Membrane Filtration and Desiccation for Optimization of Urine Concentration
Gurmukh Singh*, Roni J. Bollag
Department of Pathology, Medical College of Georgia at Augusta University, BI 2008A. 1120 15th Street, Augusta, GA 30912, USA
*Corresponding author: Gurmukh Singh, Department of Pathology, Medical College of Georgia at Augusta University, BI 2008A. 1120 15th Street, Augusta, GA 30912, USA.
Received: 29 September 2025; Accepted: 06 October 2025; Published: 21 October 2025
Article Information
Citation: Gurmukh Singh, Roni J Bollag. Membrane Filtration and Desiccation for Optimization of Urine Concentration. Archives of Clinical and Biomedical Research. 9 (2025): 412-418.
View / Download Pdf Share at FacebookAbstract
Background: For urine immunofixation electrophoresis a 100-fold urine concentration is recommended. This is not always feasible. Based on prior findings, we considered whether lack of optimal concentration of urine may have resulted in missing pathogenic monoclonal light chains in some urine specimens.
Methods: Membrane filtration urine concentration provides a 25-fold concentration. Residual urine was further concentrated by desiccation to achieve higher-fold concentration for 170 specimens that were negative for monoclonal light chains following membrane filtration concentration. Immunofixation electrophoresis was repeated after further concentration by desiccation. Data from previous observations were reevaluated to assess the importance of native urine protein concentration.
Results: Of the 170 specimens subjected to further concentration by desiccation, monoclonal kappa light chains were detected in one specimen that was negative by the routine method. The most common probable explanation for discordant results in multiple specimens from a given patient, in the earlier report, was low urine protein concentration.
Conclusions: While it would be ideal to achieve 100-fold concentration of urine for immunofixation electrophoresis, a 25-fold concentration has a minimal failure rate. We recommend that if the native urine protein concentration is <20 mg/dL, further concentration by desiccation should be applied to membrane filtration-concentrated urine specimens to avoid potential false negative result.
Keywords
<p>Membrane filtration; Urogenital disorders; Urine; Examination; Renal function; Neoplastic disorders; Monoclonal immunoglobulins; International Myeloma Working Group (IMWG)</p>
Article Details
1. Introduction
Urine examination is a common laboratory test used mostly to assess renal function and urogenital disorders. Systemic disorders, with or without direct renal involvement can result in abnormal findings in urine: e.g., diabetes, jaundice, osteoporosis, metabolic disorders of amino acid metabolism, metabolites of medications and illegal drugs, dehydration, and disorders of immunoglobulins etc. [1]. Neoplastic disorders of lympho-plasmacytic cells usually result in the presence of monoclonal light chains in the urine [2]. Autoimmune disorders, lymphomas, amyloidosis, and immunoglobulin chain deposition disorders also result in detection of abnormal immunoglobulins or immunoglobulin components in urine [3]. Urine examination for the presence of monoclonal immunoglobulins in general and monoclonal light chains in particular is recommended by the International Myeloma Working Group (IMWG) in the diagnostic workup of multiple myeloma and related pre-malignant disorders [4].
Neoplastic monoclonal gammopathic disorders consist of three lesions, in increasing order of severity: monoclonal gammopathy of undetermined significance (MGUS), smoldering/asymptomatic myeloma (SMM) and multiple myeloma (MM) [5]. Multiple myeloma is the second most common hematological malignancy in adults and while treatable, is generally incurable [6,7]. MGUS and SMM are premalignant conditions yet both may be associated with detectable monoclonal immunoglobulins in serum and/or urine. Serum protein electrophoresis (SPEP), serum immunofixation electrophoresis (SIFE) urine protein electrophoresis (UPEP) and urine immunofixation electrophoresis (UIFE) are commonly used to investigate monoclonal disorders of immunoglobulins [8,9]. UIFE is the optimal test for detection of monoclonal light chains in urine. Use of serum free light chain concentration (SFLC) and ratio of kappa and lambda light chain concentrations, κ/λ ratio, have been promoted as proxies for UIFE [10,11]. However, an abnormal κ/λ ratio is not diagnostic of monoclonal gammopathy and a normal ratio does not exclude monoclonal gammopathy [9].
There are differences of opinion in conducting urine examination for detection of monoclonal light chains. IMWG recommends 24-hour urine, whereas random urine has been found to be equally useful and avoids the logistical complications of 24-hour urine collection [12]. Urine needs to be concentrated to optimize the results of UIFE interpretation for monoclonal free light chains. A recommended 100-fold concentration is desirable but is not always feasible [13,14]. The usual method of membrane filtration for urine concentration becomes ineffective at high urine protein concentration. Alternative concentration methods, e.g., different types of membrane filtration devices, salt and alcohol precipitation, and desiccation, have similar issues with effectiveness [15].
In an earlier study, exploring the comparison of UIFE and SFLC for detection of monoclonal light chains in urine, it was discovered that discordance in UIFE results for a given patient may be driven by variations in native urine protein concentrations [16]. We explored this issue of possible false negative results by: (a) Further concentration, by desiccation, of urine concentrated by membrane filtration followed by repeat UIFE and comparison with original UIFE results. (b) Review of UIFE data over 14.5 years to examine causes of discordant results at repeat UIFE examinations [16]. For the latter, data from a previously reported study were re-reviewed, especially to assess the comparison of results of UIFE in patients who had multiple examinations. The likely reasons for the few disparities in the repeat results were ascertained. The text and data related to the previously published manuscript are segregated in the various sections and labeled appropriately.
2. Methods
This study was conducted at a 500+ bed tertiary care medical center affiliated with a medical school in Southeastern USA. The protocol was approved by the Institutional Review Board.
The study was carried out in two phases:
(a) Clinical specimens tested by UIFE that had negative results for monoclonal light chains: Urine specimens submitted for routine clinical care examination were concentrated by membrane filtration, as described previously and below. The residual, concentrated and unconcentrated, specimens were stored at -40°C. 170 Specimens that tested negative for monoclonal light chain were further concentration by desiccation of urine specimens, concentrated by membrane filtration, followed by urine immunofixation electrophoresis using antisera specific for free light chains. (Sebia Laboratories Inc. Peachtree Corners, GA.). The urine specimens were previously tested following membrane filtration concentration using VIVA membrane filtration concentrators. (VIVA Products, Littleton, MASS.) Using antisera specific for free light chains improved the sensitivity of UIFE by 18% and this has been adopted as the standard protocol in our laboratory [17].
Stored urine specimens concentrated by membrane filtration and unconcentrated residual urine were subjected to further concentration by desiccation by incubation at 37°C. Residual membrane concentrated and unconcentrated urine specimens were loaded into 96 well plates and incubated at 37°C overnight, to dryness. The dry residue was dissolved in 50 µL of deionized water and subjected to UIFE with Sebia antibodies to free light chains. Due to variations in volume of concentrated and unconcentrated urine, 100-fold concentration could not be achieved in many specimens. The final concentration and the number of specimens involved are listed in Table 1. UIFE was conducted according to a previously described protocol using conventional Helena antisera to IgG, IgA and IgM and Sebia antisera specific to free kappa and lambda light chains [17].
(b) Retrospective re-review of data collected from urine analysis. The previously published study was done to compare the results of UIFE with serum free light chain κ/λ ratio in order to assess the rate of false positive results from using κ/λ ratio [16]. In that study, that spanned 14.5 years, many patients had UIFE done more than once. The results of multiple tests were not reported or discussed in the earlier publication but were analyzed for this report to assess the concordance of results from multiple UIFE tests. The results from multiple tests were compared to assess the suitability of membrane filtration as the concentration method. The instances of discordant results among repeat UIFEs were addressed by examining the results of SPEP, SIFE, serum free light chains and urine protein concentration among other clinical parameters, at the different times of urine collection.
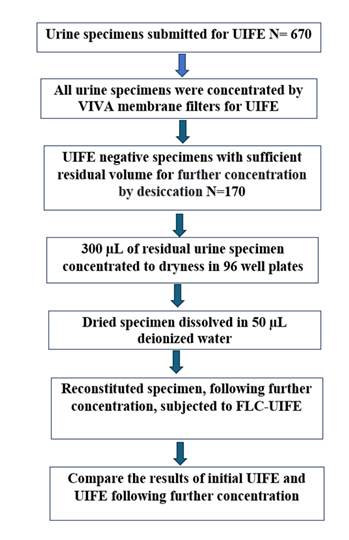
(c) UIFE with further concentration by desiccation: One hundred seventy, of the 670, urine specimens from January 1, 2024, to November 30, 2024, that were negative on routine UIFE, were stored frozen at -40°C following initial UIFE testing as part of routine patient care (See Flow Chart (Figure 1)). Both concentrated and native urine specimens were saved during this period. The initial urine concentration with VIVA membrane filter concentrators employed charging the concentrator with 5.0 ml of native random urine and concentrating the sample to 200µL for a 25-fold concentration. Following UIFE analysis for routine patient care, the residual concentrated specimen and any unconcentrated sample were stored. The final volume of concentrated urine was larger in specimens with high urine protein concentration and the concentration level was correspondingly lower. If the total urine protein was >1,000 mg/dL the specimen was not concentrated. Sometimes there was insufficient urine volume for concentration. For this study, the residual urine samples were concentrated by desiccation to achieve additional concentration with a goal of 100-fold concentration; however, specimen volume limitations did not always yield the desired concentration. Residual concentrated urine and unconcentrated urine samples were inoculated into 96 well plates, at a total volume of 300 µL and incubated at 37°C overnight to achieve desiccation. The resulting dry specimens were dissolved in 50 µL of deionized water and subjected to UIFE using Sebia antisera specific to free light chains [17]. The electrophoretic gels were subjected to one manual wash using Clear Wash buffer from Helena Laboratories. (Helena Laboratories, Beaumont TX) [18]. The initial UIFE results were compared with those from samples subjected to further concentration by desiccation.
Only the urine specimens giving a negative result on UIFE from initial examination were subjected to further concentration by desiccation and repeat UIFE to ascertain that the usual concentration protocol did not yield false negative results due to insufficient concentration of urine. Specimens testing positive on initial UIFE were not examined further as we did not have any reason to suspect false positive results. Finding of monoclonal light chains in urine were always supported by other findings, e.g. elevated serum levels of involved light chain, presence of light chain band on SIFE or the presence of monoclonal immunoglobulin on SIFE. The anti-kappa and anti-lambda antisera to free light chains served as controls for each other. In over 11 years of tracking, and we did not observe any false positive results.
(d) Retrospective re-review of UIFE results: UIFE results at this institution over 14.5 years were reviewed for correlation of serum free light chain values and UIFE results. Some of these data were reported in an earlier publication [16]. Description of various specimen types is repeated to provide continuity in reading this manuscript. The 4998 specimens, on which UIFE was done. were segregated into two main groups of UIFE 0 and UIFE 1 consisting of 3230 and 1723 specimens, respectively. The remaining 45 specimens were not analyzed further, due to biclonal or unclear results, as described earlier [16]. Specimens designated UIFE 0 were from patients without evidence of, or history of monoclonal gammopathy, except occasional incidental finding of monoclonal light chains in urine. Specimens designated UIFE 1 were from patients with an extant, or history of, monoclonal gammopathy.
UIFE 0 and UIFE 1 specimens were queried for instances in which two of more specimens had been analyzed by UIFE from a given patient [16]. This facet of data analysis was not reported in the earlier publication. The results of duplicate or more analyses per patient were compared and data reviewed for potential exploration of discordant results among the multiple specimens. In evaluating the urine protein concentration, specimens with urine protein of <20 mg/dL were labeled as low total urine protein specimens. This value was chosen based on the findings of the earlier study.16 Briefly, the prevalence of UIFE positivity showed a biphasic pattern with the highest levels in the urine with total protein concentration of 21-25 mg/dL. UIFE positivity rate for specimens with urine protein <20.0 mg/dL was 1.2% and for the group with higher total urine protein the positivity rate was 3.2% [16].
3. Results
3.1 Secondary/further concentration of urine by desiccation:
Further concentration of 170 urine specimens by desiccation resulted in final concentration of urines from 6 to 150-fold. The average and median concentrations were 73.6 and 59-fold, respectively. The number of specimens and range of total concentrations are given in Table 1. The 150-fold concentration is likely an overestimate when 300 µL of membrane filtration concentrated urine was desiccated. The availability of 300 µL membrane filtration concentrated urine implied that the urine did not reach the intended 25-fold concentration by membrane filtration due to high protein content of urine. A 100-fold concentration could not be achieved in some specimens due to lack of sufficient volume of membrane filtration-concentrated urine. Out of 170 specimens only one specimen gave a positive result for monoclonal kappa light chains on UIFE following desiccation when the initial result was negative. As stated earlier, all of the specimens were negative for monoclonal light chains in initial testing. Further examination of the records revealed that the patient’s urine had tested positive for monoclonal kappa light chains in December while the urine specimen in January was negative on initial testing but gave a positive result on further concentration by desiccation. The main difference between the positive December specimen and the negative January specimen was the concentration of total urine protein in native specimen. The December and January specimens had urine protein concentration of 305 and 12 mg/dL respectively. The post-desiccation concentration of the specimen was 63-fold. The likely explanation for the negative result in the January specimen was that a dilute native urine specimen had not been adequately concentrated by the usual protocol. Thus, we recommend that urine specimens with native protein concentration of <20mg/dL and giving a negative result on UIFE be subjected to further concentration by desiccation and repeat UIFE to avoid false negative results. It is noted that this recommendation is based on a limited data of one result out of 170 comparisons.
|
Fold conc |
Number |
|
6 to 20 |
11 |
|
21 to 40 |
17 |
|
41 to 60 |
39 |
|
61 to 80 |
27 |
|
81 to 100 |
16 |
|
101 to 120 |
35 |
|
≥ 121 |
25 |
Table 1: Further urine concentration by desiccation: Urine specimens concentrated by membrane filtration and unconcentrated urine were subjected to desiccation by incubation at 37°C, the resulting pellet was dissolved in 50 µL of deionized water and subjected to UIFE with Sebia antibodies specific to free light chains. Due to variations in the available volumes of concentrated and unconcentrated urine 100-fold concentration could not be achieved in many specimens. The final concentration and the number of specimens involved are listed in Table 1.
3.2 UIFE 0 specimens from the previously reported study:
In 162 patients in this category there were two or more urine specimen results by UIFE, at different times [16]. The results were concordant in 159 patients, Table 2. The results were not concordant in three patients. One of the discordant results reflected two specimens positive for lambda light chains and one negative specimen for one patient. As detailed in the earlier report, the negative specimen had higher κ/λ ratio, had low urine protein of ≤4 mg/dL and polyclonal hypergammaglobulinemia, as compared to the positive specimens.16 The other two discordant results were in patients with an oligoclonal pattern in serum and the discordance is likely due to variations in the levels of free monoclonal light chains in serum.
|
UIFE 0 |
N=162 |
|
2 neg |
141 |
|
3 neg |
8 |
|
2 pos |
8 |
|
3 pos |
2 |
|
Discordant |
3 |
|
Discordant |
|
|
1/2 pos@ |
Hodgkin’s & oligoclonal |
|
2/3 pos# |
Oligoclonal |
|
2/3 pos$ |
Lambda mono |
|
@: Patient had Hodgkin’s disease with oligoclonal pattern on SIFE. One of the two urine specimens was positive for Kappa monoclonal light chains. #: Oligoclonal pattern was seen in all SIFE tests. Two of the three urine specimens displayed monoclonal kappa light chains. $: This set of specimens was noted and discussed in an earlier publication.16. Two of the three urine specimens displayed monoclonal lambda light chains. The negative specimen had total urine protein of ≤4.0 mg/dL [16]. |
|
Table 2: Discordant results in specimens meeting UIFE 0 criteria: In the only bona fide instance of discordant results in one patient with monoclonal lambda light chains in two of three urine specimens the disparity could be explained by the low urine protein concentration in the negative specimen. Both specimens were negative in 141 patients, all three were negative in 8 patients, both and all three specimens were positive in 8 and 2 patients, respectively. Two of the three discordant results were in patients who did not have a documented monoclonal gammopathic disorder.
3.3 UIFE 1 specimens from the previously reported study:
In this category there were 332 patients with results for two or more UIFE analyses. The results were concordant in 234 patients with up to eight specimens from a given patient, Table 3 [16]. The results in all specimens were negative for 100 patients and positive in all specimens for monoclonal light chains in 134 patients. The probable explanations for the discordant results in 98 patients are listed in Table 3. The most frequent probable cause for negative results in discordant groups was low urine protein concentration, implying dilute urine, resulting in ostensibly false negative results. Other probable and contributory causes were changes in the concentration of serum free light chains and negative SIFE results due to oncology therapy in the negative specimens. The ten negative SPEP/SIFE listed in the lower part of Table 3 were for specimens that had low total urine protein as well [16].
|
UIFE 1 |
N=332 |
|
|
Concordant |
N= 234 |
|
|
N of Specimens/replicates |
Neg (100) |
Pos (134) |
|
2 |
70 |
90 |
|
3 |
17 |
27 |
|
4 |
5 |
9 |
|
5 |
3 |
4 |
|
6 |
3 |
2 |
|
7 |
1 |
2 |
|
8 |
1 |
0 |
|
Discordant |
N =98 |
|
|
Potential Primary explanation |
Number |
|
|
Low total urine protein |
77 |
|
|
Negative SPEP/SIFE |
7 |
|
|
Low serum free light chain level |
5 |
|
|
IgM monoclonal Ig |
2 |
|
|
Amyloidosis |
1 |
|
|
No explanation |
6 |
|
|
Secondary/additional explanation |
Number |
|
|
Low serum free light chain level |
18 |
|
|
Negative SPEP/SIFE |
10 |
|
Table 3: Discordant results in specimens meeting UIFE 1 criteria. In 234 of the 332 patients the results of multiple urine specimens were concordant for replicate of up to 8 samples per patient. The commonest probable explanation, in more than 70% of instances, for discordant results was the low urine protein concentration, implying dilute urine in specimens with negative UIFE.
4. Discussion
Multiple myeloma accounts for about 2% of cancer deaths and it has been posited that all MM cases are preceded by MGUS and SMM [19]. However, screening for MGUS is not warranted as there is no difference in the outcomes between MGUS discovered by active screening vs. incidental finding [20]. In fact, a case can be made to avoid screening for MGUS as this diagnosis generates as much mental anguish as a diagnosis of MM, without providing any benefit from “early” diagnosis [21]. A major reason for an erroneous diagnosis of MGUS is the use of κ/λ ratio in making a diagnosis without documentation of monoclonality by an orthogonal assay. Initially, results outside the reference range of 0.26 to 1.65, for κ/λ ratio. were considered evidence of light chain MGUS. This criterion was recently revised to designate specimens with κ/λ ratio ≥3.0 as having kappa MGUS. Even the revised criterion κ/λ ratio ≥3.0 as having kappa MGUS generates more than 80% false positive results [16,22].
Detection of monoclonal light chains in urine is important in that “Bence Jones Protein” the quintessential original tumor marker is a valid risk factor for renal disease. About 15% of the MM lesions secrete light chains only (LCMM) and the only diagnostic laboratory marker may be monoclonal light chains in urine [9,23]. In addition, about 18% of MM lesions secreting intact immunoglobulins secrete an excess of free monoclonal light chains, categorized as light chain predominant MM (LCPMM) [23-25]. Thus, about 30% of MM lesions secrete only or predominantly monoclonal light chains. Both LCPMM and LCMM with higher levels of free monoclonal light chains have been shown to have a significantly worse prognosis and may warrant customized treatment [23-26]. Therefore, accurate detection of monoclonal light chains in urine is an important issue in the diagnosis and follow up of monoclonal gammopathic disorders. An important reason for accurate diagnosis of monoclonal free light chains in urine is to avoid erroneous diagnosis of light chain MGUS based only on an abnormal κ/λ ratio [16,22].
False positive results on UIFE have not been identified to be a meaningful issue; however, false negatives are more abundant. For example, using Sebia antibodies to free light chains in lieu of conventional antisera from Helena Laboratories resulted in identification of monoclonal light chains in 18% greater number of specimens [17]. The yield by MASS FIX MALDI is about equal, if not slightly worse, than that by conventional UIFE [17,27].
Concentrating urine, be it 24-hour urine, first morning specimen, or random sample, is essential to improve the yield of UIFE for monoclonal free light chains. While it has been stated that monoclonal light chains are detectable in all newly diagnosed LCMM in unconcentrated urine, it is generally recommended that urine be concentrated 100-fold prior to subjecting to UIFE to avoid false negative results [11,13,14]. In one part of this study, it was shown that 25-fold urine concentration is generally adequate, though this method did miss one specimen out of 170, that was positive in an earlier test when native urine had higher total protein and serum had higher level of free kappa light chain. Repeat testing with further concentration by desiccation did correct the false negative result. A false negative result on membrane concentrated urine specimens was noted in only one of 170 cases. It warrants noting that this patient had a diagnosis of Kappa multiple myeloma and was under treatment. His serum kappa free light chain levels had decreased from 318 to 0.5 and urine protein decreased from 305 mg/dL to 12.0 mg/dL. Both these factors combined to produce a negative UIFE in January, on initial testing. However, it is worthy of note that he still had detectable monoclonal kappa light chains in urine on further concentration of the specimen. The persistence of detectable monoclonal light chains in urine attests to residual disease and is an important finding. This finding of monoclonal kappa light chains in urine would obviate the need for a bone marrow examination to ascertain minimal residual disease. While a solitary finding, it does support the case of further concentration by desiccation in urine specimens with <20 mg protein/dL.
In the earlier study of UIFE 0 specimens it was discovered that the urine protein concentration of 21-25 mg/dL provided the highest yield of positive UIFE results.16 The lower yield at higher native urine protein concentrations may have been due the difficulty in obtaining optimal urine concentration by membrane filtration.
From the combination of findings in this and earlier studies, we propose that urine should be concentrated by at least 25-fold, by membrane filtration, and in specimens with native urine protein concentration of < 20 mg/dL, further concentration by desiccation to achieve 100-fold or so concentration would optimize UIFE results. An additional essential consideration is the use of antisera specific to free light chains for UIFE, as the sensitivity of immunofixation via antibodies to conventional light chains is lower than that using antibodies specific to free light chains [17].
There are several shortcomings in this study that was conducted at a single medical center using a limited number of specimens to compare the UIFE results by routine processing to those obtained following further concentration by desiccation. This shortcoming is further compounded by the inability to have uniform urine concentrations due to marked variations in the total protein concentration in native urine specimens. The use of antisera specific to free light chains in UIFE was implemented in 2023; thus, earlier results were with a less sensitive method. The comparison was limited to testing by UIFE only; however conventional mass spectrometry may have higher sensitivity, but that has not been documented yet. MASS FIX MALDI has lower sensitivity than UIFE using antisera specific to free light chains [17]. Comparative studies with alternative sources of anti- free light chain antibodies, e.g., from the Binding Site, may improve sensitivity, should such reagents become available.
In summary, 25-fold concentration of random urine by membrane filtration is generally adequate for UIFE. In specimens with a native urine protein concentration of <20 mg/dL, further concentration by desiccation of membrane concentrated specimens would avoid the occasional false negative results. Use of antisera specific to free light chains for UIFE is strongly recommended [17].
Funding:
There was no external source of funding for the study.
Conflict of interest:
GS served as a consultant to Sebia Inc, Diazyme Laboratories Inc, Helena Laboratories and Beckman-Coulter.
References
- Karcher DS, McPherson RA, Pincus MR. Urine and other body fluids. In: Henry’s Clinical Diagnosis and Management by Laboratory Methods. 24th Chapter 29. Elsevier (2021): 468-509.
- Bradwell AR. Serum free light chain analysis plus Hevylite. 7th The Binding Site (2015).
- Wang C, Su W, Zhang W, et al. Serum immunoglobulin free light chain and heavy/light chain measurements in POEMS syndrome. Ann Hematol 93 (2014): 1201-1206.
- Dimopoulos MA, Sonneveld P, Leung N, et al. International Myeloma Working Group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol 34 (2016): 1544-1557.
- Kyle RA, Durie BG, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 24 (2010): 1121-1127.
- Crassini K, Gibson J. Pathogenesis and management of immune dysfunction secondary to B cell haematological malignancies. Intern Med J 54 (2024): 16-25.
- Islami F, Ward EM, Sung H, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. J Natl Cancer Inst 113 (2021): 1648-1669.
- Keren DF. Electrophoresis and mass spectrometry. Monoclonal Gammopathies and Protein Disorders. ASCP Press (2024).
- Singh G. Serum and urine protein electrophoresis and serum-free light chain assays in the diagnosis and monitoring of monoclonal gammopathies. J Appl Lab Med 5 (2020): 1358-1371.
- Katzmann JA, Dispenzieri A, Kyle RA, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 81 (2006): 1575-1578.
- Dejoie T, Corre J, Caillon H, et al. Serum free light chains, not urine specimens, should be used to evaluate response in light-chain multiple myeloma. Blood 128 (2016): 2941-2948.
- Natsuhara KH, Huang CY, Knoche J, et al. Significance of the pee-value: relevance of 24-hour urine studies for patients with myeloma. Leuk Lymphoma 64 (2023): 1186-1193.
- Shaheen SP, Levinson SS. Serum free light chain analysis may miss monoclonal light chains that urine immunofixation electrophoreses would detect. Clin Chim Acta 406 (2009): 162-166.
- Keren DF. Electrophoresis and mass spectrometry. Monoclonal Gammopathies and Protein Disorders. Chapter 10. ASCP Press (2024): 241.
- Ye Mon M, Ufondu O, Mortley S, et al. Urine immunofixation electrophoresis for diagnosis of monoclonal gammopathy: evaluation of methods for urine concentration. J Appl Lab Med 9 (2024): 350-356.
- Singh G, Xu H, Bollag R. Screening for light chain monoclonal gammopathy of undetermined significance: pseudo epidemic based on current diagnostic criteria. Cell Mol Med Res 3 (2025): 1-12.
- Singh G, Arinze N, Manthei DM, et al. Urine protein immunofixation electrophoresis: free light chain urine immunofixation electrophoresis is more sensitive than conventional assays for detecting monoclonal light chains and could serve as a marker of minimal residual disease. Lab Med 54 (2023): 527-533.
- Singh G, Saldaña EJ, Spencer J, et al. Automated detection of free monoclonal light chains by enhanced-sensitivity modified immunofixation electrophoresis with antisera against free light chains. Lab Med 56 (2025): 536-540.
- Heider M, Nickel K, Högner M, et al. Multiple myeloma: molecular pathogenesis and disease evolution. Oncol Res Treat 44 (2021): 672-681.
- Visram A, Larson D, Norman A, et al. Comparison of progression risk of monoclonal gammopathy of undetermined significance by method of detection. Blood 145 (2025): 325-333.
- Maatouk I, He S, Hummel M, et al. Patients with precursor disease exhibit similar psychological distress and mental HRQOL as patients with active myeloma. Blood Cancer J 9 (2019): 9.
- Singh G. Screening for monoclonal gammopathy of undetermined significance is contraindicated. Br J Haematol 207 (2025): 43-45.
- Lee WS, Singh G. Serum free light chain assay in monoclonal gammopathic manifestations. Lab Med 50 (2019): 381-389.
- Singh G, Xu H. Light chain predominant intact immunoglobulin monoclonal gammopathy disorders: shorter survival in light chain predominant multiple myelomas. Lab Med 52 (2021): 390-398.
- Singh G, Savage NM, Jillella AP, et al. Light chain–predominant multiple myeloma subgroup: impaired renal function correlates with decreased survival. Lab Med 53 (2022): 145-148.
- Jin Y, Savage NM, Bollag RJ, et al. Light chain multiple myeloma: high serum free light chain concentrations portend renal damage and poorer survival. J Appl Lab Med 6 (2021): 1592-1600.
- Wilhite D, Arfa A, Cotter T, et al. Multiple myeloma: detection of free monoclonal light chains by modified immunofixation electrophoresis with antisera against free light chains. Pract Lab Med 27 (2021): e00256.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 