Efficacy of Triple Therapy with Proton Pump Inhibitor, Levofloxacin and Amoxicillin as First Line Treatment to Eradicate Helicobacter Pylori: An Observational Study
Dr. Mohammad Asadur Rahman1*, Dr. Mahedi Hasan2, Dr. ABM Safiullah3, Dr. Mohammad Shoaib Chowdhury4, Dr. Mohammad Syedul Islam5, Dr. Ahmad Monjurul Aziz6, Dr. Rana Jahangir Alam7, Dr. Khalada Binte Khair8
1Associate Professor, Department of Gastroenterology, Bangladesh Medical University, Dhaka, Bangladesh.
2 Senior Consultant and Head, Central Police Hospital, Dhaka, Bangladesh.
3Associate Professor, Department of Gastroenterology, Bangladesh Medical University, Dhaka, Bangladesh.
4Associate Professor, Department of Gastroenterology, Bangladesh Medical University, Dhaka, Bangladesh.
5Associate Professor, Department of Internal Medicine, Bangladesh Medical University, Dhaka, Bangladesh
6Assistant Professor, Department of Medicine, Shaheed Suhrawardy Medical College and Hospital, Dhaka, Bangladesh.
7Assistant Professor, Department of General Surgery, Bangladesh Medical University, Dhaka, Bangladesh.
8Senior Consultant, Department of Clinical Pathology, Bangladesh Medical College Hospital, Dhaka, Bangladesh
*Corresponding author: Dr. Mohammad Asadur Rahman, Associate Professor, Department of Gastroenterology, Bangladesh Medical University, Dhaka, Bangladesh.
Received: 03 May 2025; Accepted: 23 May 2025; Published: 04 July 2025
Article Information
Citation: Dr. Mohammad Asadur Rahman, Dr. Mahedi Hasan, Dr. ABM Safiullah, Dr. Mohammad Shoaib Chowdhury, Dr. Mohammad Syedul Islam, Dr. Ahmad Monjurul Aziz, Dr. Rana Jahangir Alam, Dr. Khalada Binte Khair; Efficacy of Triple Therapy with Proton Pump Inhibitor, Levofloxacin and Amoxicillin as First Line Treatment to Eradicate Helicobacter Pylori: An Observational Study. Archives of Clinical and Biomedical Research. 9 (2025): 280-285.
View / Download Pdf Share at FacebookAbstract
Microglial cells constitute the largest number of non-neuronal cells in the brain. As part of their immune surveillance function, they are responsible for detecting the presence of both external and internal danger signals, stimulating a defense response through the release of pro- inflammatory cytokines. Once the damage is controlled, microglia stimulate a reparative response that allows tissue homeostasis to be maintained. When this balance is not physiologically achieved, the use of drugs or other non-pharmacological therapies is needed to promote tissue repair and prevent the appearance of complications secondary to the primary damage. In the particular case of traumatic brain injury (TBI), the application of low frequency electromagnetic field (EMF) has proven very helpful in reducing the levels of inflammatory mediators. In the present study we investigated the effect of EMF in an “in vitro” model of tumor necrosis factor alpha (TNF-α)-induced neuroinflammation. Human microglial cells (HMC3) were treated with TNF-α (50 ng/mL) and, after 20 minutes, were exposed to 2.5 or 5 Hz EMF for 3 min. The effect of both treatments on survival, migration capacity and transcriptional expression of M1/M2 phenotypic markers was evaluated at 6, 24 and 48 hours. The exposure to EMF increased the survival rate of cells incubated with high doses of TNF-α and significantly reduced the migration rate of TNF-α treated cells. The analysis of expression patterns in different time points showed that EMF promoted the expression of M1 and M2 phenotypic markers in a timedependent manner, suggesting a stimulating effect on the phagocytic capacity of microglial cells. Further studies are necessary to fully characterize the effects of EMF on the function of primary microglial cells within a brain injury-like microenvironment.
Keywords
<p>Helicobacter pylori; levofloxacin; proton pump inhibitor; amoxicillin; eradication therapy; antibiotic resistance; gastritis</p>
Article Details
1. Introduction
Helicobacter pylori (H. pylori) infection is one of the most prevalent bacterial infections worldwide, affecting nearly half of the global population [1]. It is strongly associated with a spectrum of gastrointestinal disorders, including chronic gastritis, peptic ulcer disease, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer [2]. The successful eradication of H. pylori has been shown to mitigate the risk of these conditions, improve clinical outcomes, and reduce healthcare burdens [3]. Despite its clinical significance, achieving consistent eradication of H. pylori remains a challenge due to the growing issue of antibiotic resistance and variations in patient compliance [1-3].
Current Treatment Landscape:
Standard first-line treatments for H. pylori typically include triple therapy comprising a proton pump inhibitor (PPI) combined with two antibiotics, most commonly clarithromycin and amoxicillin or metronidazole [4]. However, the efficacy of such regimens has been declining in many regions due to the increasing prevalence of clarithromycin resistance [5]. This decline in effectiveness underscores the urgent need for alternative therapeutic strategies to optimize eradication rates and address the limitations of traditional regimens [4-6].
Rationale for Triple Therapy with Levofloxacin:
Levofloxacin, a fluoroquinolone antibiotic, has emerged as a promising alternative in the management of H. pylori infection, particularly in settings with high clarithromycin resistance [7]. Its broad spectrum of activity and favourable pharmacokinetic profile make it a valuable candidate for inclusion in first-line eradication protocols [8-9]. When combined with a PPI and amoxicillin, levofloxacin-based triple therapy has demonstrated efficacy in overcoming resistance and achieving satisfactory eradication rates in various observational and clinical studies [8-11]. However, its role as a first-line treatment option remains underexplored and warrants further evaluation in diverse patient populations [10-11].
Study Objectives:
This observational study aims to evaluate the efficacy of triple therapy comprising a PPI, levofloxacin, and amoxicillin as a firstline treatment for H. pylori eradication. By assessing treatment outcomes in a realworld setting, the study seeks to contribute valuable insights into the viability of this regimen as an alternative to standard therapies, particularly in regions facing high clarithromycin resistance.
Additionally, the study explores factors influencing treatment success, including patient demographics, adherence, and baseline antibiotic resistance patterns.
Significance of the Study:
Given the evolving resistance patterns and the critical need for effective first-line treatments, this study has the potential to inform clinical practice and guideline development for H. pylori management. By focusing on the efficacy of levofloxacinbased triple therapy, the findings may provide a basis for tailored therapeutic approaches that improve eradication success rates and patient outcomes.
2. Methods and Materials
Study Design: This observational study was conducted at the Department of Gastroenterology, Central Police Hospital, Dhaka. The study design was prospective, with all enrolled patients undergoing endoscopy, during which H. pylori infection was diagnosed based on positive results from the rapid urease test. Written informed consent was obtained from all participants prior to enrolment, and ethical approval was secured from the institutional review board.
Study Population: Total 34 patients aged 18 years and older who tested positive for H. pylori and had no prior history of eradication therapy were eligible for inclusion. Exclusion criteria included known allergies to levofloxacin or amoxicillin, significant comorbid conditions, pregnancy, lactation, and use of antibiotics or PPIs within four weeks prior to study enrolment.
Treatment: Participants in the study received a 10-day course of triple therapy for the eradication of H. pylori, designed to maximize treatment efficacy in accordance with established clinical guidelines [12-13]. The regimen consisted of the following medications:
• Proton pump inhibitor (PPI): Either omeprazole, esomeprazole, or lansoprazole was administered at a standard dose, taken twice daily to suppress gastric acid production and enhance antibiotic efficacy [12-13].
• Levofloxacin: A fluoroquinolone antibiotic was prescribed at a dose of 500 mg, taken once daily, targeting H. pylori's DNA replication process [12-13].
• Amoxicillin: A penicillin-class antibiotic was provided at a dose of 1 g, taken twice daily, to disrupt bacterial cell wall synthesis and provide a synergistic effect with levofloxacin [12-13].
Participants were instructed on the importance of adherence to the treatment regimen to achieve optimal outcomes. Medication intake schedules and potential adverse effects were explained in detail. Follow-up visits were arranged to monitor adherence, manage any side effects, and evaluate treatment efficacy through posttherapy diagnostic testing.
Outcome Measures: The primary outcome was the eradication of H. pylori, defined as a negative result on the C13 urea breath test conducted at least four weeks after completion of therapy. Secondary outcomes included treatment adherence, incidence of adverse events, and identification of factors influencing eradication success (e.g., age, sex).
Data Collection: Data were collected through structured data collection sheets, including baseline demographics, indication of endoscopy and endoscopic findings. Adherence to therapy was assessed through patient self-reports and pill counts. Adverse events were recorded during and after the treatment period.
Statistical Analysis: Descriptive statistics were used to summarize baseline characteristics and treatment outcomes. The eradication rate was calculated as the proportion of patients achieving negative follow-up tests. Logistic regression analyses were performed to identify predictors of treatment success. All analyses were conducted using IBM-SPSS Software (Version 27), with a significance threshold set at p < 0.05.
3. Results
This observational study evaluated the efficacy of triple therapy with a proton pump inhibitor, levofloxacin, and amoxicillin as first-line treatment for Helicobacter pylori eradication. A total of 34 participants were included, with 19 males (55.9%) and 15 females (44.1%). The results are presented below in terms of demographic characteristics, indications for endoscopy, endoscopic findings, diagnostic outcomes, and adverse effects.
Participant Demographics
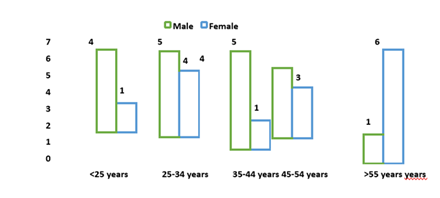
The age distribution of participants varied across sexes. Among males, the most represented age groups were 25–34 years and 45–54 years (each comprising 26.3%), while the largest proportion of females (40.0%) belonged to the >55 years age group. Notably, younger participants (<25 years) were primarily male (21.1%) compared to females (6.7%), whereas older age categories showed an opposite trend (Figure 1).
Table 1: Distribution of Participants according to Indication of Endoscopy
|
Indication of Endoscopy |
Male (n=19) |
Female (n=15) |
p value |
|
Abdominal Pain |
12 (63.2%) |
11 (73.3%) |
0.400 |
|
Dyspepsia |
5 (26.3%) |
4 (26.7%) |
0.640 |
|
Vomiting |
4 (21.1%) |
0 (0.0%) |
0.084 |
|
Anorexia |
1 (5.3%) |
1 (6.7%) |
0.695 |
|
GERD |
1 (5.3%) |
1 (6.7%) |
0.695 |
Indications for Endoscopy
The primary indication for endoscopy was abdominal pain, reported by 12 males (63.2%) and 11 females (73.3%), with no significant sex-based difference (p = 0.400). Dyspepsia was the second most common indication, affecting 5 males (26.3%) and 4 females (26.7%; p = 0.640). Vomiting was reported by 4 males (21.1%) but no females (0.0%; p = 0.084). Other indications, including anorexia, GERD, and others, were uncommon and showed no significant differences between sexes (p > 0.05) (Table 01).
Table 02: Distribution of Participants according to Endoscopic Findings
|
Endoscopic Findings |
Male (n=19) |
Female (n=15) |
p value |
|
Normal Findings |
3 (15.8%) |
2 (13.3%) |
0.616 |
|
Gastritis |
10 (52.6%) |
13 (86.7) |
0.039 |
|
Ulcer |
3 (15.8%) |
0 (0.0%) |
0.162 |
|
Gastropathy |
1 (5.3%) |
0 (0.0%) |
0.559 |
|
Gastric Polyp |
0 (0.0%) |
1 (6.7%) |
0.441 |
|
Gastric Carcinoma |
0 (0.0%) |
1 (6.7%) |
0.441 |
|
Reflux Esophagitis |
1 (5.3%) |
3 (20.0%) |
0.216 |
|
Hiatal Hernia |
3 (15.8%) |
1 (6.7%) |
0.397 |
|
Antral Erosion |
1 (5.3%) |
1 (6.7%) |
0.695 |
Endoscopic Findings
Gastritis was the most frequent endoscopic finding, significantly more common in females (86.7%) than males (52.6%; p = 0.039). Normal findings were observed in 3 males (15.8%) and 2 females (13.3%; p = 0.616). Ulcers were found in 3 males (15.8%) but not in females (0.0%; p = 0.162). Rare findings, including gastric carcinoma, gastric polyps, gastropathy, and hiatal hernia, occurred infrequently, with no statistically significant sex-based differences (p > 0.05) (Table 02).
Table 03: Distribution of Participants according to Test Result Before and After Therapy
|
|
C13 Urea Breath Test |
|
||
|
Positive |
Negative |
Loss of follow-up |
p value |
|
|
Positive Rapid Urease Test |
3 (8.8%) |
24 (70.6%) |
7 (20.6%) |
<0.001 |
Diagnostic Testing
The distribution of C13 urea breath test results among participants who initially tested positive on the rapid urease test is shown in Table 3. After completing triple therapy, 24 participants (70.6%) had a negative C13 urea breath test, indicating successful eradication of Helicobacter pylori. However, 3 participants (8.8%) remained positive, suggesting persistent infection despite therapy. Additionally, 7 participants (20.6%) were lost to follow-up and did not undergo post-treatment testing. The difference in C13 urea breath test outcomes following therapy was statistically significant (p < 0.001), reflecting the efficacy of the treatment in most cases. These results highlight the importance of post-therapy testing to confirm eradication and address cases of treatment failure.
Table 04: Distribution of Participants according to Adverse Effects related to Therapy
|
Adverse Effects |
Male (n=19) |
Female (n=15) |
p value |
|
Nausea |
14 (73.7%) |
12 (80.0%) |
0.494 |
|
Metallic Taste |
5 (26.3%) |
3 (20.0) |
0.494 |
|
Abdominal Pain |
2 (10.5%) |
4 (26.7%) |
0.220 |
|
Diarrhoea |
5 (26.3%) |
4 (26.7%) |
0.640 |
|
Vomiting |
7 (36.7%) |
5 (33.3%) |
0.561 |
|
Oral Thrush |
1 (5.3%) |
0 (0.0%) |
0.441 |
|
Myalgia |
1 (5.3%) |
1 (6.7%) |
0.695 |
Adverse Effects
The therapy was associated with mild to moderate adverse effects, predominantly nausea, which affected 14 males (73.7%) and 12 females (80.0%; p = 0.494). Other common side effects included diarrhoea (26.3% in males vs. 26.7% in females; p = 0.640), vomiting (36.7% in males vs. 33.3% in females; p = 0.561), and abdominal pain (10.5% in males vs. 26.7% in females; p = 0.220). Rare side effects such as oral thrush and myalgia were reported by only one participant of each sex (Table 04).
4. Discussion
This study evaluated the efficacy of triple therapy combining a proton pump inhibitor, levofloxacin, and amoxicillin as a first-line treatment for Helicobacter pylori eradication, providing insights into clinical outcomes, adverse effects, and diagnostic findings. The eradication rate, as evidenced by the C13 urea breath test, was 70.6%, with a statistically significant improvement in post-treatment outcomes (p < 0.001). These results align with but also deviate in notable ways from previously published studies.
The observed eradication rate of 70.6% is consistent with studies that have highlighted levofloxacin-based triple therapy as an effective alternative to traditional clarithromycin-based regimens. A study reported eradication rates between 70% and 80% when using levofloxacincontaining regimens as a first-line treatment, particularly in populations with high clarithromycin resistance [14]. Similarly, a meta-analysis demonstrated a comparable success rate, emphasizing the efficacy of levofloxacin in regions where resistance patterns favour its use [15].
However, the eradication rate in our study falls short of the >80% efficacy threshold recommended by the Maastricht V/Florence Consensus for first-line therapies. This could reflect variations in local antibiotic resistance patterns, patient adherence, or the influence of demographic factors, such as the high prevalence of gastritis observed in our female participants (86.7%).
The positive rapid urease test results in conjunction with C13 urea breath test outcomes reinforce the utility of combining diagnostic approaches for accurate assessment. A significant portion of participants (20.6%) were lost to follow-up, a challenge also noted in different study [16], which reported similar drop-off rates in multi-step eradication studies. This highlights the need for improved follow-up protocols to ensure accurate evaluation of treatment efficacy.
The adverse effect profile in our study, including nausea (76.5%) and vomiting (35.3%), mirrors findings from study [17], which reported mild to moderate side effects in 60-80% of participants receiving similar regimens. Notably, the incidence of nausea was slightly higher in our cohort, potentially reflecting demographic or genetic predispositions affecting drug metabolism. Despite these effects, no serious adverse events were reported, underscoring the tolerability of the regimen.
The significant association of gastritis with female participants in our study (p = 0.039) aligns with findings from another study [18], who observed higher rates of gastritisrelated complications in women. This may suggest sex-based differences in immune response or Helicobacter pylori pathogenesis that warrant further investigation. Additionally, our study identified successful eradication rates across varying age groups, consistent with broader population studies [19].
The findings of this study underscore the efficacy and safety of levofloxacin-based triple therapy as a first-line treatment, particularly in populations with clarithromycin resistance. However, the observed eradication rate suggests room for optimization, potentially through personalized therapy guided by resistance testing. Future research should focus on understanding the influence of demographic factors, improving follow-up adherence, and evaluating alternative regimens such as quadruple therapies or novel agents to enhance treatment outcomes.
This study contributes valuable data to the growing body of evidence supporting the use of levofloxacin-based triple therapy while highlighting areas for improvement in patient management and therapeutic strategies.
5. Conclusion
This study demonstrates that triple therapy with a proton pump inhibitor, levofloxacin, and amoxicillin is effective and welltolerated as a first-line treatment for Helicobacter pylori eradication, achieving a 70.6% eradication rate. While the regimen showed significant efficacy, its success rate fell below the optimal threshold recommended by international guidelines, highlighting the need for tailored therapies based on local resistance patterns. The findings also underscore the importance of follow-up testing to confirm eradication and manage treatment failures. Future research should focus on optimizing therapeutic strategies and addressing demographic and clinical factors that may influence treatment outcomes
Conflict of interest:
The authors declare no conflict of interest
Funding:
Self
References
- Kusters JG, van Vliet AH, Kuipers EJ: Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 19 (2006): 449-490.
- Alkhaldi NK, Alghamdi WK, Alharbi MH, et al: The Association Between Oral Helicobacter pylori and Gastric Complications: A Comprehensive Review. Cureus 14 (2022): e24703.
- Baj J, Forma A, Flieger W, et al: Helicobacter pylori Infection and Extragastric Diseases—A Focus on the Central Nervous System. Cells 10 (2021): 2191.
- Vieira RR, Fontes LES, Pacheco RL, et al: Proton pump inhibitor- and clarithromycin-based triple therapies for Helicobacter pylori eradication. Cochrane Database Syst Rev 9 (2020): CD013734.
- Liu L, Nahata MC: Newer Therapies for Refractory Helicobacter pylori Infection in Adults: A Systematic Review. Antibiotics. 2024 Oct; 13 (10): 965.
- Chey WD, Leontiadis GI, Howden CW, et al: ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017; 112: 212–239. Correction in Am J Gastroenterol 113 (2018): 1102.
- Azab ET, Thabit AK, McKee S, et al: Levofloxacin versus clarithromycin for Helicobacter pylori eradication: are 14-day regimens better than 10-day regimens? Gut Pathog 14 (2022): 24.
- Kim SY, Choi DJ, Chung JW: Antibiotic treatment for Helicobacter pylori: Is the end coming? World J Gastrointest Pharmacol Ther. 6 (2015): 183-198.
- Gisbert JP: Optimization Strategies Aimed to Increase the Efficacy of Helicobacter pylori Eradication Therapies with Quinolones. Molecules 25 (2020): 5084.
- Branquinho D, Almeida N, Gregório C, et al.: Levofloxacin or Clarithromycin-based quadruple regimens: what is the best alternative as first-line treatment for Helicobacter pylori eradication in a country with high resistance rates for both antibiotics? BMC Gastroenterol 17 (2017): 31.
- Khadim S, Muhammad IN, Alam T, et al: Predictors of Successful First-Line Helicobacter pylori Eradication with Fluoroquinolones in Pakistan: A Prospective Exploration of Demographic and Clinical Factors. Antibiotics. 13 (2024): 211.
- Kim BJ, Lee H, Lee YC, et al.: Ten- Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea. Gut Liver 13 (2019): 531-540.
- Yang EH, Chen WY, Chiang HC, et al.: 10-Day versus 14-day bismuth quadruple therapy for first-line eradication of Helicobacter pylori infection: a randomised, open-label, non-inferiority trial. eClinicalMedicine. 70 (2024): 102529.
- Gisbert JP, Calvet X: Helicobacter pylori infection: Diagnosis and treatment. Clin Gastroenterol Hepatol 8 (2010): 532-539.
- Liou JM, Chen CC, Chen MJ, et al.: Sequential versus triple therapy for the first-line treatment of Helicobacter pylori infection: A meta-analysis. Gut 65 (11): 1714-1722.
- Nyssen OP, Bordin D, Tepes B, et al.: European Registry on Helicobacter pylori Management (Hp-EuReg): Outcomes of empirical and susceptibility-guided therapies in 30,394 patients. Gut 70 (2021): 40-54.
- McNicholl AG, Molina-Infante J, Gisbert JP: Levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. World J Gastroenterol 20 (2014): 1511-1521.
- Taneike I, Nami A, Ogata S: Gender differences in gastritis and Helicobacter pylori infection: A histopathological analysis. Helicobacter 7 (2002): 5359.
- Malfertheiner P, Megraud F, O’Morain CA, et al.: Management of Helicobacter pylori infection— the Maastricht V/Florence Consensus Report. Gut 66 (2017): 6-30.
Related PubMed Articles
- Effectiveness of 14-day high-dose dual therapy for Helicobacter pylori infection in Vietnam.
- First-Line Levofloxacin-Based Triple Therapy Versus Standard Bismuth-Based Quadruple Therapy for Helicobacter pylori Eradication in Saudi Arabia: A Retrospective Single-Center Study.
- The Efficacy of Two Triple Therapy Regimens and One Quadruple Regimen [Omeprazole, Amoxicillin, Metronidazole with Bismuth] in Eradicating Helicobacter pylori in Patients with Peptic Ulcer: A Randomized Clinical Trial.
- Vonoprazan Dual or Triple Therapy Versus Bismuth-Quadruple Therapy as First-Line Therapy for Helicobacter pylori Infection: A Three-Arm, Randomized Clinical Trial.
- Real-world efficacy of second-line therapies for Helicobacter pylori: a population-based study.
- Antimicrobial susceptibility of clinical Helicobacter pylori isolates and its eradication by standard triple therapy: a study in west central region of Colombia.
- Therapeutic efficacy and drug safety comparison of one-week Vonoprazan triple therapy with two-weeks Esomeprazole triple therapy in Helicobacter pylori infection: Findings from a single-centre randomized clinical trial in population of Pakistan.
- Refractoriness to anti-Helicobacter pylori treatment attributed to phenotypic resistance patterns in patients with gastroduodenopathy in Guayaquil-Ecuador.
- Efficacy And Cost-Effectiveness, Comparison Of 7-Days Vonoprazan Versus 14-Days Esomeprazole Based Triple Therapies For Treating Helicobacter Pylori Infection In Pakistani Population: A Randomized Clinical Trial.
- Tailored triple plus bismuth therapy based on previous antibiotic medication history for first-line Helicobacter pylori eradication: A randomized trial.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 