Frequency and Relevance of Thyroid Dysfunction in Patients with Chronic Heart Failure: A Cross Sectional Study at Yaoundé Central Hospital, Cameroon
Kemnang Yemele Honoré1, Vicky J Ama Moor1,2, Liliane Mfeukeu Kuate1,3, Etoa Martine3,4, Tcheutchoua Nzokou Daryl1, Batakeh B Agoons1, Esther Mbono Samba1, Falmata Amazia1, Nangue Tiogoung Gaelle1, Ossongo Arnaud1, Eko Ondoua Manuela1, Elage Epie Macbrain1, Jan René Nkeck1*
1Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon
2Yaoundé University Hospital Center, Yaoundé, Cameroon
3Yaoundé Central hospital, Yaoundé, Cameroon
4Faculty of Medicine and Pharmaceutical Sciences, University of Douala, Cameroon
*Corresponding Author: Jan René Nkeck, Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon
Received: 29 November 2020; Accepted: 25 January 2021; Published: 02 February 2021
Article Information
Citation: Kemnang Yemele Honoré, Vicky J Ama Moor, Liliane Mfeukeu Kuate, Etoa Martine, Tcheutchoua Nzokou Daryl, Batakeh B Agoons, Esther Mbono Samba, Falmata Amazia, Nangue Tiogoung Gaelle, Ossongo Arnaud, Eko Ondoua Manuela, Elage Epie Macbrain, Jan René Nkeck. Frequency and Relevance of Thyroid Dysfunction in Patients with Chronic Heart Failure: A Cross Sectional Study at Yaoundé Central Hospital, Cameroon. Archives of Clinical and Biomedical Research 5 (2021): 110-124.
View / Download Pdf Share at FacebookAbstract
Background: Abnormalities of thyroid function have been reported in patients with heart failure (HF). We aimed to evaluate the frequency and clinical relevance of dysthyroidism in Cameroonians with HF.
Methods: This was a cross-sectional study from January to May 2020. Including consenting adults followed up at the Yaoundé Central hospital for HF. We assessed serum level of TSH, free T3 and free T4 using ELISA method. We sought for associations between thyroid hormones levels and HF-related variables using Pearson and Spearman correlations tests. The threshold of significance was set at 0.05.
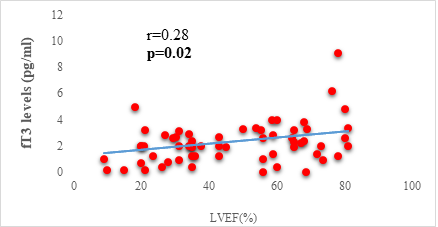
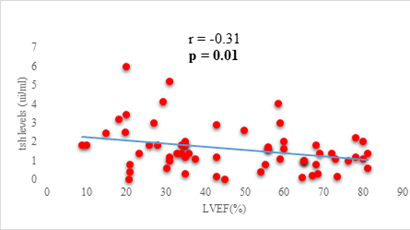
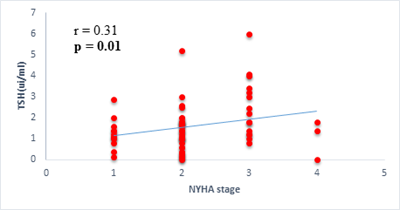
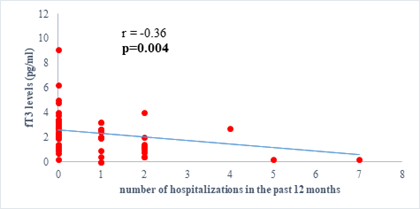
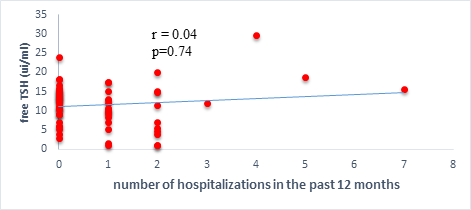
Results: 63 participants were included (33males) with a mean age of 62 ±14 years. Among them, 33 (52%) were hypertensive, 16 (25.4%) had atrial fibrillation, and half (52%) had stage II HF according to the New-York Heart Association. Dysthyroidism was found in 25 subjects (39.6%), with 17 (26.9%) and 6 (9.5%) cases of low-T3 syndrome, and subclinical hyperthyroidism respectively. TSH levels were weakly but significantly correlated with the stage of HF (r=0.31, p=0.01) and slightly correlated with left ventricular ejection fraction (LVEF) (r=-0.31, p=0.01). Free T3 levels were weakly but significantly correlated with the number of hospitalizations over the last 12 months (r=-0.36, p<0.01), and the LVEF (r=0.28, p=0.02).
Conclusion: Dysthyroidism affects nearly two out of five patients suffering from heart failure in our sample. It could be associated with a more severe clinical presentation. More studies need to be done to assess the long-term prognosis in sub-Saharan African populations with HF and dysthyroidism.
Keywords
<p>Heart failure; Thyroid hormones;Dysthyroidism; Cameroonians</p>
Article Details
Abbreviations:
HF: heart failure; TSH: thyroid stimulating hormone; FT3: free triiodothyronine; FT4: free thyroxine; LVEF: left ventricular ejection fraction; LT3S: Low T3 syndrome; NYHA: New York Heart association; YCH: Yaoundé Central Hospital; ELISA: Enzyme Linked Immunosorbent Assay
1. Introduction
Heart failure (HF) represents the final outcome of the majority of cardiovascular diseases [1]. It is a major public health problem that affects around 26 million people worldwide [2]. In Western countries, its prevalence ranges from 1.36% in people aged 25 to 49 years, to 16.14% in people over 80 years old [3]. The prevalence available in sub-Saharan Africa are from hospital-based studies, where they vary between 9.4% to 42.5% [4]. It is a disabling disease that remains one of the leading causes of death worldwide with a death rate of 17.5% within one year of diagnosis [5]. This mortality is even higher in patients with co-morbidities and can be up to 35% within one year of diagnosis [6, 7]. The comorbidities that can influence the prognosis of HF are not only limited to the presence of other cardiovascular risk factors (diabetes, arterial hypertension, dyslipidemia, renal failure), but also include endocrine disorders, and in particular thyroid dysfunction [7, 8].
The relationship between the heart and the thyroid is a two-way street: on the one hand, thyroid hormones (TH), through their positive inotropic and chronotropic effects, can be responsible for high-output HF in hyperthyroidism; hypothyroidism by its atherogenic effects increases cardiovascular risk and can thus contribute to the development of HF [9, 10]. On the other hand, in 20 to 30% of patients with chronic HF, a low T3 syndrome (LT3S) is observed, characterized by a decrease in free triiodothyronine (FT3) with normal values of free thyroxine (FT4) and thyroid stimulating hormone (TSH) [11-13]. This syndrome, initially interpreted as an adaptive phenomenon, has deleterious effects in patients with HF contributing to a progressive deterioration of cardiac function, thus representing a powerful predictive marker of mortality [8, 14-16]. Other studies show that fT3 supplementation in these patients increases their life expectancy [17-19]. In addition to LT3S, other thyroid abnormalities such as hyperthyroidism (subclinical and clinical) as well as hypothyroidism (subclinical and clinical) have been observed in patients followed up for chronic HF and are also associated with a poor prognosis [8].
Given the importance of thyroid abnormalities in patients followed up for chronic HF and their impact on prognosis, the American Heart Association recommends systematic evaluation of thyroid function in these patients [20]. In sub-Saharan Africa, especially in Cameroon, few studies have been carried out on the frequency of thyroid disorders in patients with heart failure and therefore the usefulness of this assessment remains to be demonstrated. Knowing that the presence of thyroid abnormalities during HF can influence the prognosis, we aimed to evaluate the frequency and clinical relevance of dysthyroidism in this population.
2. Material and Methods
2.1 Study design and Setting
We carried out a cross-sectional study, over a period of 5 months (January to May 2020) at the Yaoundé Central Hospital, Cameroon (YCH).
2.2 Participants
Participants were enrolled at the cardiology outpatient unit of the YCH. We included all consenting adults (≥ 21 years), who were followed up for chronic heart failure. We excluded patients with known thyroid dysfunction prior to the diagnosis of heart failure and those who were taking drugs that could modify thyroid hormones metabolism (synthetic antithyroid, amiodarone, levothyrox (free T4), synomel (free T3), corticosteroids, nonsteroidal anti-inflammatory drugs).
2.3 Sample size estimation
The sample size was estimated at 56 participants using the Cochran formula [21]. This value was obtained using a 10% precision, 5% error, and the prevalence of Akono et al. (17.5%) [22].
2.4 Data collection
Data was collected using a data collection sheet. For all the participants, we reported the age, sex, history of heart failure (baseline cardiopathy, current stage of the disease according to the New York Heart Association (NYHA) classification, the current ventricular ejection fraction assessed by ultrasonography, number of hospitalizations during the last 12 months, the current treatment), and the comorbidities. Afterwards, a physical examination was conducted, and vital signs noted. A 5 ml peripheral blood sample was obtained to assess thyroid hormones. TSH assay was done using ELISA sandwich method while FT3 and FT4 assay were done using ELISA competition method. The reagents used were branded Cypress Diagnostics® (Belgium).
2.5 Definition of terms
We defined euthyroidism as a case of TSH level between 0.4-4.2 uUI/ml. Dysthyroidism was defined for all cases out of this value range. These included Low T3 syndrome: FT3 (<2.2 pg/mL) with normal FT4 (7-22 pg/mL) and TSH (0.4-4.2 IU/mL); overt hyperthyroidism: low TSH level (<0.4 uIU/mL) with elevated FT4 (>22 pg/mL); overt hypothyroidism: elevated TSH level (>4.2 uUI/mL) with a drop in FT4 level (<7 pg/mL); subclinical hyperthyroidism: low TSH level (< 0.4 IU/mL) with a normal FT4 level (between 7 and 22 pg/mL); or subclinical hypothyroidism: elevated TSH level (> 4.2 IU/mL) with a normal FT4 level (between 7 and 22 pg/mL) [23-25].
2.6 Statistical analysis
All the data collected were analyzed using the software SPSS version 21.0. Quantitative variables were expressed in terms of means and standard deviations, while qualitative variables were expressed as counts and proportions. The association between different variables was assessed using the Pearson and Spearman correlations tests. Spearman test was used for non-parametric variables. Results are presented using the correlation coefficient (r). The threshold of significance was set at 0.05.
3. Results
3.1 Characteristics of the sample
Overall 63 participants were included in the study. Among them, 33 (52.4%) were males. Their mean age was 62 ± 14 years. More than half of the participants (52.3%) were classified as stage II according to the NYHA. We found that 33 participants (52.3%) had not been hospitalized during the last 12 months. The main baseline cardiopathies were hypertensive (52.4%) and valvular (14.3%). The main co morbidities were hypertension (55.6%), diabetes mellitus (11.1%). 16 (25.3%) subjects had an associated atrial fibrillation. The clinical characteristics are summarized in Table 1.
3.2 Frequency and distribution of dysthyroidism
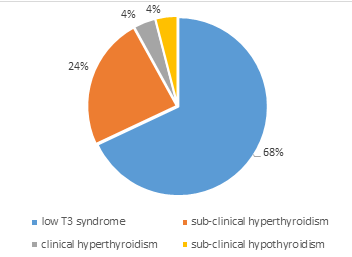
About two thirds of the sample (60.3%) were euthyroid with 25 (39.7%) being dysthyroid. In patients having thyroid abnormalities, the most frequent abnormality was the low T3 syndrome which was observed in 17 participants (68%). We observed that 6 patients (24%) had subclinical hyperthyroidism. No participant was found with clinical hypothyroidism (Figure 1).
3.3 Influence of thyroid hormones on clinical parameters
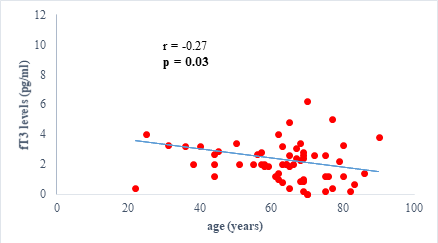
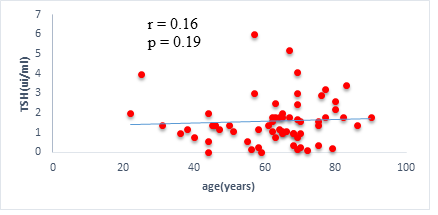
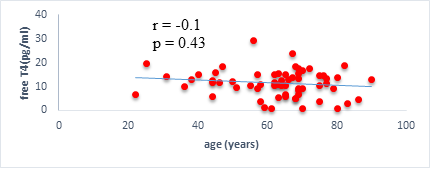
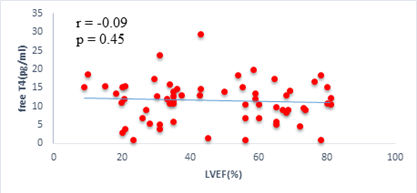
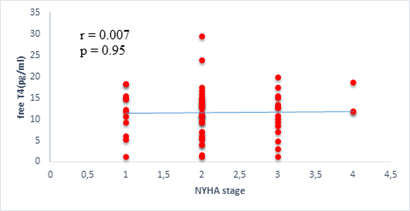
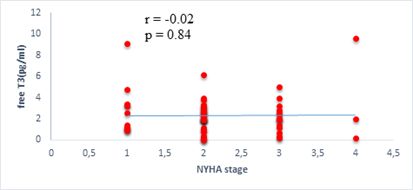
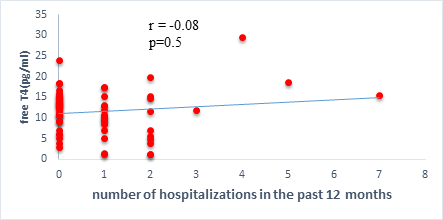
We found a negative, weak and significant correlation between FT3 levels and age (r=-0.27, p=0.03), which was not the case for the other hormones studied (Figures 2a, 2b, 2c). A weak significant correlation was also observed between left ventricular ejection fraction (LVEF) and levels of FT3 (r=-0.28, p=0.02) and TSH (r=-0.31, p=0.01) (Figures 3a, 3b). FT4 levels did not show a significant influence on LVEF (Figure 3c). The results presented in Figures 4a and 5a show respectively the significant but weak correlations between TSH levels and the clinical stage of heart failure assessed by NYHA (r=0.31, p=0.01), and FT3 levels and the number of hospitalizations in the last 12 months (r=-0.36, p=0.004).
|
Variables |
Overall |
Men |
Women |
p value |
|
N (%) |
63 (100) |
33 (52.4) |
30 (47.6) |
>0.05 |
|
Mean Age (SD), years |
62.2 (14) |
62.1 (14) |
62.3 (14.8) |
>0.05 |
|
Age, min-max, years |
22-90 |
22-83 |
31-90 |
- |
|
Heart Failure NYHA stage, n (%) |
||||
|
I |
12 (19) |
6 (18.2) |
6 (20) |
>0.05 |
|
II |
33 (52.4) |
16 (48.5) |
17 (56.7) |
|
|
III |
15 (23.8) |
9 (27.3) |
6 (20) |
|
|
IV |
3 (4.8) |
2 (6.1) |
1 (3.3) |
|
|
Baseline cardiopathies, n (%) |
||||
|
Hypertensive |
33 (52.4) |
19 (57.6) |
14 (46.7) |
>0.05 |
|
Valvular |
9 (14.3) |
2 (6.1) |
7 (23.3) |
|
|
Cardiomyopathy |
7 (11.1) |
3 (9.1) |
4 (13.3) |
|
|
Ischemic |
4 (6.3) |
2 (6.1) |
2 (6.7) |
|
|
Rhythmic |
7 (11.1) |
4 (12.1) |
3 (10) |
|
|
Toxic |
1 (1.6) |
1 (3) |
0 (0) |
|
|
Congenital |
2 (3.2) |
2 (6.1) |
0 (0) |
|
|
Mean Ventricular ejection fraction (SD), in% |
46.8 (21) |
48.7 (20) |
44.9 (22) |
>0.05 |
|
Number of hospitalizations in the last 12 months, n (%) |
||||
|
0 |
33 (53) |
17 (51.5) |
16 (53.3) |
>0.05 |
|
1 or 2 |
26 (41) |
13 (39.4) |
13 (43.3) |
|
|
> 2 |
4 (6) |
3 (9.1) |
1 (3.3) |
|
|
Mean BMI, Kg/m² (SD) |
25.4 (5.8) |
25.6 (4.1) |
25.3 (7.4) |
>0.05 |
|
Comorbidities, n (%) |
||||
|
Hypertension |
35 (55.6) |
16 (48.5) |
12 (40) |
>0.05 |
|
Atrial fibrillation |
16 (25.3) |
9 (27.3) |
7 (23.3) |
>0.05 |
|
Diabetes mellitus |
7 (11.1) |
4 (12.1) |
3 (10) |
>0.05 |
|
Dyslipidemia |
7 (11.1) |
4 (12.1) |
3 (10) |
>0.05 |
|
Chronic kidney disease |
2 (3.17) |
1 (3) |
1 (3.3) |
>0.05 |
|
Chronic liver disease |
3 (4.76) |
2 (6.1) |
1 (3.3) |
>0.05 |
|
Current treatment, n (%) |
||||
|
ACE inhibitor/ARB |
27 (42.8) |
10 (30.3) |
17 (56.6) |
>0.05 |
|
Beta blocker* |
13 (20.6) |
12 (36.4) |
13 (43.3) |
>0.05 |
|
Digoxin |
12 (19.04) |
6 (18.2) |
6 (20) |
>0.05 |
|
Diuretic |
52 (82.5) |
28 (84.2) |
23 (76.7) |
>0.05 |
|
Mean TSH (SD), uUI / ml |
1.6 (1.1) |
1.53 (1) |
1.67 (1.2) |
>0.05 |
|
Mean Free T3 (SD), pg / ml |
2.3 (1.8) |
2.4 (1.7) |
2.2 (1.8) |
>0.05 |
|
Mean Free T4 (SD), pg / ml |
11.4 (5.5) |
11.4 (5.6) |
11.4 (5.5) |
>0.05 |
SD: Standard deviation; NYHA: New York Heart Association; ACE: Angiotensin converting enzyme; ARB: angiotensin receptor blocker; *Beta blocker other than propranolol
Table 1: Clinical characteristics of the sample.
4. Discussion
Dysthyroidism has been reported as deleterious in patients with heart failure. The aim of our study was to evaluate the thyroid function of patients followed for chronic heart failure in order to determine the frequency of those abnormalities and their clinical implications, in our context. We observe a high frequency of LT3S. Already known to be frequent in people with chronic diseases [26-28], LT3S is the most common thyroid disorder found in Caucasians patients having heart failure [7, 9, 11, 29, 30]. A possible explanatory mechanism for this, is a decreased peripheral conversion of T4 to T3 due to a decrease in intra-tissue (hepatic, myocardial, renal) expression of 5'deiodase, an enzyme which allowing the peripheral conversion of T4 to T3 [12, 17, 29, 31]. There is also an increased catabolism of thyroid hormones due to an ectopic induction of 5 desiodase which transforms T4 into reverse T3, its inactive form [12, 13, 15, 31]. This phenomenon result in a biological fall in the concentration of FT3 and thus leads to a low FT3. Other thyroid abnormalities were found but at lower frequencies. They may precede the onset of HF, or even be a cause, but may also appear concomitantly or after its onset [5, 15]. The large proportion of these various abnormalities can also be explained by the increased age of our study population, as evidence exists to prove that abnormalities in thyroid hormones metabolism are more frequent in the elderly [20]. Additionally, we found a negative correlation between FT3 levels and age. This is explained by age-induced changes in the thyroid gland. Increasing age causes a gradual decrease in thyroid hormone levels due to progressive fibrosis of the thyroid gland. Also, progressive muscle wasting occurs with increasing age, leading to a reduction in peripheral muscle conversion of T4 to T3 [20]. We did not have any cases of clinical hypothyroidism. A potential reason for this is the fact that in Cameroon, since 1991, iodine has been systematically introduced into cooking salt, considerably contributing to the low prevalence of hypothyroidism [32]. With the high occurrence of dysthyroidism, it highlights the importance of systematic evaluation of thyroid function in patients with HF as recommended by the American Heart Association [20]. However, with the lack of universal health coverage, routinely thyroid hormones assessment is difficult or even impossible. To this end, we evaluated the clinical impact of thyroid hormones variations in patients suffering from heart failure, and found that an elevated TSH level was associated with a more advanced stage of the disease. This result is similar to that obtained by Kannan et al. [7]. Basically, it could be explained by the fact that FT3 exerts negative feedback on TSH and therefore a fall in FT3 leads to an increase in TSH. Since FT3 is generally lower in patients with advanced stages, a concomitant increase in TSH is therefore observed [33]. We found a significant correlation between the FT3 concentrations and the number of hospitalizations during the last twelve months which are similar to the findings of other studies presenting low FT3 as a marker of poor prognosis in patients with HF, associated with a higher long-term mortality, more severe symptoms and a higher number of re-hospitalizations [7, 12, 13, 29]. In addition, our study found a positive correlation between FT3 concentration and LVEF which is similar to the results obtained by Saurav et al.; FT3 has a direct and indirect action on systolic function, thus it is capable of exerting changes to LVEF. This provides further proof that myocardial contractility is significantly influenced by variations in thyroid hormone levels. A decrease in FT3 leads to a decrease in myocardial contractility, an increase in afterload and therefore a decrease in LVEF [29]. We did not find a significant association between thyroid hormone levels and the presence of arrhythmias. This is contrary to the results obtained by Kannan et al. [7] who observed that an elevated FT4 concentration was associated with the presence of atrial fibrillation [7]. This difference can be explained by the fact that we carried out only one set-point electrocardiogram analysis, as opposed to a 24 hour holter, which would have better assessed the rhythm disturbances. However, atrial fibrillation was linked to their cardiac diseases (valve disease, cardiomyopathy, etc.).The findings presented in this study raise possible clinical implications related to dysthyroidism, as it draws attention to the benefits of hormone supplementation in patients with these abnormalities. Some authors in western studies have revealed that FT3 supplementation in patients with HF having a LT3S could improve the prognosis [29, 30]. There is therefore a need to perform such a study in our context to assess the benefit of such supplementation and the cost of routinely testing in thyroid hormones.
The results presented here should be interpreted in the light of some study limitations. The small size of our sample did not permit overall assessment of all the thyroid functional abnormalities. During the recruitment period, the onset of the Covid 19 pandemic led to a decrease in the flow of patients in our hospital due to fears of contamination, which made enrollment difficult. The biological analysis was carried out by the ELISA method. This technique, although not being the reference, has several advantages like its accessibility, its good specificity and sensitivity [24]. However, interference with non-esterified fatty acids have been reported [23-25].
5. Conclusion
Functional thyroid abnormalities are frequent in patients followed for CHF in our setting. The most common abnormality is LT3S and is associated with a more serious clinical presentation. This therefore raises the question of systematic screening for these abnormalities as well as a possible correction that could have a beneficial effect on the prognosis.
Declaration
Acknowledgement
The authors would like to thank the staff of the biochemistry laboratory of the Yaoundé University Hospital Center, and the staff of the cardiology unit of the Yaoundé Central Hospital.
Authors Contribution
Conception and design: VJAM, LMK, MCE;
Data collection: KYH, LMK, FA, NTG, VJAM;
Data analysis and interpretation: KYH, TND, JRN, MCE;
Manuscript drafting: KYH, JRN, BBA;
Manuscript revision: VJAM, EMS, OA, EOM, EEM
Approval of the final manuscript: All the authors.
Funding
This research did not receive fund from any organization.
Availability of Data and Materials
The datasets used for this study are available from the corresponding author on request.
Ethical Approval and Consent to Participate
The study was approved by the Institutional Ethical Review Board of Faculty of Medicine and Biomedical Sciences, University of Yaoundé I (Cameroon). All the participants read and signed an informed consent before their inclusion in the study.
Consent for Publication
Not applicable.
Competing Interest
The authors declare that they have no competing interests.
References
- Velagaleti R, Vasan RS. Heart Failure in the 21st Century: Is it a Coronary Artery Disease Problem or Hypertension Problem? Cardiol Clin 25 (2007): 487-v
- Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 8 (2011): 30-41
- Ceia Fatima. Prevalence of chronic heart failure in Southwestern Europe: the EPICA study.Disponible sur: Eur J heart failure 4 (2002): 531-539.
- Nyaga UF, Bigna JJ, Agbor VN, et al. Data on the epidemiology of heart failure in Sub-Saharan Africa. Data Brief 17 (2018): 1218-1239
- Savarese G, Lund LH. Global Public Health Burden of Heart Failure. Card Fail Rev 3 (2017): 7-11
- Ergatoudes C, Schaufelberger M, Andersson B, et al. Non-cardiac comorbidities and mortality in patients with heart failure with reduced vs. preserved ejection fraction: a study using the Swedish Heart Failure Registry. Clin Res Cardiol 108 (2019): 1025-1033
- Kannan L, Shaw PA, Morley MP, et al. Thyroid Dysfunction in Heart Failure and Cardiovascular Outcomes. Circ Heart Fail 11 (218): e005266.
- Angermann CE. Comorbidities in heart failure: a key issue. Eur J Heart Fail Suppl 8 (2009): 5-10
- Danzi S, Klein I. Thyroid hormone and the cardiovascular system. Minerva Endocrinol 29 (2004): 139-150
- Biondi B. Mechanisms in endocrinology: Heart failure and thyroid dysfunction. Eur J Endocrinol 167 (2012): 609-618
- Opasich C, Pacini F, Ambrosino N, et al. Sick euthyroid syndrome in patients with moderate-to-severe chronic heart failure. Eur Heart J 17 (1996): 1860-1866
- Ascheim DD, Hryniewicz K. Thyroid hormone metabolism in patients with congestive heart failure: the low triiodothyronine state. Thyroid Off J Am Thyroid Assoc 12 (2002): 511-515
- Saurav S, Singh A, Debnath A, et al. Prevalence of Low T3 syndrome and its association with severity in patients of Chronic heart failure: A hospital based study. J Assoc Physicians India (2014).
- Simonides WS, Mulcahey MA, Redout EM, et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 118 (2008): 975-983
- Iervasi G, Molinaro S, Landi P, et al. Association Between Increased Mortality and Mild Thyroid Dysfunction in Cardiac Patients. Arch Intern Med 167 (2007): 1526-1532
- Iervasi Giorgio, Pingitore Alessandro, Landi Patrizia, et al. Low-T3 Syndrome. Circulation 107 (2003): 708-713
- Gerdes Anthony Martin, Iervasi Giorgio. Thyroid Replacement Therapy and Heart Failure. Circulation 122 (2010): 385-393
- Malik FS, Mehra MR, Uber PA, et al. Intravenous thyroid hormone supplementation in heart failure with cardiogenic shock. J Card Fail 5 (1999): 31-37
- Savinova OV, Liu Y, Aasen GA, et al. Thyroid Hormone Promotes Remodeling of Coronary Resistance Vessels. PLoS One 6 (2011): e25054.
- Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 38 (2001): 2101-2113
- Elise Whitley, Jonathan Ball. Statistics review 4: Sample size calculations. Critical Care 6 (2002).
- Akono MN, Simo LP, Agbor VN, et al. The spectrum of heart disease among adults at the Bamenda Regional Hospital, North west Cameroon: a semi urban setting. BMC Res Notes 12 (2019): 761.
- Thakur C, Saikia TC, Yadav RN. Total serum levels of triiodothyronine (T3) thyroxine (T4) and thyrotropine (TSH) in school going children of Dibrugarh district: an endemic goitre region of Assam Indian J Physiol Pharmacol 41 (1997): 167-170.
- Sakamoto S, Putalun W, Vimolmangkang S, et al. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J Nat Med 72 (2018): 32-42
- Maes M, Mommen K, Hendrickx D, et al. Components of biological variation, including seasonality, in blood concentrations of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers [Internet]. Vol. 46, Clinical endocrinology. Clin Endocrinol (Oxf) 46 (1997): 587-598.
- Ruiz-Núñez B, Tarasse R, Vogelaar EF, et al. Higher Prevalence of “Low T3 Syndrome” in Patients With Chronic Fatigue Syndrome: A Case–Control Study. Front Endocrinol. Front Endocrinol (Lausanne) 9 (2018): 97.
- Moura Neto A, Zantut-Wittmann DE. Abnormalities of Thyroid Hormone Metabolism during Systemic Illness: The Low T3 Syndrome in Different Clinical Settings. Int J Endocrinol (2016).
- Punekar P, Ashvanee Kumar Sharma, Jain A. A Study of Thyroid Dysfunction in Cirrhosis of Liver and Correlation with Severity of Liver Disease. Indian J Endocrinol Metab 22 (2018): 645-650.
- Galli E, Pingitore A, Iervasi G. The role of thyroid hormone in the pathophysiology of heart failure: clinical evidence. Heart Fail Rev 15 (2010): 155-169.
- Pantos C, Mourouzis I, Markakis K, et al. Thyroid hormone attenuates cardiac remodeling and improves hemodynamics early after acute myocardial infarction in rats. Eur J Cardiothorac Surg 32 (2007): 333-339.
- Wassen FWJS, Schiel AE, Kuiper GGJM, et al. Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology 143 (2002): 2812-2815.
- Lantum D. Supplement: The conquest of Iodine deficiency in Cameroon 1990-2007. Journal of the Cameroon Academy of Sciences 7 (2008): 237-248.
- Mariotti S, Beck-Peccoz P. Physiology of the Hypothalamic-Pituitary-Thyroid Axis. In Eds.: Feingold KR, Anawalt B, Boyce A, et al. South Dartmouth (MA): MDText.com, Inc (2000).


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 