Efficacy, Safety and Tolerability of Dapagliflozin and Teneligliptin Fixeddose Combination in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin Monotherapy: A Double Blind, Four-arm, Randomized Phase 3 Clinical Study
Dr Vipul Khandelwal MBBS, MD1, Dr Arindam Naskar MBBS, MD2, Dr Swapna Borthakur MBBS, MD3, Dr Rahee Borulkar MBBS, MD4*, Dr Mayur Jadhav MBBS, MD5, Dr. Sumit Bhushan MD Physician, PGDCR, MBA6, Dr Sanjay Choudhari MBBS, MD7, Dr Saiprasad Patil MBBS, MD8, Dr Hanmant Barkate MBBS, MD9
1Internal Medicine-Director - Medical Services & Internal Medicine, Apex Hospital, SP-4 & 6, Central Road, Malviya Nagar Industrial Area, Malviya Nagar, Jaipur, Rajasthan, India
2General Medicine, MRCP (London), FRCP (Edinburgh), CCEBDM (Diabetes), MRCP (SCE Endocrinology and Diabetes)- Consultant endocrinologist, School of Tropical Medicine, Endocrinology, Nutrition and Metabolic Diseases, Chittaranjan Ave, Calcutta Medical College, College Square, Kolkata, West Bengal, India
3Medicine Senior Consultant, Department of Medicine cum Deputy Medical Superintendent, GS Rd, Bormotoria, Guwahati, Assam, India
4Pharmacology Manager, Global Medical Affairs, Glenmark Pharmaceuticals Ltd., Mumbai, India
5Pharmacology Senior Manager, Global Medical Affairs, Glenmark Pharmaceuticals Ltd., Mumbai, India
6Deputy General Manager- Clinical Studies/ Global Medical Affairs, Mumbai, India
7Pharmacology General Manager, Global Medical Affairs, Glenmark Pharmaceuticals Ltd., Mumbai, India
8Microbiology Senior General Manager, Global Medical Affairs, Glenmark Pharmaceuticals Ltd., Mumbai, India
9Pharmacology-Group Vice President & Global Head of Global Medical Affairs, Glenmark Pharmaceuticals Ltd., Mumbai, India
*Corresponding author: Dr Rahee Borulkar MBBS, MD, Pharmacology Manager, Global Medical Affairs, Glenmark Pharmaceuticals Ltd., Mumbai, India.
Received: 03 September 2025; Accepted: 16 September 2025; Published: 30 September 2025
Article Information
Citation: Dr Vipul Khandelwal, Dr Arindam Naskar, Dr Swapna Borthakur, Dr Rahee Borulkar, Dr Mayur Jadhav, Dr. Sumit Bhushan, Dr Sanjay Choudhari, Dr Saiprasad Patil, Dr Hanmant Barkate. Efficacy, Safety and Tolerability of Dapagliflozin and Teneligliptin Fixeddose Combination in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin Monotherapy: A Double Blind, Four-arm, Randomized Phase 3 Clinical Study. Archives of Clinical and Biomedical Research. 9 (2025): 440-447.
View / Download Pdf Share at FacebookAbstract
Introduction: The failure of metformin monotherapy and intolerance to metformin combinations have necessitated the evaluation of various fixeddose combinations (FDC) of oral hypoglycaemic agents. The newer FDCs belong to the class of Sodium-Glucose Cotransporter Type 2 (SGLT2) inhibitors and Dipeptidyl Peptidase-4 Inhibitor (DPP4) inhibitors. In this study the efficacy and safety of the FDC of Dapagliflozin and Teneligliptin in Indian Type 2 Diabetes Mellitus (T2DM) patients were evaluated.
Methods: This multicentric, randomized, prospective, double-blind, fourarm, phase III study included inadequately controlled T2DM patients on metformin monotherapy (≥ 1500 mg/day). Subjects were randomized into four groups and received either the FDC or the monotherapy of Dapagliflozin and Teneligliptin over 24 weeks. The study endpoints included improvement in glycaemic parameters, reduced body weight, and occurrence of adverse events.
Results: FDC group of Dapagliflozin 5/10mg + Teneligliptin 20mg demonstrated a significant reduction in HbA1C by 1.5% (P < 0.0001) compared to the individual groups of Dapagliflozin (-1.1%) & Teneligliptin (-0.9%). A similar trend was observed for Fasting Plasma Glucose (FPG) and Postprandial Plasma Glucose (PPG) with the FDC groups (P < 0.0001). The mean reduction in body weight from baseline to week 24 was prominent in the Dapagliflozin 10mg (-2.16 kg) group. When compared amongst the groups, weight reduction in Dapagliflozin 10mg + Teneligliptin 20mg group was significantly more than Dapagliflozin 5mg + Teneligliptin 20mg and Teneligliptin 20mg (P <0.01) monotherapy. The reported adverse events were mild and uneventful.
Conclusion: The FDC of Dapagliflozin and Teneligliptin effectively improved glycaemic parameters and body weight in T2DM patients. The FDC of Dapagliflozin and Teneligliptin based on the current evidence can be considered for T2DM patients inadequately controlled on metformin monotherapy.
Keywords
<p>Uncontrolled T2DM; FDC; Glycaemic control; Safety; Dapagliflozin; Teneligliptin; India</p>
Article Details
1. Introduction
Diabetes mellitus (DM) is one of the most significant global health emergencies of this century, ranking among the ten leading causes of mortality [1]. In 2022, an estimated 828 million (95% credible interval [CrI] 757–908) adults (those aged 18 years and older) had diabetes, an increase of 630 million (554–713) from 1990 [1,2]. The Indian Council of Medical Research–India Diabetes (ICMR-INDIAB) study has reported a prevalence of 11.5% diabetic patients in the Indian population [3].
The primary pathological focus in Type 2 Diabetes mellitus (T2DM) revolves around diminished insulin sensitivity and deteriorating beta-cell function [4]. The prevalence of uncontrolled diabetes varies from 46.43% to 76.6% in the Indian T2DM population [5,6]. The mean HbA1c levels in Indian T2DM patients have been reported to be around 7.7% to 8.9% [6,7]. Uncontrolled diabetes increases the risk of macrovascular and microvascular complications such as cardiovascular (CV), cerebrovascular, and peripheral artery disease (PAD), and diabetic retinopathy, nephropathy, and neuropathy, respectively [8-10]. Provided there are no contraindications, the American Diabetes Association (ADA) recommends using metformin monotherapy as the first-line medication for individuals with type 2 diabetes mellitus (T2DM) [11]. The primary reason cited for discontinuation of metformin therapy is gastrointestinal intolerance, which affects approximately 25% of patients undergoing treatment with metformin, often resulting in these individuals being unable or reluctant to continue this pharmacotherapy [12]. When monotherapy does not yield the desired glycemic control, it is crucial to promptly adopt a combination therapy that employs diverse mechanisms of action and demonstrates synergistic and complementary actions [13]. Thus, considering the above factors, there is a need for fixed-dose combinations (FDC) of oral anti-diabetic agents (OAD).
The clinical guidelines recommend a patient-centric approach and individualizing the treatment to achieve and maintain glycemic goals. The choice of pharmacological agents should also consider cardio-renal protection and effects on other comorbidities, efficacy, risk of hypoglycaemia, impact on weight, cost, access, risk of side effects, and individual preference [11]. Among the various OADs, Dipeptidyl Peptidase-4 Inhibitor (DPP4i) and Sodium-Glucose Cotransporter Type 2 Inhibitor (SGLT2i) have demonstrated clinical efficacy with an acceptable safety profile [11]. These two classes of OADs possess different mechanisms that stimulates insulin secretion; DPP4 inhibitors by inhibiting glucagon secretion and SGLT2i by inhibiting of glucose reabsorption in proximal convoluted tubules. Apart from demonstrating efficacy in T2DM patients and safety in patients, these two agents have also shown favourable cardiovascular outcomes [14,15]. Due to these beneficial effects, two fixed-dose combinations (FDCs) containing DPP4i and SGLT2i have been approved by the FDA (U.S. Food and Drug Administration) and the EMA (European Medicines Agency).
The FDC of SGLT2i Dapagliflozin with DPP4i Teneligliptin is the first such FDC in the world and this study presents the results of the Phase III double-blind randomized clinical trial conducted in T2DM patients. The objective of the study was to evaluate the efficacy, safety, and tolerability of FDC of Dapagliflozin 5 mg + Teneligliptin 20 mg Tablets and FDC of Dapagliflozin 10 mg + Teneligliptin 20 mg Tablets versus monotherapy with Dapagliflozin Tablets 10 mg and Teneligliptin Tablets 20 mg in T2DM patients inadequately controlled on stable Metformin monotherapy.
2. Methods
This was a multicentric, randomized, double-blind parallel-group, active-controlled, four-arm, comparative phase III clinical study (CTRI/2021/11/038050). The four treatment arms included Arm 1 (A1)- FDC of Dapagliflozin 5 mg + Teneligliptin 20 mg Tablets, Arm 2 (A2)- FDC of Dapagliflozin 10 mg + Teneligliptin 20 mg Tablets, Arm 3 (A3)- Dapagliflozin Tablets 10 mg and Arm 4 (A4)- Teneligliptin Tablets 20 mg.
The male and female patients aged between 18 to 65 years (both inclusive) with a confirmed diagnosis of T2DM receiving a stable dose of Metformin ≥ 1500 mg/day as monotherapy for at least three months before screening and having inadequate glycemic control at screening defined as glycosylated hemoglobin (HbA1c) levels of ≥ 7.5% to ≤ 10.0% were included in the study. Patients with FPG > 220 mg/dL, obese patients (BMI ≥ 45.0 kg/m), patients with impaired renal function with estimated glomerular filtration rate (eGFR) <60 mL/min, clinically significant impaired hepatic function (SGOT & SGPT more than 3X the UNL and/or Total bilirubin more than 2X the UNL) at screening were excluded. Patients with uncontrolled hypertension with sitting systolic BP ≥ 160 mmHg and/or diastolic BP ≥ 100 mmHg and any ECG abnormality on 12-lead ECG at screening that, in the opinion of the investigator, was clinically significant and was judged as a potential risk for the patient’s participation in the study were also excluded from this study.
The subject’s consent, demographic data, and medical history were taken on the screening visit/baseline visit (visit 1). After providing informed consent, all patients were screened, and eligible patients were randomized at Visit 2, assigned to either of the study arms by a computer-generated randomization process. Each patient was provided with study medication and a diary to record the drug treatment compliance and report any specific observations. On the day of randomization, patients received the investigational product (IP) as assigned for the treatment groups. All patients were advised to take one tablet orally in the morning around the same time daily. Patients were prohibited from taking any other OADs as well as insulin. Open-label Metformin was dispensed in multiples of Metformin 500 mg IR Tablets to all patients to be consumed thrice daily as advised by the study investigator.
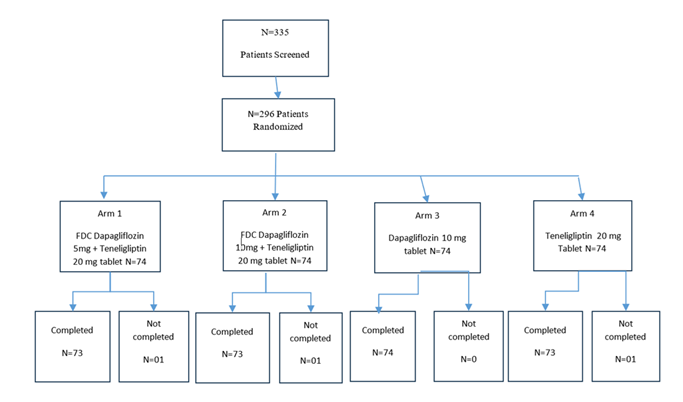
The total treatment duration was 24 weeks, and patients were required to visit the centre on predefined follow-up days. Day 168±2 days (Visit 7) was the end of the study visit. Please refer to Figure 1 for the study flow chart. Vital Signs and Physical examination were done at all visits. Body weight and BMI were recorded at all visits except the randomization visit. Drug accountability was performed using the patient diary and used/unused strips or study drug bottles at all visits.
The primary efficacy endpoint for the study was the mean change in HbA1c from baseline to the end of week 24. The secondary efficacy endpoints were mean change in FPG, PPG, body weight and proportion of patients achieving a therapeutic glycemic response, defined as HbA1c < 7% at the end of week 24. The safety assessment included hypoglycemic episodes, adverse events, serious adverse events, and any changes in the clinical laboratory parameters during the entire study period.
This sample size was assumed to detect a mean difference of at least 0.5 between the Test and comparator groups in the mean change of glycosylated hemoglobin (HbA1c) from baseline to week 24 (at 90% power and 2-sided significance level of 0.05). Based on published literature, a standard deviation of ± 0.9 was considered to calculate the sample size. The estimated number of patients in each group was deduced to be 70. The sample size consisted of 296 T2DM patients, who were subsequently assigned to 4 groups in a 1:1:1:1 ratio after the screening procedure during visit 1.
2.1 Statistical analysis:
Statistical analysis was performed using SAS 9.4. Continuous variables were statistically tested using ANOVA or a 2-sample t-test. Categorical variables were tested using the Chi-square test. Primary and secondary efficacy endpoints were analyzed using repeated measures analysis of covariance (ANCOVA) or a 2-sample t-test. All safety parameters were analyzed using ANOVA or a 2-sample t-test and descriptive statistics.
3. Results
This study was conducted from February 2022 to October 2022 at 13 study sites across India. The total duration of the study was 08 months and 22 days. A total of 335 patients were screened, and 296 patients were randomized in the study. 74 patients were randomized in each arm.
All patients were 18 to 65 years of age (both inclusive) as per protocol. No statistical difference was observed across the four treatment groups in patient demographics (Table 1). Demographic characteristics of the enrolled patients were comparable at baseline. Out of 296 randomized patients, 03 patients were lost to follow-up from the study. The study included patients of both genders, with an average age of 44 years.
|
Characteristics |
Statistics |
Arm A1 |
Arm A2 |
Arm A3 |
Arm A4 |
P-Value |
|
Age (Year) |
N |
74 |
74 |
74 |
74 |
0.606 |
|
Mean (SD) |
44.91 (10.52) |
44.07 (11.05) |
46.27 (10.78) |
44.38 (10.36) |
||
|
(Min, Max) |
(21.00,64.00) |
(22.00, 65.00) |
(19.00, 64.00) |
(22.00,65.0) |
||
|
HbA1c (%) |
Mean (SD) |
8.85 (0.55) |
8.86 (0.59) |
8.61 (0.62) |
8.68 (0.62) |
|
|
(Min, Max) |
(7.82, 9.90) |
(7.76, 10.00) |
(7.66, 10.00) |
(7.70, 10.00) |
||
|
N |
74 |
74 |
74 |
74 |
0.8205 |
|
|
Height (Cm) |
Mean (SD) |
162.03 (7.44) |
162.70 (8.11) |
162.34 (8.05) |
161.47 (8.70) |
|
|
(Min, Max) |
(146.00,177.00) |
(145.00, 181.00) |
(142.00, 183.00) |
(135.00, |
||
|
180.00) |
||||||
|
Weight (Kg) |
N |
74 |
74 |
74 |
74 |
0.9162 |
|
Mean (SD) |
61.23 (8.87) |
61.22 (8.44) |
60.71 (7.29) |
60.43 (8.49) |
||
|
(Min, Max) |
(44.00, 86.00) |
(44.00, 79.80) |
(47.00, 84.00) |
(44.00, 85.00) |
||
|
BMI (Kg/m2) |
N |
74 |
74 |
74 |
74 |
0.918 |
|
Mean (SD) |
23.32 (3.09) |
23.15 (2.96) |
23.10 (2.83) |
23.22 (3.10) |
||
|
(Min, Max) |
(18.43, 31.96) |
(18.20, 32.34) |
(18.75, 30.63) |
(18.26, 32.39) |
||
|
Sex |
N |
74 |
74 |
74 |
74 |
0.3123 |
|
Male |
41 (55.41%) |
37 (50.00%) |
48 (64.86%) |
40 (54.05%) |
||
|
Female |
33 (44.59%) |
37 (50.00%) |
26 (35.14%) |
34 (45.95%) |
||
|
Note: For age, height, weight, and BMI: P-value was obtained using the ANOVA test between A1, A2, A3, and A4. For Sex: P-value is calculated using the Chi-square test. Arm A1 = FDC of Dapagliflozin 5 mg + Teneligliptin 20 mg Tablets Arm A2 = FDC of Dapagliflozin 10 mg + Teneligliptin 20 mg Tablets Arm A3 = Dapagliflozin Tablets 10 mg Arm A4 = Teneligliptin Tablets 20 mg |
||||||
Table 1: Demographic Characteristics by Treatment Group.
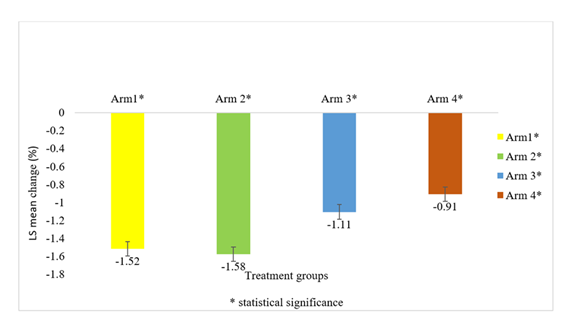
The mean change in HbA1c from baseline to end of study at week 24 (Figure 2), between Arm 1 (-1.52%) and Arm 2 (-1.58%) receiving FDC of Dapagliflozin 5mg + Teneligliptin 20 mg and Dapagliflozin 10 mg + Teneligliptin 20 mg, respectively, was comparable and was not statistically significant ((p-value 0.6063 95% CI (-0.1690, 0.2894)); however, for the same parameter, the mean change from baseline to end of study between Arm 1 and Arm 3 (-0.4%) ((p-value 0.0004 95% CI (-0.6432, -0.1832)), as well as Arm 1 and Arm 4 (-0.6%) ((p-value < 0.0001 95% CI (-0.8421, -0.3829)), was statistically significant. A similar trend was observed in population between Arm 2 and Arm 3 ((<0.0001 95%CI (-0.7035, -0.2434)) as well as Arm 2 and Arm 4 ((p-value < 0.0001 95% CI (-0.9023, -0.4431)).
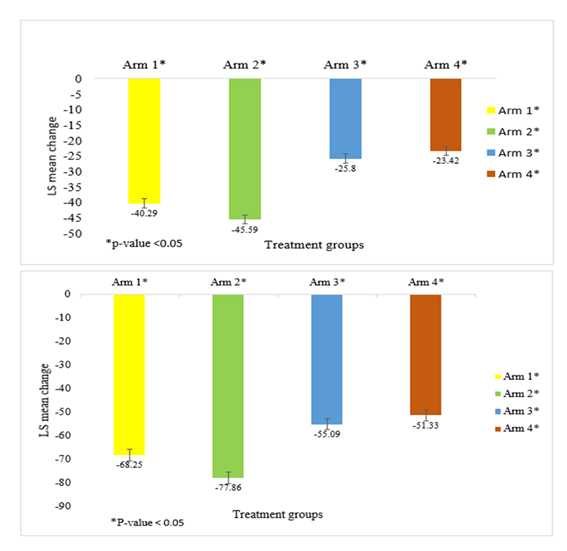
There was a significant reduction in FPG in all the treatment groups when compared between the baseline and end of study (Figure 3a). The comparison between Arm 1 (-40.28 mg/dL and Arm 2 (-45.58 mg/dL) showed statistical significance for FPG ((p-value 0.0082 95% CI (1.3722, 9.2302)); significance was also observed between Arm 1 and Arm 3 (-25.8 mg/dL) ((p-value < 0.0001 95% CI (-18.4087, -10.5661)) and Arm 1 and Arm 4 (-23.4 mg/dL) ((p-value < 0.0001 95% CI (-20.7817, -12.9472)). Similar statistical significance was also observed between Arm 2 and Arm 3 ((p-value < 0.0001 95% CI (-23.7360, -15.8411)) and Arm 2 and Arm 4 ((p-value < 0.0001 95% CI (-26.0978, -18.2334)).
All the treatment groups were effective in reducing the HbA1c level from baseline, and the reduction was statistically significant (P <0.0001) (Figure 2).
A similar trend was observed for PPG reduction from baseline to the end of the study across all the treatment groups (Figure 3b). In the Arm 1 (-68.2 mg/dL) and Arm 2 (-77.8 mg/dL) there was a statistically significant difference (difference ((p-value 0 0.0053 95% CI (2.8586, 16.3586)); significance was also observed between Arm 1 and Arm 3 (-55.09 mg/dL) ((p-value 0.0001 95% CI (-19.9115, -6.4127)) and Arm 1 and Arm 4 (-51.3 mg/dL) ((p-value < 0.0001 95% CI (-23.6826, -10.1683)). Similar statistical significance was also observed between Arm 2 and Arm 3 ((p-value < 0.0001 95% CI (-29.5207, -16.0207)) and Arm 2 and Arm 4 ((p-value < 0.0001 95% CI (-33.2875, -19.7806)).
Thirty-nine (52.70%) patients from Arm 1, 42 (58.11%) patients from Arm 2, 25 (33.78%) patients from Arm 3, and 21 (28.38%) patients from Arm 4 achieved a therapeutic glycemic response defined as HbA1c < 7% at the end of the study visit (week 24). A higher proportion of patients in the FDC groups achieved therapeutic glycemic response control compared to the monotherapy (p-value 0.0003). The mean body weight reduction at week 24 from baseline was -1.49 Kg in Arm 1, -1.91 Kg in Arm 2, and -2.16 Kg in Arm 3, however, in group 4, who received 20 mg of Teneligliptin, the mean body weight was raised by 0.23 Kg. When compared amongst the groups the weight reduction in Arm 2 was significantly more than Arm 1 (P= 0.007) and Arm 4 (P < <0.0001), whereas there was no significant difference between Arm 2 and Arm 3. The weight reduction in Arm 3 was significantly more than Arm 1 and Arm 4 (P <0.0001).
The adverse events observed were headache, diarrhoea, dyspepsia, abdominal pain, nausea, asthenia, pyrexia, nasopharyngitis, genital fungal infection, urinary tract infection, hyperglycaemia, arthralgia, back pain, and dizziness. All AEs were mild and were completely resolved. No serious adverse events (SAE) were reported during the study. All the adverse events were followed up until they were completely resolved (Table 2).
|
Arm A1 (N=74) |
Arm A2 (N=74) |
Arm A3 (N=74) |
Arm A4 (N=74) |
|
|
Nervous system disorders |
03 (4.05%) |
04 (5.41%) |
02 (2.70%) |
02 (2.70%) |
|
Gastrointestinal disorders |
04 (5.41%) |
04 (5.41%) |
03 (4.05%) |
02 (2.70%) |
|
Infections and infestations |
06 (8.11%) |
07 (9.46%) |
06 (8.11%) |
03 (4.05%) |
|
Musculoskeletal and connective tissue disorders |
02 (2.70%) |
03 (4.05%) |
00 (00.00%) |
01 (1.35%) |
|
Metabolism and nutrition disorders |
03 (4.05%) |
03 (4.05%) |
02 (2.70%) |
01 (1.35%) |
|
General disorders and administration site conditions |
01 (1.35%) |
01 (1.35%) |
00 (00.00%) |
00 (00.00%) |
|
Total |
19 (25.68%) |
22 (29.73%) |
13 (17.57%) |
09 (12.16%) |
Table 2: Adverse Events in the study population.
Nineteen (25.68%) adverse events were reported in the Dapagliflozin 5mg + Teneligliptin 20mg group, 22 (29.73%) in the Dapagliflozin 10mg + Teneligliptin 20mg group, 13 (17.57%) in the Dapagliflozin 10mg group, and 9 (12.16%) in the Teneligliptin group. The occurrence of genital tract infection was ~5% in the Dapagliflozin 10mg group and 2.7% in both the FDC groups. 1.35% patients in all the group each reported for urinary tract infection except for Teneligliptin 20 mg group. The overall incidence of hypoglycaemia was more with the FDC groups (2.7%) as compared to individual groups (1.35%).
4. Discussion
The adoption of fixed-dose combinations of antidiabetic agents is on the rise, and recognized as an effective means to address multiple pathophysiological issues in T2DM with improved medication adherence [16]. The dual combination therapy approach, which involves the use of SGLT2 inhibitors alongside DPP4 inhibitors, has demonstrated its efficacy in improving glycemic parameters and achieving target values, with additional benefits including weight reduction.
In this prospective study, we aimed to assess the efficacy and safety of the FDC of Dapagliflozin and Teneligliptin in T2DM Indian patients. The study demonstrated that this FDC improves glycemic parameters and reduces weight in T2DM patients. The outcomes are advantageous, particularly for patients who have persistent uncontrolled parameters despite being on monotherapy with recommended first-line metformin treatment. There is compelling evidence supporting the clinical effectiveness and safety of combining SGLT2 inhibitors with DPP4 inhibitors. A meta-analysis that included 2,082 patients with inadequately controlled type 2 diabetes has reported that those on the FDC of SGLT2i and DPP4i regimen achieved greater reductions in HbA1c, fasting plasma glucose, 2-hour postprandial plasma glucose, and body weight compared to those on DPP4i alone or a placebo. The findings indicate that this combination not only enhances glycemic control but also promotes weight loss while maintaining a low risk of hypoglycaemia and urinary tract infections [17].
In the current study, the FDC of Dapagliflozin and Teneligliptin demonstrated a significant decline in HbA1c levels from baseline, with a substantial number of patients reaching the glycaemic control with an HbA1c target of under 7%. In a study by Jabbour et al, the effects of dapagliflozin 10 mg were compared to a placebo in individuals diagnosed with T2DM who were already receiving sitagliptin, with or without metformin therapy. The results demonstrated a significant reduction in HbA1c levels, with dapagliflozin leading to a decrease of △ -0.4% (p<0.0001) when combined with sitagliptin and metformin, and △ -0.6% (p<0.0001) when used with sitagliptin alone, relative to the placebo group at 24 weeks. Furthermore, the addition of dapagliflozin resulted in 10% of patients achieving the HbA1c target goal of < 7% [18]. In studies evaluating combinations of Empagliflozin (10mg) with Linagliptin (5mg) and Dapagliflozin (10mg) with Saxagliptin (5mg), 43.4% and 29.8% patients demonstrated optimal glycemic response (HbA1C < 7%) at the 52nd week, respectively [19,20]. Comparing the results of our study with the reported results, our combination outperforms the above-mentioned ones, as a greater proportion of patients from our study demonstrated a desirable glycemic response by the end of 24 weeks. Similarly, a significant reduction in fasting and post-prandial blood glucose levels was observed in our study by the end of 24 weeks.SGLT2 inhibitors have been associated with weight loss attributed to urinary glucose loss, alteration of the insulin-to-glucagon ratio, increased lipolysis, activation of the AMPK enzyme, inhibition of mTORC1, improvement of mitochondrial function, inhibition of leptin, and increased expression of adiponectin, as well as activation of FGF-21 expression [21]. The findings of this study indicate a significant decrease in body weight of 2 kgs among patients on FDC therapy. In contrast, patients receiving Teneligliptin monotherapy showed a slight increase in body weight. These results are consistent with the real DAPSI study, where 93% of patients on Sitagliptin and Dapagliflozin FDC for 7 months experienced significant weight loss of 4 kg [22].
Urinary tract infections (UTIs), Genital tract infections (GTIs), and nasopharyngitis are among the most commonly reported adverse events associated with the use of SGLT2 and DPP4 inhibitors [23]. In a study, evaluating FDC of a SGLT2 inhibitor (Ertugliflozin) and DPP4 inhibitor (Sitagliptin), approximately 3-8% of patients experienced UTI, while 1-7% of patients experienced GTI symptoms [17]. In our study, 1.35% patients experienced UTI, whereas 2.7% patients reported GTI in both FDC groups. In the evaluation of hypoglycaemic episodes, a significant difference was observed where 2.7% of individuals receiving the fixed-dose combination (FDC) of Dapagliflozin (5 or 10 mg) alongside Teneligliptin (20 mg) reported experiencing hypoglycaemia. This rate was notably more than the 1.35% incidence found in the monotherapy groups. The systematic review and meta-analysis by Min et.al included 2082 patients, indicating a potentially diminished risk of genital tract infections, along with a modest decrease in the occurrence of urinary tract infections, when employing a simultaneous combination of SGLT-2 inhibitors and DPP-4 inhibitors, rather than a sequential approach [17].
With a plethora of data at our disposal regarding the FDCs of SGLT2I and DPP4I, it is clear that this combination can be considered for early initiation in T2DM patients as well as for those who have encountered failures to achieve glycemic control with monotherapy or have shown intolerance to metformin combinations and require dual therapy. The evidence suggests a low rate of adverse events, indicating that this combination is likely to remain in the therapeutic landscape. Overall, the FDC of Dapagliflozin and Teneligliptin demonstrated efficacy and safety, suggesting a promising choice for patients with uncontrolled T2DM.
5. Conclusion
The FDC Dapagliflozin and Teneligliptin has demonstrated effective glycemic control, weight loss and tolerability, thereby establishing this fixed-dose combination as an alternative for T2DM patients uncontrolled on metformin monotherapy.
6. Funding Source
Limited: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
7. Conflict of Interest:
Rahee Borulkar, Mayur Jadhav, Sanjay Choudhari, Sumit Bhushan, Saiprasad Patil and Hanmant Barkate are employees of Glenmark. All other investigators/authors have no conflicts of interest that are directly relevant to the content of this article.
8. Acknowledgement:
We would like to extend our thanks to all the institutes and respective investigators and team members for their support. Investigators: Dr D Anil Kumar, Dr G Gouri Prasad, Dr Saurabh Agarwal, Dr Sanjiv Maheshwari, Dr Prabhat Kumar Sharma, Dr Vikas Reddy Maddali, Dr Shankar Ramgouda Patil.
References
- IDF Diabetes Atlas. Brussels: International Diabetes Federation (2021).
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 404 (2024): 2077-2093.
- Anjana RM, Unnikrishnan R, Deepa M, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol 11 (2023): 474-489.
- Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci 21 (2020): 6275.
- Anusuya GS, Shobhana M, Suresh V, et al. Prevalence of undiagnosed and uncontrolled diabetes mellitus among adults in South Chennai. Int J Community Med Public Health 5 (2018): 5200-5204.
- Borgharkar SS, Das SS. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diabetes Res Care 7 (2019): e000654.
- Mohan V, Shah SN, Joshi SR, et al. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: results from the DiabCare India 2011 study. Indian J Endocrinol Metab 18 (2014): 370-378.
- Tan JK, Salim NNM, Lim GH, et al. Trends in diabetes-related complications in Singapore, 2013-2020: a registry-based study. PLoS One 17 (2022): e0275920.
- Pradeepa R, Mohan V. Prevalence of type 2 diabetes and its complications in India and economic costs to the nation. Eur J Clin Nutr 71 (2017): 816-824.
- van Dieren S, Beulens JW, van der Schouw YT, et al. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil 17 Suppl 1 (2010): S3-S8.
- American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2025. Diabetes Care 48 Suppl 1 (2025): S181-S206.
- Murphy PZ, Bramwell-Shittu A, Boehmer K, et al. Assessment of metformin intolerance: a retrospective chart review. Innov Pharm 15 (2024): 5779.
- Borse SP, Chhipa AS, Sharma V, et al. Management of type 2 diabetes: current strategies, unfocussed aspects, challenges, and alternatives. Med Princ Pract 30 (2021): 109-121.
- Gallwitz B. Clinical use of DPP-4 inhibitors. Front Endocrinol (Lausanne) 10 (2019): 389.
- Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 17 (2020): 761-772.
- John M, Gopinath D, Kalra S. Triple fixed drug combinations in type 2 diabetes. Indian J Endocrinol Metab 19 (2015): 311-313.
- Min SH, Yoon JH, Moon SJ, et al. Combination of sodium-glucose cotransporter 2 inhibitor and dipeptidyl peptidase-4 inhibitor in type 2 diabetes: a systematic review with meta-analysis. Sci Rep 8 (2018): 4466.
- Jabbour SA, Hardy E, Sugg J, et al. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care 37 (2014): 740-750.
- Kawamori R, Haneda M, Suzaki K, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab 20 (2018): 2200-2209.
- Vilsbøll T, Ekholm E, Johnsson E, et al. Efficacy and safety of dapagliflozin plus saxagliptin versus insulin glargine over 52 weeks as add-on to metformin with or without sulphonylurea in patients with type 2 diabetes: a randomized, parallel-design, open-label, Phase 3 trial. Diabetes Obes Metab 22 (2020): 957-968.
- Feder D, Gouveia MFV, Govato TCP, et al. SGLT2 inhibitors and the mechanisms involved in weight loss. Curr Pharmacol Rep 6 (2020): 346-353.
- Bhattacharjee R, Rai M, Joshi P, et al. The Real DAPSI: a real-world retrospective study on assessing the efficacy and safety of a fixed-dose combination of dapagliflozin and sitagliptin in the Indian population. Cureus 15 (2023): e46767.
- Pittampalli S, Upadyayula S, Mekala HM, et al. Risks vs benefits for SGLT2 inhibitor medications. Fed Pract 35 (2018): 45-48.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 