialysate from calf blood on human monocyte differentiation in vitro. Arzneimittelforschung 43 (1993): 747-751.
- Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med 39 (2011): 1226-1232.
- Jarvinen TA, Kaariainen M, Jarvinen M, et al. Muscle strain injuries. Curr Opin Rheumatol 12 (2000): 155-161.
- Verrall GM, Slavotinek JP, Barnes PG, et al. Clinical risk factors for hamstring muscle strain injury: a prospective study with correlation of injury by magnetic resonance imaging. Br J Sports Med 35 (2001): 435-439
- Askling C, Karlsson J, Thorstensson A. Hamstring injury occurrence in elite soccer players after preseason strength training with eccentric overload. Scand J Med Sci Sports 13 (2003): 244-250.
- Woods C, Hawkins RD, Maltby S, et al. Football Association Medical Research P (2004) The Football Association Medical Research Programme: an audit of injuries in professional football–analysis of hamstring injuries. Br J Sports Med 38 (2004): 36-41.
- Abramson S, Weissmann G. The mechanisms of action of non- steroidal antiinflammatory drugs. Clin Exp Rheumatol 7 (1989): S163-170.
- Obremsky WT, Seaber AV, Ribbeck BM, et al. Biomechanical and histologic assessment of a controlled muscle strain injury treated with piroxicam. Am J Sports Med 22 (1994): 558-561.
- Reurink G, Goudswaard GJ, Moen MH, et al. Myotoxicity of injections for acute muscle injuries: a systematic review. Sports Med 44 (2014): 943-956.
- Prohibited List January 2019. World Anti-Doping Agency (2019).
- Pfister A, Koller W. Treatment of fresh muscle injury. Sportverletz Sportschaden 4 (1990): 41-44.
- Pforringer W, Pfister A, Kuntz G. The treatment of achilles paratendinitis: results of a double-blind, placebo-controlled study with a deproteinized hemodialysate. Clin J Sport Med 4 (1994): 92-99
- Wright-Carpenter T, Klein P, Schaferhoff P, et al. Treatment of muscle injuries by local admin- istration of autologous conditioned serum: a pilot study on sportsmen with muscle strains. Int J Sports Med 25 (2004): 588-593.
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270 (1977): 725-727.
- Sondergard SD, Dela F, Helge JW, et al. Actovegin, a non-prohibited drug increases oxidative capacity in human skeletal muscle. Eur J Sport Sci 16 (2016): 801-807.
- Reichl FX, Holdt LM, Teupser D, et al. Comprehensive Analytics of Actovegin (R) and Its Effect on Muscle Cells. Int J Sports Med 38 (2017): 809-818.
- Maillo L. Anaphylactic shock with multiorgan failure in a cyclist after intravenous administration of Actovegin. Ann Intern Med 148 (2008): 407.
- Ziegler D, Movsesyan L, Mankovsky B, et al. Treatment of symptomatic polyneuropa- thy with actovegin in type 2 diabetic patients. Diabetes Care 32 (2009): 1479-1484.
- Shen W, Li Y, Tang Y, et al. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol 167 (2005): 1105-1117.
- Summer B, Paul C, Mazoochian F, et al. Nickel (Ni) allergic patients with complications to Ni containing joint replacement show preferential IL-17 type reactivity to Ni. Contact Dermatitis 63 (2010): 15-22.
- Thomas P, Iglhaut G, Wollenberg A, et al. Allergy or tolerance: reduced inflammatory cytokine response and concomitant IL-10 production of lymphocytes and monocytes in symptom-free titanium dental implant patients. Biomed Res Int 2013 (2013): 539834.
- Ständer S, Oppel E, Thomas P, et al. Evaluation of lymphocyte transformation tests as compared with patch tests in nickel allergy diagnosis. Contact Dermatitis 76 (2017): 228-234.
- Summer B, Stander S, Thomas P. Cytokine patterns in vitro, in particular IL-5/IL-8 ratio, to detect patients with nickel con- tact allergy. J Eur Acad Dermatol Venereol 32 (2018): 1542-1548.
- Tidball JG Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 27 (1995): 1022-1032
- Castagna M, Takai Y, Kaibuchi K, et al. Direct activation of calcium-activated, phospholipid- dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem 257 (1982): 7847-7851.
- Blumberg PM. Protein kinase C as the receptor for the phorbol ester tumor promoters: sixth Rhoads memorial award lecture. Cancer Res 48 (1988): 1-8.
- Niedel JE, Kuhn LJ, Vandenbark GR. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci USA 80 (1983): 36-40.
- Ghamlouch H, Ouled-Haddou H, Guyart A, et al. Phorbol myristate acetate, but not CD40L, induces the differ- entiation of CLL B cells into Absecreting cells. Immunol Cell Biol 92 (2014): 591-604.
- Huang R, Zhao L, Chen H, et al. Megakaryocytic differentia- tion of K562 cells induced by PMA reduced the activity of res- piratory chain complex IV. PLoS ONE 9 (2014): e96246.
- Swindle EJ, Hunt JA, Coleman JW. A comparison of reactive oxygen species generation by rat peritoneal macrophages and mast cells using the highly sensitive real-time chemiluminescent probe pholasin: inhibition of antigen-induced mast cell degranulation by macrophage-derived hydrogen peroxide. J Immunol 169 (2002): 5866-5873.
- Bastian NR, Hibbs JB Jr. Assembly and regulation of NADPH oxidase and nitric oxide synthase. Curr Opin Immunol 6 (1994): 131-139.
- Segal AW. The NADPH oxidase of phagocytic cells is an elec- tron pump that alkalinises the phagocytic vacuole. Protoplasma 184 (1995): 86-103.
- Jones RD, Hancock JT, Morice AH. NADPH oxidase: a universal oxygen sensor? Free Radic Biol Med 29 (2000): 416-424.
- Segal AW, Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci 18 (1993): 43-47.
- Basyreva LY, Brodsky IB, Gusev AA, et al. The effect of Intravenous Immunoglobulin (IVIG) on \textit{ex vivo} activation of human leukocytes. Hum Antibodies 24 (2016): 39-44.
- David KC, Brady MT, Weimer LK, et al. Characterization of the in vitro anti-inflammatory activity of AL-5898 and related benzopyranyl esters and amides. Inflammation 27 (2003): 31-43.
- Jeurink PV, Vissers YM, Rappard B, et al. T cell responses in fresh and cryopreserved peripheral blood mononu- clear cells: kinetics of cell viability, cellular subsets, proliferation, and cytokine production. Cryobiology 57 (2008): 91-103.
- Wu X, Herndon DN, Wolf SE. Growth hormone down-regulation of Interleukin-1beta and Interleukin-6 induced acute phase protein gene expression is associated with increased gene expression of suppressor of cytokine signal-3. Shock 19 (2003): 314-320.
- Glebov AN, Zinchuk VV. Prooxidant-antioxidant state of the organism during oxidative stress and correction of the larginine NO system. Bull Exp Biol Med 141 (2006): 387-389.
- Lavieri R, Rubartelli A, Carta S. Redox stress unbalances the inflammatory cytokine network: role in autoinflammatory patients and healthy subjects. J Leukoc Biol 99 (2016): 79-86.
- Paul BD, Sbodio JI, Snyder SH. Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol Sci (2018).
- Goodsell DS. Catalase. Molecule of the Month (2004).
- Smulevich G, Jakopitsch C, Droghetti E, et al. Probing the structure and bifunctionality of catalase-peroxidase (KatG). J Inorg Biochem 100 (2006): 568-585.
- Boon EM, Downs A, Marcey D. Catalase: H2O2: H2O2 Oxidoreductase (2018).
- Niu Z, Tang J, Zhang W, et al. Caspase-1 promotes monocyte-macrophage differentiation by repressing PPARgamma. FEBS J 284 (2017): 568-585.
- Ribeiro MC, Peruchetti DB, Silva LS, et al. LPS Induces mTORC1 and mTORC2 Activation During Monocyte Adhesion. Front Mol Biosci 5 (2018): 67.
- Leonard B, McCann JL, Starrett GJ, et al. The PKC/NF-kappaB signaling pathway induces APOBEC3B expression in multiple human cancers. Cancer Res 75 (2015): 4538-4547.
- Madarova M, Mucha R, Hresko S, et al. Identification of new phosphorylation sites of CD23 in B-cells of patients with chronic lymphocytic leukemia. Leuk Res 70 (2018): 25-33.
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117 (2011): 3720-3732.
- Schroder K, Tschopp J. The inflammasomes. Cell 140 (2010): 821-832.
- Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol 6 (2006): 308-317.
- Denes A, Lopez-Castejon G, Brough D. Caspase-1: is IL-1 just the tip of the ICEberg? Cell Death Dis 3 (2012): e338.
- Pomerantz BJ, Reznikov LL, Harken AH, et al. Inhibition of caspase 1 reduces human myocardial ischemic dys- function via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci USA 98 (2001): 2871-2876.
- Frantz S, Ducharme A, Sawyer D, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol 35 (2003): 685-694.
- Holly TA, Drincic A, Byun Y, et al. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol 31 (1999): 1709-1715.
- Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev 22 (2011): 189-195.
- Druilhe A, Srinivasula SM, Razmara M, et al. Regulation of IL-1beta generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ 8 (2001): 649-657.
- Humke EW, Shriver SK, Starovasnik MA, et al. ICEBERG: a novel inhibitor of interleukin-1beta genera- tion. Cell 103 (2000): 99-111.
- Hotfiel T, Seil R, Bily W, et al. Nonoperative treatment of muscle injuries - recommendations from the GOTS expert meeting. J Exp Orthop 5 (2018): 24.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 