Impact of End-Effector Device on Gait Restoration in Children Suffering from Neurological Disorders
Martin Malovec*
Head of Physiotherapy, RehaZentrum Malovec, Korneuburger Str., Bisamberg, Austria
*Corresponding author: Martin Malovec, Head of Physiotherapy, RehaZentrum Malovec, Korneuburger Str., Bisamberg, Austria.
Received: 25 October 2024; Accepted: 01 Novemebr 2024; Published: 12 November 2024
Article Information
Citation: Martin Malovec. Impact of End-Effector Device on Gait Restoration in Children Suffering from Neurological Disorders. Archives of Clinical and Biomedical Research. 8 (2024): 371-378.
View / Download Pdf Share at FacebookAbstract
Background: Gait disorders in children with neurological conditions significantly impact their independence and quality of life. Traditional rehabilitation methods have several limitations, highlighting the need for innovative solutions. Robotic-assisted gait training appears to be a promising approach for improving gait difficulties in this population.
Objective: This study aims to evaluate the effectiveness of a novel endeffector type of robot-assisted gait training system in addressing gait difficulties in pediatric patients with neurological disorders.
Material and methods: Twenty-five patients with gait-related neurological disorders underwent 10 therapy sessions (each lasting 30 minutes) using an end-effector RAGT device in conjunction with conventional therapy. Gait parameters, including the number of steps, distance walked, and speed, were compared based on data collected during the first and last therapy sessions.
Results: Statistically significant improvements were observed in all assessed parameters for both patient groups, with an average increase of approximately 48% in the number of steps, 63% in distance walked, and 39% in walking speed. The differences between the groups were minimal, with slightly better outcomes noted in the CP group.
Conclusion: Following therapy with the end-effector-based RAGT system, all assessed parameters showed notable improvements in both patient groups, indicating its positive impact on gait ability across various pediatric neurological conditions.
Keywords
RAGT; End-effector; Neurological disorders; Cerebral palsy; Gait parameters; Children
RAGT articles; End-effector articles; Neurological disorders articles; Cerebral palsy articles; Gait parameters articles; Children articles
Article Details
1. Introduction
Although many neurological disorders affect overall independence, gait disorders are a significant concern in pediatric neurology, presenting considerable challenges in management and rehabilitation [1]. For healthy children, walking is a natural and effortless activity, but for those with gait disorders, it can be a major obstacle. These disabilities hinder the ability to perform daily activities independently, affecting physical and social development, mental health, and overall quality of life [2].
Gait disorders in the pediatric population can arise from a myriad of neurological conditions, including several genetic disorders, spina bifida (SB), cerebral palsy (CP), as well as brain and spinal cord injuries [3]. The most common genetic disorders affecting the ability to walk in children include Duchenne Muscular Dystrophy and Charcot-Marie-Tooth disease, however, similar symptoms can also be seen in some rarer conditions such as Angelman syndrome or Rett syndrome. Although the incidence of SB has decreased over the years due to folic acid supplementation and active screening during pregnancy, the current prevalence is still about 1 in 1,000 live births in Europe [4]. Myelomeningocele, where the spinal cord extends through a partially closed spine, accounts for 80-90% of all SP cases and is the most common and severe form [5]. This condition often leads to muscle weakness or paralysis in the lower extremities, significantly impairing gait and mobility [6].
CP represents the most common and costly motor disability of childhood, with an overall prevalence worldwide of 2-3 per 1,000 live births, and a 50 times higher prevalence among neonatal survivors weighing less than 1500 grams at birth [7,8]. The estimated lifetime cost for a patient with CP in the USA and Europe exceeds $900,000 [9]. CP refers to a group of permanent, non-progressive neurological disorders caused by damage to the developing brain, typically occurring in the prenatal, perinatal, or early postnatal stages [10]. It stems from varied etiologies affecting different brain regions, resulting in diverse clinical presentations. Despite known risk factors like preterm birth, infections, and asphyxia, approximately 80% of cases are idiopathic [11]. All children diagnosed with CP face challenges in gross motor function, with the majority presenting significant gait and balance impairments. Consequently, it is estimated that one out of every three children with CP is non-ambulatory [12,13]. Gait disorders in individuals with CP are complex, arising from primary issues like muscle spasticity and weakness, along with secondary problems such as contractures and bony deformities [14]. Approximately 80% of children with CP experience spasticity, with spastic diplegia being the most common subtype, affecting 32% of patients and primarily impacting the lower limbs [11,15].
Treatment of gait disorders in children with neurological conditions often requires a multidisciplinary approach, tailored to the child's specific needs. Common interventions include orthopedic surgery, pharmacological treatments (e.g., botulinum toxin for spasticity management in CP), physical therapy, and the use of orthotics. Physical therapy aims to address structural and functional impairments through exercises like stretching and strengthening and enhances neuroplasticity with task-specific training to improve gait speed, endurance, stability, and motor skills [16-18]. Traditional body weight support treadmill training (BWSTT) is a task-specific approach that has proven safe and effective for gait therapy in children with neurological disorders. This method enables controlled and repetitive practice of walking patterns, with weight support making it suitable even for patients with more severe motor impairments [19,20]. However, BWSTT has limitations, including the need for significant therapist involvement (sometimes requiring two therapists), variability in movement patterns, and limited real-time feedback on performance [21-23].
To address the limitations of traditional BWSTT, robot-assisted gait training (RAGT) devices have been developed. These devices offer high-intensity, consistent, and reproducible interactive training, enabling precise control over leg movement during walking. RAGT devices generally fall into two categories: exoskeletons and end-effector systems. Exoskeletons are designed to align with the patient's anatomical axes, providing direct joint control. In contrast, end-effector systems primarily engage the patient's lower limb at the most distal segment, typically via footplates. This allows for free movement of the hip and knee joints, facilitating greater patient involvement in walking training [24]. Compared to established indications for conditions like stroke and spinal cord injury, which predominantly focus on adult patients, the use of these devices for pediatric patients with neurological disorders is relatively new and evolving [25]. The majority of studies conducted thus far have concentrated on examining the benefits of exoskeletons, primarily in patients with CP, while there is a noticeable lack of research investigating the effects of end-effector devices in this field [26,27]. The findings from existing studies on children with CP indicate a favorable impact of the end-effector device on various gait-related parameters, including locomotion function, balance parameters, gait speed, and step length [28-31]. Regarding studies focusing on neurological disorders in children other than CP, the evidence is even more limited, with almost no studies available, particularly on the use of end-effector devices. While the current evidence is encouraging, ongoing research is crucial to provide a comprehensive understanding of the role of end-effector therapy in the treatment of neurological gait disorders in children.
The objective of the present study is to evaluate the effectiveness of a new end-effector type of RAGT device in children diagnosed with various neurological conditions experiencing walking impairments. The primary aim is to assess the impact of the therapy on gait parameters, including a number of steps, distance walked, and walking speed.
2. Material and Methods
The study was conducted at RehaZentrum Malovec, a private neurorehabilitation center situated in Bisamberg, Austria, spanning from November 2023 to July 2024. A total of 25 pediatric patients diagnosed with various neurological gait disorders received treatment utilizing an end-effector-based robotic device. The primary inclusion criteria for participation were a diagnosed neurological condition with compromised ability to walk, an age range of 3 to 17 years, height> 80 cm, and the ability to follow the study protocols.
The exclusion criteria for the study included the presence of any of the following contraindications: contractures in the large joints, unhealed fractures, reduced bone density (osteoporosis, osteopenia), severe cardiovascular diseases, open wounds or skin lesions in the treatment area, severe disc herniation, colostomy bags, acute thrombosis, osteomyelitis, pregnancy, or epilepsy. Furthermore, patients with any medical condition preventing participation in active gait training, such as respiratory diseases, orthopedic conditions, cognitive deficits affecting communication, neuropsychological disorders, infections, or inflammatory disorders, were also excluded.
Before providing written informed consent, the legal representatives of all participants received comprehensive information about the treatment regimen, possible risks, and results. The consent form included an agreement regarding participation and potential publication of results [19]. The treatment program was compatible with the 1975 Declaration of Helsinki ethical guidelines adopted by the General Assembly of the World Medical Association (1997-2000) and by the Convention on Human Rights and Biomedicine of the Council of Europe (1997).
Each patient meeting the inclusion criteria participated in 10 therapy sessions using an end-effector-based gait rehabilitation robotic device (R-Gait, BTL Industries Ltd.). The sessions were conducted at least three times a week, with each session lasting 30 minutes. RAGT was combined with the standard rehabilitation program used at the facility. The robotic device is designed for re-education in neurological patients to walk in a more physiological gait pattern. The primary therapeutic principle involves allowing unrestricted movement of the lower extremities and pelvis in all three anatomical planes. During the session, the patient wears a harness connected to an unweighting system capable of offloading up to 100% of the patient's weight, see Figure 1. The system dynamically adjusts the offloading based on the phase of the gait cycle. Additionally, it aids in patient transfer onto the device and provides support until the patient is correctly positioned, with feet secured in the footplates. These footplates simulate the stance and swing phases of the gait cycle through movement. Each footplate is equipped with 8 load cells, which, along with a sensor in the unweighting system, monitor the activity of the right and left foot and measure the percentage of the patient's weight supported by the unweighting system. Patient motivation can be enhanced by incorporating a gaming environment into the therapy. The level of weight support, step length, and speed during therapy are adjusted according to the individual condition and capabilities of each patient.
The device automatically collected data on the number of steps, distance walked, and average step speed. The values for the initial and final therapy sessions were subsequently compared as indicators of improvement in the quality of walking in patients.
For the statistical analysis, the normality of the data distribution was first evaluated using the Shapiro-Wilk test. Upon confirming that the data followed a normal distribution, a paired t-test was conducted to determine the statistical significance of the differences between the initial and final therapy sessions. A significance threshold of p < 0.05 was applied, meaning that any result with a p-value below 0.05 was considered statistically significant.
3. Results
Patient characteristic
The study included a total of 25 pediatric patients, with an overall average age of 10.6 ± 3.74 years and a gender distribution of 16 males and 9 females. All patients successfully completed the entire course of treatment without any adverse events. The patients were divided into two groups: the first group comprised 14 children diagnosed with CP, while the second group, referred to as "Others," included 11 children with a range of conditions such as SB, genetic disorders, developmental disorders, and other neurological conditions. The distribution of individual indications and patient demographics is shown in Table 1.
|
Indication |
Total number |
Gender, male/female |
Age, mean (SD) |
|
Cerebral palsy |
14 |
9/5 |
10.86 (3.65) |
|
Others |
11 |
7/4 |
10.27 (3.67) |
|
Developmental disorders |
2 |
2/0 |
12 (2.18) |
|
Genetic disorders |
3 |
2/1 |
8 (3.82) |
|
Other neurological conditions |
3 |
2/1 |
8.33 (3.62) |
|
Spina bifida |
3 |
1/2 |
13.33 (3.60) |
|
Total |
25 |
16/9 |
10.6 (3.74) |
Table 1: Demographics and distributions of indications in study participations.
Gait parameters
All observed parameters demonstrated improvements when comparing measurements taken during the first and final therapy sessions. Statistically significant differences were detected across all measured gait parameters in both study groups. A summary of these values is presented in Table 2.
|
Parameter |
Indication |
Before |
After |
Difference (%) |
P-value |
|
Walked steps |
CP |
1082.57 ± 317.29 |
1616.21 ± 356.75 |
49.29 |
< 0,001 |
|
Others |
967.09 ± 275.87 |
1408.81 ± 326.93 |
45.68 |
0.002 |
|
|
Total |
1031.76 ± 312.01 |
1524.96 ± 352.87 |
47.8 |
< 0,001 |
|
|
Walked distance (m) |
CP |
383.79 ± 122.60 |
632.12 ± 210.88 |
64.71 |
< 0,001 |
|
Others |
339.76 ± 112.45 |
544.69 ± 197.54 |
60.31 |
0.001 |
|
|
Total |
364.42 ± 121.81 |
593.65 ± 206.80 |
62.91 |
< 0,001 |
|
|
Speed (steps/min) |
CP |
40.01 ± 10.38 |
55.32 ± 11.99 |
38.26 |
< 0,001 |
|
Others |
34.89 ± 9.18 |
48.52 ± 10.89 |
39.06 |
0.003 |
|
|
Total |
37.76 ± 10.19 |
52.33 ± 11.81 |
38.58 |
< 0,001 |
Table 2: Average gait parameter values obtained during the first (before) and last therapy (after) for each group, categorized by indication.
Following the course of treatment, both groups demonstrated a statistically significant increase in the number of steps, with an overall improvement of 47.80% (p < 0.001). Patients with CP showed a 49.29% improvement (p < 0.001), which was slightly greater than that observed in the 'Others' group. A detailed comparison of pre-and post-treatment values is shown in Figure 2. Improvement in a number of steps was noted in nearly all patients, except for one who experienced mild deterioration, as illustrated in Figure 3.
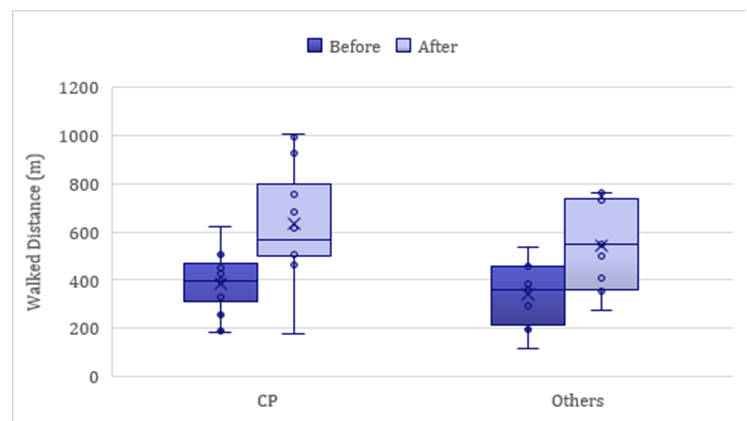
A statistically significant improvement in the distance walked was observed for both patient groups. The most notable improvement was seen in patients with a diagnosis of CP, who demonstrated an almost 65% increase (p < 0.001). Patients in the "Others" group showed a 60.31% improvement (p = 0.001), as illustrated in Figure 4. The overall improvement for both groups combined was 62.91% (p < 0.001). Out of 25 patients, 23 (92%) showed improvement, while one patient from each group experienced slight deterioration in walked distance after the treatment program (see Figure 5).
A statistically significant increase in walking speed was observed across all indications, reflecting a 38.58% improvement (p < 0.001). The recorded difference in the average increase in walking speed between the groups was minimal (see Figure 6). Only one patient in the CP group did not show positive progress following the course of treatment. The remaining 24 patients exhibited an increase in walking speed of varying degrees (Figure 7).
4. Discussion
The objective of this study was to evaluate the efficacy of a novel end-effector device for gait rehabilitation in children with various neurological disorders. The progress of participants was assessed using gait parameters recorded directly by the device during each therapy session. To the best of the author’s knowledge, this is the first study to date that includes children with gait impairments resulting from a range of neurological conditions, as previous research on end-effector devices has predominantly focused on children with CP.
Following 10 therapy sessions, the results demonstrated statistically significant improvements across all evaluated parameters. Specifically, there was an average increase of approximately 48% in the number of steps, 63% in distance walked, and 39% in walking speed. The differences in improvements between the first and final sessions were minimal across the groups, with slightly superior outcomes observed in the CP group.
The positive effects of RAGT can be attributed to several factors. One key aspect is its impact on neuroplasticity in the brain and spinal cord. Due to its design, RAGT allows for higher intensities and longer durations of repetitive gait training while maintaining a physiological walking pattern, which provides an ideal stimulus for creating lasting structural and functional changes in the CNS [32,33]. A study by Perpetuini et al. [34] demonstrated that RAGT induces modifications in the motor and prefrontal cortices, thereby enhancing motor control and attention in children with CP during therapy. This highlights the essential role of neuroplasticity in the recovery process for these patients. In addition, CNS exhibits a high degree of plasticity during the early stages of development, making early rehabilitation in children essential for maximizing its effectiveness [34]. Automatic data collection provided by some RAGT devices, including the technology used in this study, enables detailed analysis and progress tracking, allowing for adjustments to therapeutic interventions to achieve optimal outcomes. Moreover, actively engaging patients, combined with the increased motivation fostered by incorporating games into therapy, can further enhance its overall effectiveness [35,36].
Most prior research on the impact of RAGT for gait rehabilitation in children with neurological disorders has focused primarily on CP. A significant proportion of these studies used exoskeleton devices [26,27,37,38], with fewer employing end-effector systems [28-31]. The evidence suggests that RAGT positively affects gait parameters, overall mobility, and gross motor skills in pediatric CP patients, which aligns with the findings of the current study. However, the studies differ in the extent of achieved results, which may be attributed to various factors, such as differences in protocols or the severity of patients's conditions. Smaina et al. [29] investigated an end-effector device in 18 children with CP, reporting a 9% increase in gait speed and a 23% increase in walking distance after 10 sessions. Hwang et al. [28] observed a 17% improvement in gait speed following 24 sessions. Variability in outcomes for exoskeleton devices is noted, with Meyer-Heim et al. [37] showing a 16.6% increase in gait speed and a 13.1% increase in distance after 20 sessions, while Ma et al. [38] reported improvements of 37% and 48% in speed and distance, respectively, after 40 sessions. There is a very limited number of studies involving pediatric patients with various neurological conditions and all of them utilize exoskeletons. In the study by Beretta et al. [39] comprising patients with CP and acquired brain injury (ABI), a 21.5% improvement after 20 therapy sessions in walking distance was noted, with better outcomes in the ABI group. Similarly, another study by Gazzellini et al. [40] reported a 24% increase in walking distance and a 29% improvement in walking speed among 47 pediatric patients with unspecified neurological conditions.
Despite fewer therapy sessions, the current study achieved superior outcomes compared to the studies mentioned above. This difference may partly be due to the direct assessment of gait parameters through device-generated data, as opposed to the 10MWT and 6MWT tests used in most of the previous research. The robotic device provides patients with enhanced support and stability compared to overground walking. This helps reduce the risk as well as fear of falling which can cause hesitation and slower walking, ultimately leading to increased walking speed and greater distance covered. In addition, the patient's lower limbs in an end-effector device are fixed only at the feet, which provides distinct advantages over exoskeleton systems. This configuration allows for greater freedom of movement in all anatomical planes, facilitating more active patient participation during therapy, which is directly related to improved therapeutic outcomes [41].
The primary limitation of this study is the absence of a control group, which would allow for a more robust evaluation of the effectiveness of RAGT compared to the conventional approach. Furthermore, progress was assessed only based on device-generated values from the first and last therapy sessions, without any follow-up evaluation. To better capture the therapy's benefits, it would be useful to incorporate additional assessment methods that reflect the patient's functional abilities and independence in daily life over a longer-term scale. Another drawback is the broad diversity of diagnoses within the "Others" group, which, due to the small sample size for each condition, prevents drawing meaningful conclusions about these specific disorders. Despite these limitations, this study contributes to the field as it is the first to explore the effectiveness of end-effector RAGT across various neurological disorders in children. While the sample sizes for individual conditions in the "Others" group were limited, the results suggest a trend indicating the device's potential effectiveness for a range of neurological conditions. For future research, it would be valuable to include a larger sample of patients for each condition. Combined with economic evaluations, this could assist potential clients in determining whether RAGT is a worthwhile investment based on the distribution of patients by diagnosis in their specific facilities.
5. Conclusion
Pediatric patients with various neurological disorders demonstrated statistically significant improvements in all assessed parameters following rehabilitation program including a novel end-effector-based RAGT system. Based on the evaluation of gait parameters generated by the device, increases of approximately 48%, 63%, and 39% were observed in the number of steps, distance walked, and walking speed, respectively. These findings indicate a positive impact of the RAGT system on gait, benefiting not only pediatric patients with CP but also those with other neurological conditions, which may contribute to enhancing functionality and independence in daily life.
References
- Smith M, Kurian MA. Neurological gait disorders in childhood. Paediatrics and Child Health 28 (2018): 454-458.
- Begnoche DM, Chiarello LA, Palisano RJ, et al. Predictors of independent walking in young children with cerebral palsy. Physical Therapy 96 (2016): 183-192.
- Graser JV, Letsch C, Van Hedel HJA. Reliability of timed walking tests and temporo-spatial gait parameters in youths with neurological gait disorders. BMC Neurology 16 (2016).
- Parvin A, Hasan MM. An overview of spina bifida. Open Journal of Orthopedics 13 (2023): 443-456.
- Fletcher JM, Brei TJ. Introduction: Spina bifida—A multidisciplinary perspective. Developmental Disabilities Research Reviews 16 (2010): 1-5.
- Mueske NM, Õunpuu S, Ryan DD, et al. Impact of gait analysis on pathology identification and surgical recommendations in children with spina bifida. Gait & Posture 67 (2018): 128-132.
- Carvalho I, Pinto S, Chagas DV, et al. Robotic gait training for individuals with cerebral palsy: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation 98 (2017): 2332-2344.
- Zhou J, Butler EE, Rose J. Neurologic correlates of gait abnormalities in cerebral palsy: Implications for treatment. Frontiers in Human Neuroscience 11 (2017).
- Moll F, Kessel A, Bonetto A, et al. Safety and Feasibility of Robot-assisted Gait Training in Adults with Cerebral Palsy in an Inpatient Setting - an Observational Study. Journal of Developmental and Physical Disabilities 35 (2023): 1091-1106.
- Sadowska M, Sarecka-Hujar B, Kopyta I. Cerebral palsy: Current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatric Disease and Treatment 16 (2020): 1505-1518.
- Duman K. Cerebral Palsy: An Overview. Hamidiye Medical Journal 3 (2022): 1-6.
- Novak I. Evidence-based diagnosis, health care, and rehabilitation for children with cerebral palsy. Journal of Child Neurology 29 (2014): 1141-1156.
- Piitulainen H, Kulmala J, Mäenpää H, et al. The gait is less stable in children with cerebral palsy in normal and dual-task gait compared to typically developed peers. Journal of Biomechanics 117 (2021): 110244.
- Armand S, Decoulon G, Bonnefoy-Mazure A. Gait analysis in children with cerebral palsy. EFORT Open Reviews 1 (2016): 448-460.
- Patel DR, Neelakantan M, Pandher K, et al. Cerebral palsy in children: a clinical overview. Translational Pediatrics 9 (2020): S125-S135.
- Rasmussen HM, Pedersen NW, Overgaard S, et al. The use of instrumented gait analysis for individually tailored interdisciplinary interventions in children with cerebral palsy: a randomised controlled trial protocol. BMC Pediatrics 15 (2015).
- Moreau NG, Bodkin AW, Bjornson K, et al. Effectiveness of Rehabilitation Interventions to Improve Gait Speed in Children with Cerebral Palsy: Systematic Review and Meta-analysis. Physical Therapy 96 (2016): 1938-1954.
- Shobeiri P, Presedo A, Karimi A, et al. Orthopedic management of myelomeningocele with a multidisciplinary approach: a systematic review of the literature. Journal of Orthopaedic Surgery and Research 16 (2021).
- Butt Y, Saghir M, Waseem I, et al. Effects of Treadmill Training on Gross Motor Function, Spasticity and Gait Speed in Ambulatory Children with Spastic Cerebral Palsy. Journal Riphah College of Rehabilitation Sciences 12 (2024): 60-65.
- Gandhi P, Chan K, Verrier MC, et al. Training to Improve Walking after Pediatric Spinal Cord Injury: A Systematic Review of Parameters and Walking Outcomes. Journal of Neurotrauma 34 (2016): 1713-1725.
- Schwartz I, Meiner Z. Robotic-Assisted GAIT training in Neurological patients: Who may benefit? Annals of Biomedical Engineering 43 (2015): 1260-1269.
- Ammann-Reiffer C, Bastiaenen C, Meyer-Heim A, et al. Effectiveness of robot-assisted gait training in children with cerebral palsy: a bicenter, pragmatic, randomized, cross-over trial (PeLoGAIT). BMC Pediatrics 17 (2017).
- Eguren D, Contreras-Vidal JL. Navigating the FDA medical Device regulatory pathways for pediatric lower limb exoskeleton devices. IEEE Systems Journal 15 (2020): 2361-2368.
- Valè N, Gandolfi M, Vignoli L, et al. Electromechanical and Robotic Devices for Gait and Balance Rehabilitation of Children with Neurological Disability: A Systematic Review. Applied Sciences 11 (2021): 12061.
- Hunt M, Everaert L, Brown M, et al. Effectiveness of robotic exoskeletons for improving gait in children with cerebral palsy: A systematic review. Gait & Posture 98 (2022): 343-354.
- Llamas-Ramos R, Sánchez-González JL, Llamas-Ramos I. Robotic Systems for the physiotherapy treatment of children with cerebral palsy: A systematic review. International Journal of Environmental Research and Public Health 19 (2022): 5116.
- Bonanno M, Militi A, La Fauci Belponer F, et al. Rehabilitation of gait and balance in Cerebral Palsy: A scoping review on the use of robotics with biomechanical implications. Journal of Clinical Medicine 12 (2023): 3278.
- Hwang J. Effect of an End-effector Type of Robotic Gait Training on Stand Capability, Locomotor Function, and Gait Speed in Individuals with Spastic Cerebral Palsy. Daehan Mulli Uihag Hoeji/Daehan Mulli Uihak Hoeji 16 (2021): 123-130.
- Smania N, Bonetti P, Gandolfi M, et al. Improved Gait After Repetitive Locomotor Training in Children with Cerebral Palsy. American Journal of Physical Medicine & Rehabilitation 90 (2011): 137-149.
- Tarakç1 D, Emir A, Avcil E, et al. Effect of robot assisted gait training on motor performance in cerebral palsy: a pilot study. Journal of Exercise Therapy and Rehabilitation 6 (2020): 156-162.
- Yaz1c1 M, Livanelioglu A, Gücüyener K, et al. Effects of robotic rehabilitation on walking and balance in pediatric patients with hemiparetic cerebral palsy. Gait & Posture 70 (2019): 397-402.
- Lim J, Kang E, Park S, et al. Effects of robot rehabilitation on the motor function and gait in children with cerebral palsy: a systematic review and meta-analysis. Journal of Exercise Rehabilitation 20 (2024): 92-99.
- Sucuoglu H. Effects of robot-assisted gait training alongside conventional therapy on the development of walking in children with cerebral palsy. Journal of Pediatric Rehabilitation Medicine 13 (2020b): 127-135.
- Perpetuini D, Russo EF, Cardone D, et al. Identification of Functional Cortical Plasticity in Children with Cerebral Palsy Associated to Robotic-Assisted Gait Training: An fNIRS Study. Journal of Clinical Medicine 11 (2022): 6790.
- Gonzalez A, Garcia L, Kilby J. et al. Robotic devices for paediatric rehabilitation: a review of design features. BioMed Eng OnLine 20 (2021): 89.
- Cortés-Pérez I, González-González N, Peinado-Rubia AB, et al. Efficacy of Robot-Assisted Gait Therapy Compared to Conventional Therapy or Treadmill Training in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Sensors 22 (2022): 9910.
- Meyer-Heim A, Ammann-Reiffer C, Schmartz A, et al. Improvement of walking abilities after robotic-assisted locomotion training in children with cerebral palsy. Archives of Disease in Childhood 94 (2009): 615-620.
- MA T, Zhang H. Effect of Robotic-assisted Gait Training on Motor and Walking for Children with Spastic Cerebral Palsy. Chinese Journal of Rehabilitation Theory and Practice (2021): 1260-1265.
- Beretta E, Storm FA, Strazzer S, et al. Effect of Robot-Assisted GAIT training in a large population of children with motor impairment due to cerebral palsy or acquired brain injury. Archives of Physical Medicine and Rehabilitation 101 (2020): 106-112.
- Gazzellini S, Andellini M, Falco FD, et al. Clinical study and Health Technology Assessment (HTA) of a Robot-Assisted Gait Training on children with neurological disorders: A quasi-experimental study. Edizioni FS (2023).
- Diego P, Herrero S, Macho E, et al. Devices for GAIT and Balance Rehabilitation: General Classification and A Narrative Review of End Effector-Based Manipulators. Applied Sciences 14 (2024): 4147.








 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 