Efficacy of Ibrutinib in the Treatment of Monoclonal Gammopathy of Renal Significance with C3 Deposits Secondary to Chronic Lymphocytic Leukemia
Laura De Padua1*, Francesca Apponi2, Gabriella Tomei3, Franco Bondatti2, Antonella Ferrari1
1Hematology, Fabrizio Spaziani Hospital, Frosinone, Italy
2Nephrology, Fabrizio Spaziani Hospital, Frosinone, Italy
3Hematology, Santa Rosa Hospital, Viterbo, Italy
*Corresponding author: Laura De Padua, Hematology, Fabrizio Spaziani Hospital, Frosinone, Italy.
Received: 26 August 2025; Accepted: 02 September 2025; Published: 22 September 2025
Article Information
Citation: Laura De Padua, Francesca Apponi, Gabriella Tomei, Franco Bondatti, Antonella Ferrari. Efficacy of Ibrutinib in the Treatment of Monoclonal Gammopathy of Renal Significance with C3 Deposits Secondary to Chronic Lymphocytic Leukemia. Archives of Clinical and Biomedical Research. 9 (2025): 360-363.
View / Download Pdf Share at FacebookAbstract
Background: The new International Kidney and Monoclonal Gammopathy Research Group consensus definition of monoclonal gammopathy of renal significance includes all B cell or plasma cell proliferative disorders that produce a nephrotoxic monoclonal immunoglobulin. A novel entity characterized by glomerulonephritis with isolated glomerular C3 deposits has been described.
Methods: Here we present the case of a patient with a previous diagnosis of Chronic Lymphocytic Leukemia with associated Monoclonal Gammopathy, who showed onset of proteinuria and was diagnosed with C3 glomerulopathy on renal biopsy. Since early treatment tailored to the to underlying clonal B-cell disorder can improve renal outcome, we started treatment with ibrutinib.
Results: Disease assessment after 6 months of therapy showed partial hematological response and renal response with normalization of proteinuria. Conclusions: In our patient with Monoclonal Gammopathy of Renal Significance secondary to Chronic Lymphocytic Leukemia and aggressive renal disease, the ibrutinib based regime proved to be an appropriate strategy to target the lymphocyte clone and to improve renal damage.
Keywords
Chronic lymphocytic leukemia; Monoclonal gammopathy of renal significance; C3 glomerulopathy; Ibrutinib
Article Details
1. Introduction
The new Monoclonal Gammopathy Research Group (IKMG) consensus definition of monoclonal gammopathy of renal significance (MGRS) includes all B cell or plasma cell proliferative disorders that produce a nephrotoxic monoclonal immunoglobulin [1]. A novel MGRS entity characterized by glomerulonephritis with isolated glomerular complement fraction 3 (C3) deposits has been descrived [2,3]. It is associated with a circulating monoclonal Immunoglobulin G (IgG), most often with monoclonal gammopathy of undetermined significance or indolent multiple myeloma. Hypocomplementemia secondary to activation of the complement alternative pathway is usual, in the absence of detectable anti-C3 convertase activity (nephritic factor).
Chemotherapy tailored to the underlying B-cell clonal disorder improves renal outcome and should be considered early in the disease course [1,2,4]. There are few data on treatment of MGRS with bruton tyrosine kinase inhibitors [5,6]. Here we present the case of a patient with C3 glomerulopathy secondary to chronic lymphocytic leukemia (CLL), who received ibrutinib therapy.
2. Matherials and Methods
In september 2023, a 76 year old male was referred to us by nephrology department of our hospital because he had submitted to renal biopsy for renal failure and nephrotic syndrome (serum creatinine level 2,1 mg/dl, proteinuria of 11 g/day, microalbuminuria of 2900 mg/day (range < 300 mg/day)), with anasarcatic state. He reported a four years history of asymptomatic CLL associated with monoclonal immunoglobulin (Ig)G-k and essential hypertension.
The total body CT scan performed on february 2023 showed: ‘Abundant right pleural effusion, minimal on the left. Some increased lymphadenopathy in the mediastinal region, the largest measuring 29 millimetres (mm) subcarinal, 14 mm paratracheal on the right. Spleen with longitudinal diameter of 15 centimeters (cm); kidneys normal in size and morphology. Some increased lymphadenopathy in the lumboaortic region (12 mm), interaortocaval region (11 mm), and at the root of the mesentery (10 mm). Minimal fluid accumulation in the perihepatic region and pelvic cavity. Examination of pleural fluid does not revealed neoplastic cells. He underwent a minor salivary gland biopsy which resulted negative for the Congo red stain.
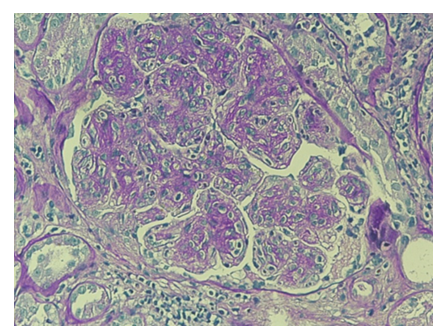
For further diagnostic investigation of nephrotic syndrome, a renal biopsy was performed (june 2023), which showed mesangial hypercellularity with interposition along the wall of the glomerular capillaries. (Figure 1,2). Immunofluorescence revealed finely granular, peripheral, segmental, diffuse positivity for C3 (++) and C1q (+), and negative IgA, IgG, IgM, fibrinogen and kappa and lambda light chains. The patient was diagnosed with C3 glomerulopathy.
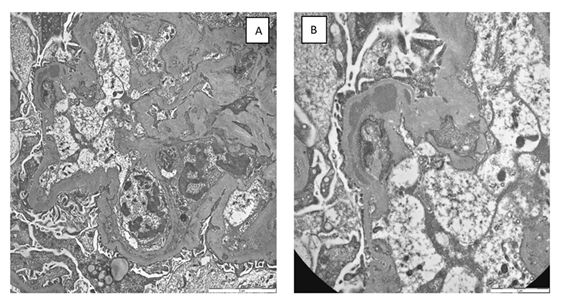
Figure 2: a) Transmission electron microscopy: Evidence of diffuse folding of the glomerular basement membrane and interposition of endothelial and mesangial cell cytoplasm. Rare segmentally distributed subendothelial and intramembranous deposits are present. Extensive fusion of the podocyte pedicels. b) At high magnification, a large intramembranous deposit is evident.
On september 2023 he presented with kidney disease stage 4 (kidney disease improving global outcomes (KDIGO) guidelines) [7]. His medication included beta blockers (nebivololo), metolazone, furosemide, erythropoietin. Laboratory tests on admission showed hemoglobin 9,6 gr/dl, serum creatinine 3,14 mg/dl, urea 300 mg/dl (range < 50 mg/dl), proteinuria 7,5 g in 24 hours, a white blood cell count of 19 x 103/µL, a lymphocyte count of 9,7 x 103/µL, a platelet count of 260 x 103/µL (Table 1). The physical examination revealed a blood pressure of 130/80 mmHg, with peripheral edema; on physical examination he presented superficial lymphadenopathy with a maximum diameter of 1.5 cm and palpable splenomegaly 4 cm from the costal arch. Bone marrow biopsy showed a 60% of small lymphocytes with positivity for CD20 and CD5, and negative cyclin D1. Immunophenotype on peripheral blood confirmed the presence of a B lymphoid population with a CD5 positive, CD200 positive, CD23 negative, CD10 negative phenotype, with restriction for the expression of kappa chains, compatible with chronic lymphocytic leukemia.
|
First Medical Evaluation |
1 Month Later |
6 Months Later |
|
|
Blood Cell count (4-10 x 103/µL) |
19 |
4,1 |
7,9 |
|
Haemoglobin (12-18 g/dl) |
9,6 |
10,2 |
8,9 (Hemolytic Anemia) |
|
Platelet count (140-440 x 103/µL) |
260 |
101 |
167 |
|
Lymphocyte count (1,0-4,8 x 103/µL) |
9,7 |
2,59 |
3,16 |
|
Serum albumine (3,4-4,8 g/dl) |
2,6 |
3,3 |
3,3 |
|
Serum creatinine (0,5- 1,2 mg/dl) |
3,14 |
2,7 |
2,52 |
|
Serum potassium (3,5-5,1 mEq/l) |
5,6 |
4,6 |
4 |
|
24 hours urine total protein excretion (< 0,5 g) |
7,5 |
3 |
0,37 |
|
Free light chains (FLC) kappa (0,67-2,24 mg/dl) |
5,17 |
4,23 |
2,7 |
|
Free light chains lambda (0,83-2,7 mg/dl) |
1,41 |
2,22 |
1,68 |
|
FLC ratio kappa/lambda (0,31-1,56) |
3,67 |
1,9 |
1,61 |
|
Splenomegaly ( longitudinal diameter, cm) |
18 |
17 |
15 |
|
Lymphoadenopathy (maximum diameter, cm) |
3 |
2 |
1,5 |
Table 1: Analytical parameters at the first evaluation, 1 month after and 6 months after starting ibrutinib
Abdominal ultrasound showed splenomegaly, with longitudinal diameter of 18 cm. A seric monoclonal band in the gamma fraction was detected with a monoclonal spike of 0.03 g/dl. Quantification of the free light chains showed kappa chains 5,17 mg/dl; lambda chains 1,41 mg/dl and free kappa/free lambda ratio of 3,67 (Table 1). Urine immunoelectrophoresis and bence jones were negative. Serum levels of immunoglobulin were IgG 132 mg/dl (range 700-1600 mg/dl), IgM 11 mg/dl (range 40-230 mg/dl), IgA 29 mg/dl (range 70-400 mg/dl), and raised levels of beta2 microglobulin (14 µg/ml) (range < 3 µg/ml) was found. He had marked hypocomplementemia, with reductions in complement C3 (52 mg/dl) (range > 80 mg/dl) and complement C4 (8 mg/dl) (range > 15 mg/dl). Estimated glomerular filtration rate (eGFR) was reduced (25 ml/min per 1,73 mq). Complementary blood tests showed the following: lactate dehydrogenase (LDH) 235 U/l (range < 225 U/l), potassium 5,6 mEq/L, albumin 2,6 g/dl, uricemia 5,8 mg/dl (range 3,4-7 mg/dl), magnesium 2,2 mg/dl (range 1,6-2,3 mg/dl), calcium 8 mg/dl (range 8,6-10,2 mg/dl), coombs test negative; the other chemical parametres were normal or negative. Arterial blood gas analysis revealed: pH 7,379 (range 7,350-7,450), pO2 65,2 mmHg (range 83-108 mmHg), pCO2 34,4 mmHg (range 32-48 mmHg), bicarbonates 19,8 mmol/l (range 21-28 mmol/l), Base Excess -4,6 mmol/l (range -2 / -3 mmmol/l).
In summary, we had a patient with asymptomatic CLL in RAI/Binet stage B/II with splenomegaly 18 centimetres and lymphadenopathy maximum 3 centimetres. The molecular biology test for TP53 mutation in peripheral blood was negative.
Based on the clinical, analytical and histological findings from the renal biopsy, in conjunction with the nephrology service, a diagnosis of C3 glomerulopathy secondary to CLL was established. The patient underwent treatment with ibutinib at a reduced initial dose of 140 mg per day in August 2023. Lamivudine prophylaxis was started due to positive anti-HBc antibodies (HBV DNA negative).
3. Results
The treatment was well tollerated by the patient. The laboratory investigations after one month showed substantial renal function and proteinuria improvement (serum creatinine 2,7 mg/dl, arterial pH 7,428; pO2 93,2 mmHg, pCO2 35,1 mmHg, bicarbonates 22,7 mmmol/l, Base Excess -1,3 mmol/l; proteinuria 3 g in 24 hours) with stability of hemoglobin levels, thus we decided to increase the dosage of ibrutinib to 240 mg per day (Table 1).
Disease assessment at 6 months showed reduction of lymphadenomegaly (1,5 cm) and splenomegaly (15 cm) with normalization of the lymphocyte count. Serum electrophoresis was negative for monoclonal band. Quantification of the serum free light chains showed kappa chains 2,7 mg/dl; lambda chains 1,68 mg/dl and free kappa/free lambda ratio of 1,61. Proteinuria was 0,37 g in 24 hours (Table 1). Urine immunoelectrophoresis and bence jones were negative.
Since February 2024, due to the onset of loss of appetite and fatigue, ibrutinib has been reduced to 140 mg per day with partial improvement of symptoms. At the end of february the patient developed hemolytic anemia, with positive direct Coomb’s test, and an episode of autoimmune hemolytic anemia was assumed. Corticosteroid therapy was started with benefit on hemolysis and ibrutinib dose increased to 280 mg per day.
Since March 2024, due to persistent non-infectious diarrhea, the dosage of ibrutinib has been reduced again to 140 mg per day and the patient has been referred for gastroenterological evaluation. The total body CT scan of april 2024 showed: ‘No laterocervical lymphadenopathy; large neoplasm in the proximal sigma extending for more than 10 cm, with signs of infiltration of the adipose tissue and apparently inseparable from the walls of the bladder dome. Liver is heterogeneous due to the presence of multiple secondary lesions, the majority measuring 8 mm in the hepatic duct. Splenomegaly 15 cm. Kidneys are in normal position in terms of size and morphology. Some enlarged lymphonodes: lumbar-aortic (15 mm), inter-aortocaval (15 mm), and at the level of the mesenteric root (10 mm). No intraperitoneal fluid deposits.
Therefore the patient obtained a renal response and partial hematological response but developed colon cancer and unfortunately died on May 2024.
4. Discussion
Due to the rarity of MGRS to date, the therapeutic approaches for this pathology are not well stated. The treatment of MGRS is directed at the underlying B-cell or plasma cell clones and is based on a combination of various chemotherapy agents, aiming to preserve kidney function. No formal guidelines exist for this disease and different regimens have been described [4-6].
Based on the efficacy of bruton tyrosine kinase inhibitors in CLL we decided to employ an ibrutinib-based regimen, directed against the pathologic clone [8]. Other bruton tyrosine kinase inhibitors were available for CLL, but ibrutinib has more data in the treatment of MGRS [6].
Considering the renal insufficiency we decided to start the treatment with a reduced dose of ibrutinib. The laboratory investigations after one month of treatment showed substantial renal function and proteinuria improvement, thus we increased the dosage of ibrutinib to 280 mg per day. Disease assessment at 6 months showed resolution of nephrotic syndrome and stabilization of renal function with partial hematological response (Table 1).
The therapy was well tolerated, and the gastrointestinal symptoms and fatigue can be traced back to the intestinal pathology. After 6 months of therapy, an episode of autoimmune hemolytic anemia occurred, responsive to corticosteroid therapy, with maintenance of hematological and renal response. Unfortunately, the patient developed concomitant colon cancer and died 9 months after starting treatment.
5. Conclusion
We presented the case of a patient with MGRS secondary to CLL. The use of bruton tyrosine kinase inhibitors in this context is rarely reported. In our patient, the lymphoproliferative disease did not have clinical-laboratory criteria that would indicate the need to start treatment. It is crucial for patients with MGRS to undergo comprehensive evaluation by a multidisciplinary team comprising nephrologists, hematologists, and nephropathologists to elucidate the causative role of the Monoclonal protein in renal disease pathogenesis. Currently, clone-directed therapy stands out as the most effective treatment strategy for these cases. In our patient with aggressive renal disease, the ibrutinib based regime proved to be an appropriate strategy to target the lymphocyte clone and to improve renal damage. We conclude that the early use of ibrutinib in MRGS secondary to CLL can be considered a valid therapeutic approach with good tolerability.Conflicts of interest:The authors declare no conflict of interest
References
- Leung N, Bridoux F, Batuman V, et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol 15 (2019): 1-15.
- Fermand JP, Bridoux F, Kyle RA, et al. How I treat monoclonal gammopathy of renal significance (MGRS). Blood 122 (2013): 3583-3590.
- Nasr SH, Royal V, Best Rocha A, et al. Renal Pathology Society/International Kidney and Monoclonal Gammopathy Research Group consensus on pathologic definitions and terminology of monoclonal gammopathy-associated kidney lesions. Kidney Int 108 (2025): 217-229.
- Chauvet S, Frémeaux-Bacchi V, Petitprez F, et al. Treatment of B cell disorder improves renal outcome of patients with monoclonal gammopathy-associated C3 glomerulopathy. Blood 129 (2017): 1437-1447.
- Castillo JJ, Callander NS, Baljevic M, et al. The evaluation and management of monoclonal gammopathy of renal significance and monoclonal gammopathy of neurological significance. Am J Hematol 96 (2021): 872-884.
- Nie G, Sun L, Zhang C, et al. Clinicopathological features and individualized treatment of kidney involvement in B cell lymphoproliferative disorders. Front Immunol 13 (2022): 1012345.
- Eknoyan G, Lameire N. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 105 (2024 Suppl 4): S117-S314.
- Eichhorst B, Ghia P, Niemann CU, et al. ESMO clinical practice guideline interim update on new targeted therapies in the first line and at relapse of chronic lymphocytic leukaemia: ESMO Guidelines Committee. Ann Oncol 35 (2024): 987-999.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 