Long Lasting Chronic Resistive Training Effects on Circulating S-Klotho and IGF-1
Moran Saghiv1*, Chris Sherve1, Ehud Goldhammer2, David Ben-Sira3, Michael Sagiv3
1Exercise Physiology Department, Casey center, Room 141B, University of Mary, 7500 University Drive, Bismarck, ND 58504, USA
2Heart Institute Bnai-Zion Haifa Medical Center, Technion, Haifa, Israel
3Life Sciences Department, Wingate College, Netanya, Israel
*Corresponding Author: Moran Saghiv, Exercise Physiology Department, Casey center, Room 141B, University of Mary, 7500 University Drive, Bismarck, ND 58504, USA, Tel: 702-908-2390;
Received: 05 March 2017; Accepted: 13 March 2017; Published: 17 March 2017
Article Information
View / Download Pdf Share at FacebookAbstract
Purpose: The purpose of the present study was to examine the effects of long lasting chronic resistive training on circulating s-klotho s and IGF-1 levels in young adult.
Methods: 50 national level powerlifters and 50 age matched untrained young adults (27.1±1.0 and 26.5±1.0 years respectively). Following overnight fasting forearm vein blood samples were taken, circulating s- Klotho were examined by means of a-klotho Enzyme Linked Immunosorbent Assay ELISA kit. A chemiluminescent immunometric method was applied in order to define serum IGF-1 levels.
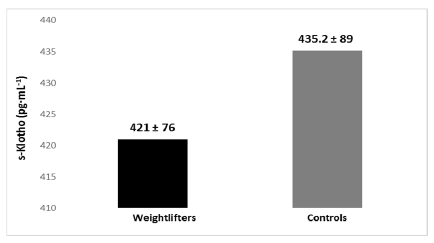
Results: No significant differences were seen between the weightlifters and untrained young adults for s-Klotho (421.0±76.0 and 435.2 ±89.0 pg·mL-1 respectively). However, IGF-1 levels were significantly (p<0.05) higher in the weightlifters compared to the untrained subjects (110.6±16.4 and 77.6±23.2 mmol·L-1 respectively).
Conclusions: It seems that long lasting resistive training did not influence circulating levels of s-Klotho, while the increased circulating IGF-I may, at least in part, mediate increases in strength, power and muscle hypertrophy.
Keywords
Untrained young adults; Anti-Aging effects; Heavy resistance training; Weightlifting
Article Details
1. Introduction
Gene expression pattern and physiological responses can be altered at rest and during exercise following resistive or aerobic exercise training namely; epigenetic [1]. Following exercise training epigenetic changes can help to improve work capacity and physical performance of humans in health and disease [2].
Multiple growth factor signaling pathways are regulated by the s-Klotho protein, namely, insulin/IGF-1 [3] in mice, lack of brings about a reduction in life span accompanied with many disorders such in chronic kidney disease and aging [4], though, the opposite means increase longevity [5]. Similar anti-aging effects have also been ascribed to exercise and physical activity [6].
Resistance training targets specific muscle groups to increase strength. While there might be some crossover, with certain forms of aerobic exercise increasing strength and some kinds of resistance training, improving endurance, those benefits would be minimal compared to a targeted program. Several studies on rats and humans, suggest that resistance training has beneficial effects and can potentially be an effective treatment for various clinical conditions. [7,8]. Previously it has been found that long lasting aerobic exercise training increased circulating s-Klotho level [9]. However, to the best of our knowledge, the effect of long lasting resistive training on circulating s-Klotho and IGF-1 has not been investigated. Consequently, the present study tested the effects of long lasting chronic resistive training on circulating s-klotho and IGF-1 levels in young weightlifters.
2. Methods
Subjects: One hundred healthy young male adults were subjects in this study. Fifty were recruited as active young adults following chronic powerlifting resistance training at the national level for three or more years and, 50 age matched untrained young adult subjects (27.1±1.0 and 26.5±1.0 years respectively). All subjects were healthy by documented medical history and following a maximal exercise stress test to maximal oxygen uptake. The Burdick Eclipse 400 3-channel, 12-lead ECG recorder system, was utilized continuously to measure heart rate and to detect any electrocardiogram abnormalities. At rest and at peak exercise were acquired by five-second recordings. At rest and at peak exercise, the standard sphygmomanometer cuff and mercury manometer mounted at eye level, was used to measure systolic and diastolic blood pressures [10]. A written informed consent was completed and obtained from each subject, for taking blood samples and their medical records. The research was done following the approved by the Clinical Science Center Committee on Human Subjects by the guides of the Helsinki declaration.Adipose fat assessment was performed as suggested by Behnke and Wilmore [11] which included measurement of total body weight (± 0.05 kg), skin fold thicknesses at 8 sites (± 1 mm) using the Lange Caliper and anthropometric procedures. Blood sampling and procedures: The sterile antecubital venipuncture techniques into ethylenediam-inotetraacetate (EPA) tubes were employed to draw 2.5 mL of peripheral venous blood samples. Time of day for blood sampling was kept consistent to control for problems associated with diurnal variation. Blood collection was obtained once from each subject.
3. Analysis
Following overnight fasting forearm vein blood samples were taken, circulating s- Klotho was examined by means of a-klotho Enzyme Linked Immunosorbent Assay ELISA kit. A chemiluminescent immunometric method was applied in order to define serum IGF-1 levels. S-Klotho configuration and separation were followed as described previously [12-15]. Serum IGF-1 levels were defined as described elsewhere [16].
4. Statistical methods
Data is reported as mean ± SD values. One way ANOVA was performed, post hoc analysis was performed by using the Tukey 2 multiple comparison tests. The level of significance was set at alpha<0.05.
5. Results
Subjects' mean descriptive data and physiological variables at rest are presented in Table 1.| Untrained | Trained | |
|---|---|---|
| Number of subjects | 50 | 50 |
| Age (years) | 26.5±1.0 | 27.1±1.0 |
| Weight (Kg) | 71.6±3.1 | 70.9±2.4 |
| Height (cm) | 176.9±2.0 | 168.0±2.1 |
| Fat (%) | 15.1±3.1 | 9.4±0.7a |
| Heart rate (b?min?¹) | 69.6±10.0 | 65.9±7.8 |
| Systolic BP (mmHg) | 117.4±12.1 | 121.3±8.4 |
| Diastolic BP (mmHg) | 73.5±7.7 | 78.9±9.6 |
a = Significant (p<0.05) differences between groups
Table 1: Weightlifters had significant (p<0.05) lower values for fat percentage compared to the normal subjects. No significant differences were noted between the groups in all other variables.
6. Discussion
This study suggests that circulating s-Klotho levels were not affected following chronic powerlifting resistance training. However, circulating IGF-1 levels were significantly higher in the weightlifters following chronic powerlifting resistance training compared to the untrained subjects.S-Klotho a transmembrane is unconfined in the serum. S-Klotho is a result of another uniting of the Klotho gene, leading to a complex molecules seen in kidney patients [17]. Previously it has been suggested that the level of the IGF-1 receptor activity may be constrain by s-Klotho [18].
S-Klotho may target multiple secluded tissues and organs [17], containing a wide range of biological activities, including antiaging properties [6]. Previously it has been demonstrated that recombinant Klotho proteins of full length with the extracellular domain inhibit IGF-1 signal transduction, thus increasing longevity in mice [19]. Moreover, the blockade of IGF-1 signaling induced by the released form of Klotho has been shown to increase the resistance of oxidative stress, thereby improving survival [19].
Unlike chronic powerlifting resistance training, previous studies suggested that circulating s-Klotho levels have increased in response to chronic aerobic exercise training, and the response depends on fitness level [9]. Physiological responses differ depending upon whether they result from a pressure overload (weightlifting) and increased peripheral vascular resistance or from a total peripheral resistance such as during aerobic exercise [20].
Long lasting resistive exercise training performed against a sharp increased blood pressure (pressure response) provokes physiological change adaptations that increase strength, power, muscle endurance and times muscle hypertrophy. Aerobic exercise training has different physiological effects such decrease in heart rate and stroke volume, reducing blood pressure and maximal oxygen uptake. Additional benefits of aerobic exercise include an increase in fat utilization during exercise, facilitate the endocrine system and the immune system. It seems that resistance training that involves strength and power does not elicit an increase in s-klotho. It seems that mechanical loading in humans does not influence circulating levels of s-Klotho following chronic powerlifting resistance training, since the effectiveness of mechanical stimuli on muscle tissue depends on the type of contractions [21]. Earlier it has been shown that close relationships exist between s-Klotho and IGF-1 with s-Klotho inhibiting IGF-1 and insulin receptor, IGF-1R signaling by influencing both receptors by compelling tyrosine phosphorylation action [22].
While in the well trained subjects following resistance training s-Klotho levels remained unchanged, IGF-1 levels were increased. IGF-1 is generally thought to be associated with positive attributes such as growth, health, youth and wellbeing, yet the bulk of the scientific evidence suggests that signaling through IGF-1 and insulin receptors is related to a shortened lifespan in adults [16]. Reduced levels of IGF-1 in the working muscles may result in muscle dystrophy due to a dramatic decrease ability of the muscle to synthesize protein. IGF-1 has anabolic effects on muscle protein content by inhibiting protein degradation and promoting myogenesis. Consequently, the functional ability of IGF-1 in working muscles is limited [23]. In response to resistive training high plasticity of satellite cells is noted demonstrating that changes in the size of skeletal muscle fibers can be achieved without the addition of new myonuclei.
This study showed that chronic powerlifting resistant exercise training can cause a significant increase in IGF-1 concentrations. Bamman et al. [24] reported significant elevations in muscle IGF-1 mRNA following resistance exercise, particularly during eccentric resistance exercise. Previous research correlates with this study suggesting that resistance training, trained men, have shown to have higher resting IGF-1 concentrations than untrained men. [25]. The mechanism(s) responsible for resistive training to induce skeletal muscle hypertrophy is still blurred. However, some suggest that skeletal muscle’s IGF-1 has a significant role. Following an intensive resistive training, muscle damage ensues alters muscle’s IGF-1 activity, by increasing muscle protein turnover [25). This increase in protein regeneration is closely dependent on other muscle growth factors [26]. IGF-1 is known to stimulate myoblast proliferation and differentiation in vitro [27] as well as muscle protein synthesis [25] and, as such, researchers looked more into the factors affecting muscle hypertrophy. Besides its expression the effectiveness of muscle IGF-1 action dependents on the regulation of six IGF binding proteins and by the amount and availability of its receptor.
7. Conclusions
It seems that long lasting resistive training did not influence circulating levels of s-Klotho, while the increased circulating IGF-I may, at least in part, mediate increases in strength, power and muscle hypertrophy.
References
- Ling C, Ronn T. Epigenetic adaptation to regular exercise in humans. Drug Discov Today 19 (2014): 1015-1018.
- Voisin S, Eynon N, Yan X, Bishop DJ. Exercise Training and DNA Methylation in Humans. Acta Physiol 213 (2015): 39-59.
- Brobey RK, Dheghani M, Foster PP, Kuro-O M, Rosenblatt KP. Klotho Regulates 14-3-3? Monomerization and Binding to the ASK1 Signaling Complex in Response to Oxidative Stress. PLoS One 10 (2015): e0141968.
- Torres PU, Prie D, Molina-Blétry V, Beck L, Silve C, et al. Klotho: an Antiaging Protein Involved in Mineral and Vitamin D Metabolism. Kidney Int 71 (2007): 730-737.
- Kurosu H, Yamamoto M, Clark JD, et al. Suppression of Aging in Mice by the Hormone Klotho. Science 309 (2005): 1829-1833.
- Castillo-Garzon MJ. Anti-Aging Therapy through Fitness Enhancement. Clin Interv Aging 1 (2006): 213-220.
- Nunes RB, Alves JP, Kessler LP, Dal Lago P. Aerobic Exercise Improves the Inflammatory Profile Correlated with Cardiac Remodeling and Function in Chronic Heart Failure Rats. Clinics (Sao Paulo). 68 (2013): 876-882.
- Kraemer WJ, Ratamess NA, Nindl BC. Highlighted Topics: Recovery from Exercise: Recovery Responses of Testosterone, Growth Hormone, and IGF-1 after Resistance Exercise. J Appl Physiol 17 (2016): jap-00599.
- Saghiv M, Goldhammer E, Sagiv M, Ben-Sira D. Effects of Aerobic Exercise Training on S-Klotho in Young and Elderly. J J Physiology 1 (2015): 1-6.
- ACSM's Guidelines for Exercise Testing and Prescription. 9th edition. Wolters Kluwer/Lippincott Williams & Wilkins; 2014. pp. 145-147 and 165-199.
- Behenke AR, Wilmore J (1974). Evaluation and Regulation of Body Build and Composition. Englewood Cliffs, N.J Prentile Hall, Inc.
- Heijboer AC, Blankenstein MA, Hoenderop J, de Borst MH, et al. NIGRAM consortium. Laboratory Aspects of Circulating Alpha-Klotho. Nephrol Dial Transplan 28 (2013): 2283-2287.
- Pedersen L, Pedersen SMl, Brasen CL, Rasmussen LM. Soluble Serum Klotho Levels in Healthy Subjects. Comparison of two Different Immunoassays. Clin Biochem 46 (2013): 1079-1083.
- Ranke MB, Schweizer R, Elmlinger MW, et al. Significance of Basal IGF-I, IGFBP-3 and IGFBP-2 Measurements in the Diagnostics of Short Stature in Children. Horm Res 54 (2000): 60-68.
- Yamazaki Y, Imura A, Urakawa I, et al. Establishment of Sandwich ELISA for Soluble Alpha-Klotho Measurement: Age-Dependent Change of Soluble Alpha-Klotho Levels in Healthy Subjects. Biochem Biophys Res Commun 398 (2010): 513-518.
- Jazwinski SM. Longevity, Genes, and Aging. Science 273 (1996): 54-59.
- Otani-Takei N, Masuda T, Akimoto T, Honma S, Watanabe Y, et al. Association between Serum Soluble Klotho Levels and Mortality in Chronic Hemodialysis Patients. Int J Endocrinol 2015 (2015): 406269.
- Schmid C, Neidert MC, Tschopp O, Sze L, Bernays RL. Growth Hormone and Klotho. J Endocrinol 219 (2013): R37-R57.
- Yamamoto M, Clark JD, Pastor JV, et al. Regulation of Oxidative Stress by the Anti-Aging Hormone Klotho. J Biol Chem 280 (2005): 38029-38034.
- Green DJ, Hopman MT, Padilla J, Laughlin MH, Thijssen DH. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol Rev 97 (2017): 495-528.
- Adams GR, Cheng DC, Haddad F, Baldwin KM. Skeletal Muscle Hypertrophy in Response to Isometric, Lengthening, and Shortening Training Bouts of Equivalent Duration. J Appl Physiol 96 (2004): 1613-1618.
- Chateau MT, Araiz C, Descamps S, Galas S. Klotho Interferes with a Novel FGF-Signaling Pathway and Insulin/Igf-like Signaling to Improve Longevity and Stress Resistance in Caenorhabditis Elegans. Aging. 2 (2012): 567-581.
- Hameed M, Harridge SD, Goldspink G. Sarcopenia and Hypertrophy: A Role for Insulin-Like Growth Factor-1 in Aged Muscle. Exerc Sport Sci Rev 30 (2002): 15-19.
- Bamman MM, Shipp JR, Jiang J, et al. Mechanical Load Increases Muscle IGF-I and Androgen Receptor mRNA Concentrations in Humans. Am J Physiol Endocrinol Metab 280 (2001): E383-E390.
- Rubin MR, Kraemer WJ, Maresh CM et al. High-Affinity Growth Hormone Binding Protein and Acute Heavy Resistance Exercise. Med Sci Sports Exerc 37 (2005): 395-403.
- Adams G. Role of Insulin-Like Growth Factor-I in the Regulation of Skeletal Muscle Adaptation to Increased Loading. Exerc Sports Sci Rev 26 (1998): 31-60.
- Florini JR, Ewton DZ, Coolican SA. Growth Hormone and the Insulin-Like Growth Factor System in Myogenesis. Endocrine Rev 17 (1996): 481-517.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 