Osteopetrosis: Insights and Oversights—A Case Report and its Literature Review
Mohammad Bilal Khan1, Sayed Ali2, Saddam Hussain3, Aimon Akhtar4, Taha Hassan Habib5, Majid Ali Shah6, Nouman Hasan7, Maaz Ali8, Dawood Khan8, Qazi Muhammad Farooq Wahab9, Syed Farooq Shafiq10, Avijit Debnath11*, Furqan Ul Haq12
1Windsor University School of Medicine, St. Kitts, 1621 Brighton’s Estate, Cayon, St Kitts & Nevis
2Senior Medical Officer, Pediatrics Department, Swat Medical College and Swat Medical Complex Teaching Hospital, Saidu Sharif Swat, Pakistan
3Registrar Pediatrics Govt Lady Reading Hospital Peshawar, Pakistan
4Department of Internal Medicine, Saidu Group of Teaching Hospital Swat, Pakistan
5House Officer in the Department of Internal Medicine, Niazi Medical College, Sargodha, Pakistan
6Department of Pediatrics, Hayatabad Medical Complex, Peshawar, Pakistan
7House Officer in the Department of Internal Medicine, Niazi Medical College, Sargodha, Pakistan
8Department of Medicine, Bacha Khan Medical Complex, MTI Mardan, Pakistan
9Department of Medicine, Khyber Teaching Hospital, Peshawar, Pakistan
10Gajju Khan Medical College, Shahmansoor, Shah Mansur Twp, Pakistan
11Department of Medicine, Jalalabad Ragib Rabeya Medical College, Sylhet, Bangladesh
12Radiation Oncology Department, Shifa International Hospital, Islamabad, Pakistan
*Corresponding Author: Avijit Debnath, Department of Medicine, Jalalabad Ragib Rabeya Medical College, Sylhet, Bangladesh.
Received: 12 May 2025; Accepted: 04 June 2025; Published: 11 June 2025
Article Information
Citation: Mohammad Bilal Khan, Sayed Ali, Saddam Hussain, Aimon Akhtar, Taha Hassan Habib, Majid Ali Shah, Nouman Hasan, Maaz Ali, Dawood Khan, Qazi Muhammad Farooq Wahab, Syed Farooq Shafiq, Avijit Debnath, Furqan Ul Haq. Osteopetrosis: Insights and Oversights—A Case Report and its Literature Review. Journal of Orthopedics and Sports Medicine. 7 (2025): 269-280.
View / Download Pdf Share at FacebookAbstract
Background: Osteopetrosis is a rare genetic disorder characterized by defective osteoclast function, leading to increased bone density and skeletal fragility. The condition manifests in various forms, ranging from mild adult-onset to severe infantile types, often associated with hematologic, neurological, and growth complications.
Introduction: Osteopetrosis patients will mostly suffer from pathologic fractures and progressive cranial nerve compression neuropathies. There are two sub classifications of autosomal dominant osteopetrosis, and these patients are often asymptomatic in adults.
Objective: To present a comprehensive case report of osteopetrosis, emphasizing its clinical presentation, diagnostic challenges, and therapeutic considerations. Additionally, this study provides a narrative review of the different types of osteopetrosis, their clinical manifestations, and the multidisciplinary approaches involved in their management. We have focused to discuss case analysis, diagnostic challenges, therapeutic insights and role of stem cell transplant.
Results and conclusion: Osteopetrosis is a complex genetic disorder requiring a multidisciplinary approach for effective management due to its wide-ranging complications. Pediatricians play a crucial role in early diagnosis and monitoring growth-related issues, while hematologists manage bone marrow failure and related hematologic abnormalities. Three types of osteopetrosis with different severity degrees of skeletal disorders and pathological severity are explained. BMT markedly improves cases of infantile osteopetrosis. It cures bone marrow failure and metabolic abnormalities in patients. Multidisciplinary team approach is needed for treatment and management of complications.
Keywords
<p>Osteopetrosis; Inherited bone diseases; Skeletal abnormalities; Etiology of osteopetrosis; Management of osteopetrosis</p>
Article Details
1. Introduction
Osteopetrosis is a group of rare bone disorders, characterized by reduced osteoclastic bone resorption. Osteoclast are highly specialized cells, which degrade bone minerals and bone matrix, dysfunction that causes increased bone density [1]. These processes are crucial for bone remodeling, maintenance of bone biomechanical stability, and mineral homeostasis. Expansion of bone into marrow cavities causes cranial nerve compression, anemia, bleeding, frequent infections, and hepatosplenomegaly further worsening can cause blindness, deafness, and nerve palsies [2]. Autosomal recessive osteopetrosis (ARO) ARO, also known as malignant or infantile osteopetrosis, is the most serious one. It is often diagnosed earlier and is lethal without treatment. In ARO, bone remodeling is inhibited, due to dysfunctional osteoclast but as bone synthesis continues, pressure is exerted on the nervous system. Newly synthesized bone fills all the available space, which results in denser compact bone. This condition leads to a lack of red bone marrow, which results in anemia and an immunocompromised state, due to reduced production of red and white blood cells. Deficient immune system, the patient is at high risk of severe infections. Mutations in the TCIRG1, CLCN7, OSTM1, SNX10, and PLEKHM genes, their function is limited, and they have difficulty production. Most children have a visual impairment; this impairment is caused by growing bone suppressing the optic nerve; it occurs in the child’s first year after birth. Children who retain vision after two years of age will continue to have sight. Furthermore, almost one-third of children with ARO will have deafness [3]. One theory is that hearing impairments occur due to a combination of sclerosis in the ear bones and compression of the auditory nerve. Compression of cranial nerve VII causes facial paralysis, and compression of cranial nerve VIII leads to deafness, and occasionally, balance disorders. Balance disorders can be caused by pressure on the peripheral motor nerves, which can also cause problems with swallowing. The dangerous situations when ossification of the cranium fontanels begins, the brain does not have sufficient space to grow, and cognitive disabilities happen.
As bones are denser and sensitive, bone fractures occur with little force ARO carries a risk of dental complications, such as caries, due to effects on enamel and dentin. Individuals with ARO often have little or no tooth enamel and misshaped dentin, which leads to deformed teeth. Eruption is delayed and can even cause osteoporosis of the jaw. This occurs more frequently in the mandible than in the maxilla, because the maxilla has vascular-rich areas and thin cortical bone, which prevents osteomyelitis and osteonecrosis. Intermediate autosomal recessive osteopetrosis (IARO) IARO is similar to ARO, except IARO is typically discovered during late infancy and the symptoms develop more slowly [4]. Children with this mutation are more exposed to multiple fractures and shorter bone lengths. Children with mutations in the carbonic anhydrase II gene develop renal tubular acidosis, short bone lengths, and mental retardation. Therefore, children with IARO seldom have liver and spleen enlargement or bone marrow complications. Thus, the prognosis of IARO is more favorable than that of ARO, and children are more likely to survive. The symptoms are almost the same as those associated with ARO, except this IARO subtype is not fatal in infancy. Hematopoietic Stem Cell Transplant (HSCT), is a successful and final treatment of choice in most cases. Based on radiographs of bone fractures the disease is mostly diagnosed. Patients typically have a sign of ’sandwich vertebrae’, where the vertebral endplates show parallel, dense bands of sclerosis. ADO was divided into two subtypes: ADO1 and ADO2. ADO1 is believed to be caused by a mutation in the LDL receptor-related protein 5 genes. ADO is a rare disorder, but it has two subtypes 1 and 2. ADO2 is caused by a mutation in the CLCN7 gene, which encodes an H+/Cl- exchange transporter. This mutation more often causes bone fractures, and patients carry some risk of cranial nerve compression (5%) and moderate risks of anemia and immune insufficiency. These patients have oral complications as well; femur and rib fractures can occur. Each patient had, on average, more than four bone fractures. The oral complications are; Dental fractures, multiple and severe dental decay, mandibular osteomyelitis, and multiple Disease-causing variants in several different genes can cause osteopetrosis. The severity of the disease varies depending on the gene mutated, from mild disease accidentally diagnosed at an x-ray of a traumatic fracture to severe disease that is already life-threatening in a newborn baby [5].
2. Case Report
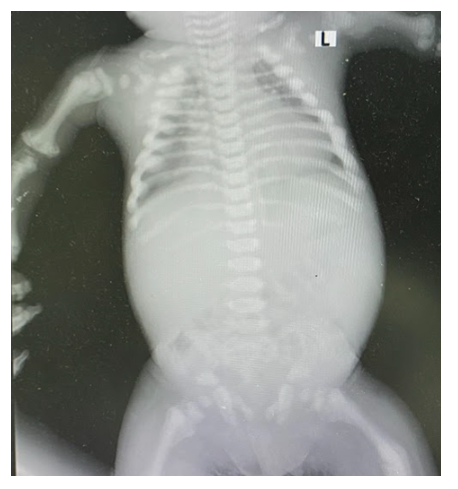
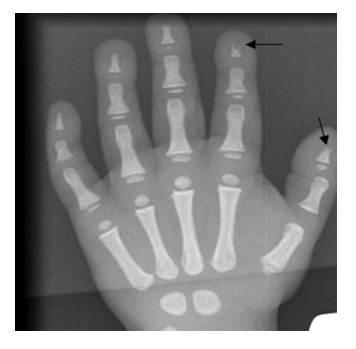
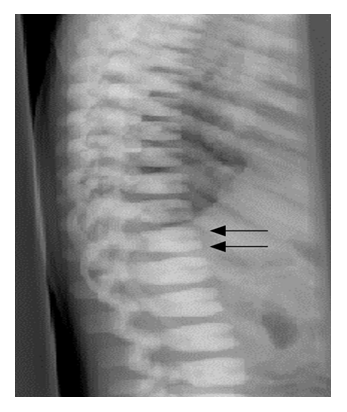
A five years old child reported with the chief complaint of intermittent and continuous pain with swelling in the right upper back tooth region for the past 1 week, fever non documented and fits since 6am in the morning. On general examination, the patient had short stature with frontal bossing, flattening of the nasal bridge, exophthalmos with proptosis and strabismus of the left eye. The parents gave a history of apparent hearing loss on the left side with complete loss of vision in the left eye. Extraoral examination revealed mild swelling in the region of the right maxilla that was soft and tender. Intraoral examination showed evidence of sinus opening with yellowish-white pus discharge in the right upper first premolar region. There was no lymphadenopathy or hepatosplenomegaly. Her blood complete picture has been mentioned in tabulated form in Table 1. X-rays of the body, hands and vertebrae or spine are given below in Figure 1, 2, 3 respectively. Radiological evaluation showed increased bone density with increased thickening of bony cortices with diagnosis of osteopetrosis. He was referred to us for bone marrow biopsy. Bony texture was stony hard. Bone marrow aspirate was hypocellular with hypoplastic erythroid, myeloid and megakaryocytic series cells. No abnormal cells were found. Histological section of trephine biopsy showed hypocellular bone marrow fragments with decrease in erythroid, myeloid and megakaryocytic series cells. Bone marrow fragments were replaced by new bone formation. Orthopantomogram revealed the presence of an unhealed socket in the right maxillary posterior region with patchy areas of radiolucency. There was evidence of generalized dense amorphous radio-opacities involving the bones. He was given birth through normal vaginal delivery at seven months gestational age but developmentally he can’t sit or stand. On examination he was afebrile, tachypneic with exaggerated reflexes. There is also some cognition defect seen as he can’t say mama baba. His mother is 15 years old and it was a consanguineous marriage.
|
Name of investigation |
Result at admission |
Result at discharge |
Normal Range |
Unit |
|
Hemoglobin |
5.8 |
9.6 |
M=14-18 |
mg/dl |
|
F= 11.7-15.7 |
||||
|
MCV |
77 |
77 |
80-100 |
fl |
|
MCH |
23.1 |
25 |
25-35 |
pg/cell |
|
MCHC |
29.9 |
29 |
32-36 |
g/dl |
|
W.B.C |
16.5 |
8.3 |
15.6 |
x103/dl |
|
Lymphocytes |
0.23 |
0.21 |
20-25% |
x103/dL |
|
Monocytes |
0.01 |
0.01 |
2-10% |
x103/dL |
|
Eosinophil |
0.01 |
0.01 |
1-2% |
x103/dL |
|
Platelets Count |
100 |
160 |
150-400 |
x106/L |
|
Sodium |
134 |
130 |
136-149 |
mmol/L |
|
Potassium |
4 |
4 |
3.8-5.2 |
mmol/L |
|
Chloride |
100 |
100 |
98-107 |
mmol/L |
|
Random Blood Sugar |
90 |
90 |
80-140 |
mg/dl |
|
Blood Urea |
40 |
40 |
18537 |
mg/dl |
|
Acid Phosphatase |
25 |
20 |
8.6-12.6 |
u/ml |
|
Serum Calcium (Total) |
10 |
9.2 |
8.8-12.0 |
mg/dl |
|
Total Bilirubin |
1 |
1 |
0.1-1 |
mg/dl |
|
Creatinine kinase |
100 |
100 |
10-120 |
micrograms per liter (mcg/L) |
|
ANF |
negative |
negative |
negative |
|
|
ESR |
10mm/hr |
10mm/hr |
0 to 15 mm/hr in men. 0 to 20 mm/hr in women |
mm/hr |
|
Ferritin |
30 |
30 |
Male: 30 to 400 nanograms per milliliter (ng/mL) Female: 13 to 150 ng/mL |
ng/mL |
|
CRP |
0.4 |
0.4 |
0.3 to 1.0 |
mg/dL |
|
LDH |
150 |
150 |
140 to 280 |
U/L |
|
Anti CCP |
10 |
10 |
less than 20 Units |
|
|
Urine R/E |
normal |
normal |
looking for any nitrates and WBC, RBC and PH most of the time or amt sediments |
|
|
Serum procalcitonin |
less than 0.1 |
less than 0.1 |
less than 0.1 |
ng/mL |
|
Abbreviations: DLC: differential leukocyte count; RDW%: red cell distribution width; MCV: mean corpuscular volume; ESR: erythrocyte sedimentation rate; ALT: alanine transaminase; HIV: human immunodeficiency virus; HCV: hepatitis C virus; HBsAg: hepatitis B surface antigen; ICT: immunochromatographic test, fl: femtoliter, mg/dl milligram per deciliter, ng/ml: nanogram per milliliter. |
||||
Table 1: Baseline investigations.
General findings, increased cortical thickening, Increased overall bone density, Loss of medullary canal diameter, Bone-in-bone appearance, Additional findings "Erlenmeyer flask" proximal humerus and distal femur, "Rugger jersey spine".
Pycnodysostosis: Right Hand radiograph, acro-osteolysis in the distal phalanx of thumb and index fingers (arrows) and generalized increase in bone density.
Sclerosis of backbone resulting in sandwich vertebrae appearance.
In hospital treatment received by the patient has been discussed in Table 2,
|
Serial no. |
Treatment |
Dose |
Frequency |
Route |
|
1 |
provas |
10ml |
TDS |
intravenous |
|
2 |
Risek |
20mg |
BD |
intravenous |
|
3 |
Nezkil |
50ml |
TDS |
intravenous |
|
4 |
Meronem |
200mg |
TDS |
injection |
|
5 |
Azomax |
½ TSF |
BD |
injection |
|
6 |
Phenobarb |
4tab |
OD |
syrup |
|
7 |
Ceftriaxone |
600mg |
BD |
injection |
|
8 |
Vancomycin |
120mg |
TDS |
injection |
|
9 |
Brivaracetam |
18mg |
BD |
injection |
|
Abbreviations: Table 3: Treatment received. Inj., injection; Syp., Inf. infusion; Tab., tablet; IV, intravenous; PO, per oral; TSF, teaspoon; OD, once daily; TDS, thrice daily; BD, twice daily. |
||||
Table 2: Treatment received during hospitalization in our patient.
3. Epidemiology
These conditions are rare, and their overall incidence is difficult to estimate. To some extent estimated data is calculated. Autosomal recessive osteopetrosis has an incidence of 1 in 250,000 births, with a high incidence in Costa Rica (3.4:100,000). Autosomal dominant osteopetrosis has an incidence of 5:100,000 births. Approximately 1 in 20,000 for autosomal dominant form, 1 in 200,000 for autosomal recessive form. Benign autosomal recessive is the most common form of osteopetrosis. It may skip generation, but gene penetration is 75%. prevalence of fracture is 40 to 50% [6].
4. Etiology
Osteoclast dysfunction leads to denser bone and obliteration of medullary canals due to the inability of osteoclast to acidify Howship's lacunae. The lower extremity is more prone to fracture as compared to the upper extremity and axial skeleton. Osteoclasts are highly specialized cells, which degrade bone mineral and organic bone matrix. These processes are crucial for bone remodeling and the maintenance of bone biomechanical stability and mineral homeostasis. It is estimated that the adult skeleton is completely regenerated every 10 years. Osteoclasts are derived from the mononuclear precursors in the myeloid lineage of hematopoietic cells that also give rise to macrophages. By contrast, osteoblasts are derived from multipotent mesenchymal stem cells, which also give rise to chondrocytes, adipocytes, and muscle cells. Other important signals for osteoclast differentiation include the ligand of receptor activator of nuclear factor-kappa B (RANKL) and M-CSF [7]. Failure of osteoclast differentiation as a result of mutations in these genes accounts for the rare osteoclast-poor forms of ARO, in which no mature osteoclasts are present. A fully differentiated osteoclast dissolves bone minerals and degrades bone matrix using specialized enzymes. The process is important in particular the formation of the ruffled border and sealing zone. These form the resorption lacuna where hydrochloric acid is actively secreted resulting in the dissolution of bone mineral hydroxyapatite [8].
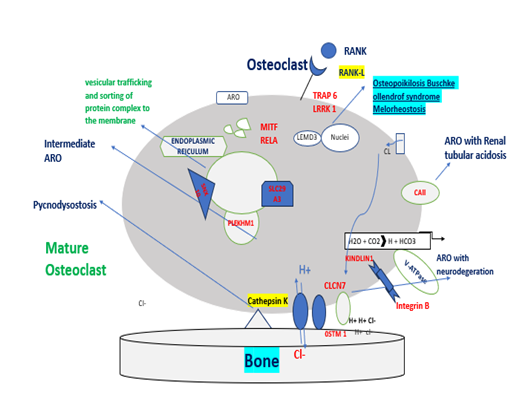
Figure 4: Those genes are involved in the pathogenesis of ARO or autosomal-rich osteopetrosis. MCSF and RANKL, cytokines secreted by osteoblasts and osteocytes, are necessary for the differentiation of osteoclast precursors into mature and resorbing osteoclasts. When these signals are absent (TNFSF11 gene mutations) or the pathway is interrupted by the lack of cytokine receptors (TNFRSF11A and CSF1R gene mutations), osteoclast precursors are not able to differentiate into mature osteoclast-causing osteoclast-poor forms of osteopetrosis. Alternatively, if osteopetrosis is caused by mutations in genes encoding for protein necessary for bone resorption, the disease is defined as osteoclast-rich osteopetrosis. On the right of the figure, are indicated genes involved in bone resorption activity with different roles: i.e., acidification of resorption lacunae and pH regulation (TCIRG1, CLCN7, OSTM1, and CAII), vesicular trafficking and sorting of protein complex to the membrane (SNX10 and PLEKHM1), cytoskeletal rearrangement for ruffle border formation (FERMT3 and LRRK1). Other molecules involved in different signal transductions, essential for osteoclast functions (MITF, LRP5, and IKBKG) are reported.
Most forms of osteoclast-rich osteopetrosis are caused by defects in gene products involved in the acidification machinery. Acid secretion is dependent on two key molecules, which facilitate proton transport: the proton pump vacuolar ATPase (V-ATPase) and the chloride-specific ion channel, chloride channel 7 (CLCN-7). Homozygous mutations in the genes encoding the subunit of V-ATPase and the CLCN-7 produce severe malignant osteopetrosis phenotypes in humans CLCN-7 on the other hand plays a key role in lysosomal acidification, which explains the severe neuronal storage and neurodegeneration in the CNS and retina. CLCN-7 is closely associated with another membrane protein, OSTM1. The protons and chloride ions that are expended in the acidification process need to be replenished intracellularly in order to avoid alkalization. This is achieved by carbonic anhydrase type II (CAII) and an anion exchanger. Given the key role of CAII in kidney function, it is not surprising that mutations in CAII result in ARO with tubular acidosis. The collagen bone matrix is dissolved by two groups of enzymes, the matrix metalloproteinases (MMPs) and lysosomal cathepsins. Cathepsin K in particular has been identified as a key enzyme. It is secreted in the resorption lacuna where it degrades collagen I at acidic pH. Inhibition of cathepsin K prevents matrix degradation. Homozygous mutations in the human cathepsin K gene lead to Pycnodysostosis. The formation and maintenance of the osteoclast polarised membrane domains requires complex vesicular trafficking mechanisms and continuous remodelling of the osteoclast cytoskeleton. One protein, which plays a critical function in vesicle trafficking and acidification is PLEKHM1, and heterozygous mutations in this have been associated with intermediate forms of osteopetrosis. Other signalling pathways are likely to be important in osteoclast function, and mutations in the LEMD3 gene which codes for an integral protein of the inner nuclear membrane thought to be involved in BMP and TGFβ signalling, result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Mutations in genes described so far only account for approximately 70% of cases and the search continues for the genes responsible for the remainder [9].
5. Associated Conditions and Complications
Cranial nerve palsies can occur due to overgrowth of bone. Caffey disease affects the mandible in addition to the clavicle, ribs, and scapulae. Lower lumbar pain increases the prevalence of spondylolysis pelvis. Coxa Vara occurs commonly due to femoral neck fracture due to repeated stress fractures and increased risk of degenerative joint arthritis. Extremities have an increased tendency for long bone fractures often causing carpal tunnel syndrome. Refracture chances increase by hard brittle bones hardware; Infection increases risk due to reduced tissue vascularity Malunion Nonunion also occurs [10].
6. Genetic Condition
Osteopetrosis is a trait that can be inherited in an autosomal dominant, autosomal recessive, X-linked, and genetic counseling will depend on the mode of inheritance in a particular. Autosomal recessive: the parents of the affected child have a 1 in 4 (25%) risk of having further affected children in each pregnancy. 2/3 of unaffected siblings are expected to be carriers. Autosomal dominant signs of osteopetrosis, including radiographic studies of the skeleton. Each child of an affected individual has a 1 in 2 (50%) risk of being affected. If the parents are unaffected, there may still be a low risk of further affected children due to gonadal mosaicism. X-linked recessive: if the mother is a carrier, 50% of male pregnancies will be affected, and 50% of female pregnancies will be carriers. If the mother is not a carrier, there may still be a small risk of further affected offspring due to gonadal mosaicism [11].
|
X-linked osteopetrosis |
X-linked osteopetrosis |
Intermediate osteopetrosis |
Autosomal dominant osteopetrosis |
|
Onset |
infancy |
infancy |
childhood |
|
Severity |
severe |
mild to moderate |
mild to moderate |
|
Treatment |
supportive |
supportive |
supportive |
|
Recurrent risk |
if mother of proband carrier: 50% OF MALE pregnancies affected |
parents of proband: 25% recurrence |
50% pregnancies if one parent affected |
|
Prognosis |
poor |
variable |
normal life expectancy |
Table 3: Clinical classification of human osteopetrosis.
|
Characteristics |
Type 1 |
Type 2 |
|
Skull sclerosis |
marked sclerosis mainly of the vault |
sclerosis mainly of base |
|
Spine |
do not show much sclerosis |
show rugged appearance |
|
Pelvis |
no endo bones |
show endo bones in pelvis |
|
Risk of fracture |
low |
high |
|
Serum acid phosphatase |
normal |
very high |
|
Transverse banding of metaphysis |
absent |
may or may not be present |
Table 4: Types of adult osteopetrosis.
7. Treatment Approach
The following have been used in the treatment of patients with osteopetrosis [12]:
1. Calcium and cholecalciferol:
a. Goal 25-hydroxy vitamin D is >30
b. Supplementation of calcium may be necessary if dietary intake is below the daily recommended amount.
2. Red blood cell transfusion
a. This is needed for patients who have symptomatic anemia.
3. Interferon gamma
a. treatment with interferon gamma can increase leukocyte superoxide activity and hematologic recovery and decrease trabecular bone volume.
4. Corticosteroids
a. There is lack of evidence at this time to support the routine use of steroids
b. Case reports have shown that steroid use in infantile osteopetrosis can cause underdeveloped bone growth, preventing the need for bone marrow transplantation.
5. Hematopoietic stem cell transplantation (HSCT)
a. Patients aged younger than 1 year and patients with bone marrow failure should be referred for HSCT.
b. For patients with malignant infantile osteopetrosis, HSCT may correct bone metabolism.
c. failed medicine treatment.
6. Surgical treatment:
In osteopetrosis, surgical treatment is sometimes necessary because of fractures. In adult osteopetrosis, surgical treatment may be needed for aesthetic reasons (eg, in patients with notable facial deformity) or functional reasons (eg, in patients with multiple fractures, deformity, and loss of function). Severe, related degenerative joint disease may warrant surgical intervention [13].
Fractures most commonly involve long bones such as the femur, tibia, humerus, and foot bones. Historically, fractures have been treated nonsurgically due to challenges associated with surgical treatment. Preoperative planning with orthopedic surgery is necessary to evaluate the best course of surgical management [14].
7. Consultations:
A multidisciplinary approach may be needed for patients with osteopetrosis. including ophthalmologist, dentist, orthopedics specialist, neurologist, otolaryngologist, hematologist, or nephrologist.
8. Diet:
Nutritional support is important to improve patient growth. It also enhances responsiveness to other treatment options. However, patients may need calcium if hypocalcemia or rickets becomes a problem [15].
9. Long-term monitoring:
Measurement of serum calcium, phosphorus, 25-hydroxy vitamin D, and parathyroid hormone levels and a complete blood cell count every 6-12 months is generally recommended.
Patients who are receiving calcitriol therapy may require monitoring with serum calcium, serum phosphorus, serum creatinine, and a urinary calcium/creatinine ratio [16].
10. Assessments for Screening and Monitoring Treatment Responses in Patients With Osteopetrosis:
The baseline assessments and monitoring of patients are done at specific intervals to check for serum calcium levels, vitamin d levels, parathyroid hormones levels, complete blood count, LDH, MRI of brain for optic nerve functioning, renal ultrasonography for renal stones and serum calcium levels as well.
|
Serial No. |
Intervention |
Timing |
|
1 |
Serum calcium |
At diagnosis and every 6–12 mo (every 3 mo during calcitriol therapy) |
|
2 |
Serum phosphorus |
At diagnosis and every 6–12 mo (every 3 mo during calcitriol therapy) |
|
3 |
Serum creatinine |
At diagnosis (every 3 mo during calcitriol therapy) |
|
4 |
Vitamin D |
At diagnosis and every 6–12 mo |
|
5 |
Parathyroid Hormone |
At diagnosis and every 6–12 months |
|
6 |
Complete blood count with differential |
At diagnosis and every 6–12 months |
|
7 |
BB isoenzyme of creatine kinase |
At diagnosis |
|
8 |
Lactate dehydrogenase |
At diagnosis |
|
9 |
MRI to evaluate optic nerves |
At the time of diagnosis and do it if clinically indicated. |
|
10 |
Urinary calcium/creatinine ratio (random or 24 h) |
At initiation of calcitriol and every 3 mo during calcitriol therapy |
|
11 |
Renal ultrasonography |
At diagnosis and every 12 mo in patients with CaII mutations (every 12 mo during calcitriol therapy) |
|
Abbreviation: MRI, magnetic resonance imaging. |
||
Table 5: Required screening.
Graphical presentations of survival and complications, age of diagnosis of osteopetrosis based on its types, categorized by income level, survival rates and long-term complications in children with osteopetrosis over time:
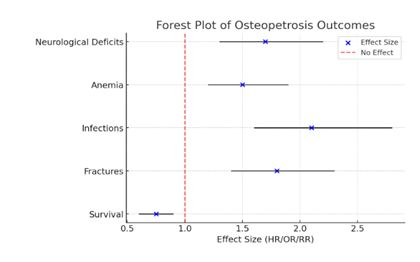
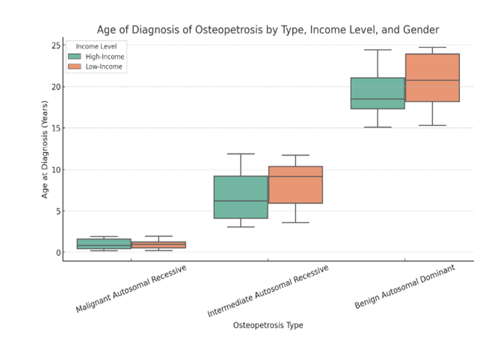
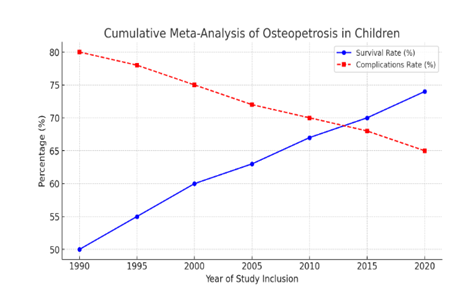
Figure 5 is a forest plot of Osteopetrosis patients' survival and their complications, Figure 6 is Box plot the age of diagnosis of osteopetrosis based on its types, categorized by income level (low-income vs. high-income countries). Figure 7 is a Cumulative Meta-Analysis Plot showing the trends in survival rates and long-term complications in children with osteopetrosis over time.
Osteopetrosis patients' survival and complications. The blue dots represent the effect sizes (HR/OR/RR), while the black horizontal lines indicate the 95% confidence intervals. The red dashed line at 1 represents the point of no effect.
Here is the box plot showing the age of diagnosis of osteopetrosis based on its types, categorized by income level (low-income vs. high-income countries).
- Malignant Autosomal Recessive (MAR): Diagnosed early (8 weeks - 2 years).
- Intermediate Autosomal Recessive (IAR): Diagnosed in childhood to adolescence (3 - 12 years).
- Benign Autosomal Dominant (BAD): Diagnosed later (15 - 25 years).
- Survival rates have gradually improved from approximately 50% in 1990 to 74% in recent years, reflecting advancements in treatment such as hematopoietic stem cell transplantation.
- Complication rates have declined from 80% to around 65%, likely due to better supportive care and early diagnosis.
11. Osteopetrosis requiring multiple subspecialities for management:
Osteopetrosis is a complex genetic disorder requiring a multidisciplinary approach for effective management due to its wide-ranging complications. Pediatricians play a crucial role in early diagnosis and monitoring growth-related issues, while hematologists manage bone marrow failure and related hematologic abnormalities. Orthopedic specialists address fractures and skeletal deformities, whereas ophthalmologists monitor and treat optic nerve compression to prevent vision loss. Dentists manage dental anomalies and osteomyelitis risks, while ENT specialists handle hearing loss due to cranial nerve compression. Nephrologists oversee renal complications linked to metabolic disturbances, ensuring comprehensive care. This coordinated approach optimizes patient outcomes and improves quality of life.
|
Serial No. |
Subspeciality |
Expected future complications |
|
1 |
Endocrinology |
Osteopetrorickets, hypocalcemia. |
|
2 |
Ophthalmology |
Papilledema, ptosis, strabismus, paralysis of extraocular muscles, optic nerve atrophy, exophthalmos, nystagmus, retinal degeneration tearing (from nasolacrimal duct obstruction). |
|
3 |
Dentistry |
Delay/failure of tooth eruption, malformed crowns/roots, periodontal ligament defects, odontoma tooth agenesis, enamel hypoplasia, tooth decay/caries, thickened lamina dura, osteomyelitis (most frequently of the mandible). |
|
4 |
Orthopedics |
Skeletal deformities, scoliosis, spondylolisthesis, fractures (particularly of the long bones), delayed union/nonunion, degenerative arthritis, spondylolysis. |
|
5 |
Neurology/neurosurgery |
Compressive cranial neuropathies (often optic and facial nerves, but can involve any of cranial nerves I–VIII), increased intracranial pressure, craniosynostosis, Arnold–Chiari I malformation, neuromuscular scoliosis, developmental delay/regression, seizures (OSTM1 mutation), calcifications of the basal ganglia, thalami (CAII deficiency), hydrocephalus, cerebrovascular stenosis/occlusion, acquired encephalocele. |
|
6 |
Otorhinology |
Conductive hearing loss, recurrent otitis media, chronic congestion (poorly pneumatized sinuses), rhinorrhea, choanal atresia, rhinosinusitis, obstructive sleep apnea. |
|
7 |
Hematology |
Thrombocytopenia with bleeding, anemia, leukopenia with frequent infections, hepatosplenomegaly, transfusion dependence. |
|
8 |
Nephrology |
Renal tubular acidosis, nephrocalcinosis, and nephrolithiasis (CAII deficiency). |
Table 6: Complications and its management by subspeciality.
12. Bone Marrow Transplantation:
BMT markedly improves cases of infantile osteopetrosis. BMT can cure bone marrow failure and metabolic abnormalities in patients. BMT is the only curative treatment for this disease. BMT may be limited in a subset of patients whose condition is unlikely to respond to medication but an appropriate bone marrow donor is not always found. Magnetic resonance imaging (MRI) can be used to assess bones over time after BMT [17].
13. Differential Diagnosis:
Primary sclerosing conditions of bone caused by osteoclast dysfunction need to be distinguished from the large number of conditions in which bone sclerosis can occur as a secondary phenomenon. Some alternative diagnoses to consider include fluorosis; beryllium, lead, and bismuth poisoning; myelofibrosis; Paget's disease (sclerosing form); and malignancies (lymphoma, osteoblastic cancer metastases). Neonatal radiographs can be particularly difficult to interpret in the absence of additional multiorgan involvement, as the normal neonatal skeleton can appear denser than normal. However, in contrast to osteopetrosis, this appearance will improve over time. Once the diagnosis of a primary osteoporotic condition is made, it is important to distinguish between different subtypes as they have different responses to treatment, prognosis, and recurrence risks [18].
14. Restoration:
Some patients go for dental fillings. Some defective teeth were protected with fissure sealing and deformed teeth were rebuilt with resin-based composite. ADO had previously been treated with amalgam and composite fillings, and after two molar extractions in the lower jaw, she developed osteomyelitis. Patients are also treated with implants. The etiology could not be determined, because no data were available on common risk factors, like smoking, dental hygiene, or diabetes. Fixed dental prostheses. removable prosthetics were implanted in both jaws [19].
15. Prognosis:
The severe infantile forms of osteopetrosis are associated with diminished life expectancy, with most untreated children dying in the first decade as a complication of bone marrow suppression. Life expectancy in the adult onset forms is normal [20].
16. Prenatal diagnosis:
Pre-implantation and prenatal diagnosis is theoretically possible in families, in whom the genetic mutation has been identified, thus allowing for reproductive decisions to be made. In families with severe ARO and unknown mutations, prenatal diagnosis may be possible using radiographs. If a family decides to continue with an affected pregnancy, haematopoietic stem cell transplantation (HSCT) before the age of 3 months can be planned with the aim of improving neurological outcomes [21].
8. Discussion
Efforts are important to highlight the evidence that does exist and how it can be used within the medical community in a good way, although not completely evidence-based but at least consistent with known physiologic principles. A patient diagnosed with osteopetrosis has fragile bones and is always prone to infections, and fractures. Oral hygiene is important for patients to minimize the risk of infections [22]. In this review, most included studies focused on osteomyelitis as a complication of osteopetrosis. There did not seem to be a uniform for treating osteomyelitis or osteonecrosis in patients with osteopetrosis. All patients who underwent a mandibular segmental resection, corticotomy, and oral hemi-mandibulectomy had an uneventful healing process, which most likely did not require are-surgery. It has been suggested that, when osteomyelitis is a complication of osteopetrosis, treatment should be similar to the treatment for osteoradionecrosis. A more aggressive surgical approach is required, with a combination of antibiotics and prolonged hyperbaric oxygen therapy [23]. In the present review, we included one case where hyperbaric oxygen therapy was applied, but the surgeon chose a less invasive approach of surgical debridement; in that case, complete [24].
Osteopetrosis varies in severity depending on the mode of inheritance and time of presentation. Based on the age, clinical, hematological, and radiological features have three distinct forms namely the infantile (AR) malignant condition, adult (AD) benign form, and intermediate Osteopetrosis. Malignant Osteopetrosis occurs in infancy and has a rapid downhill course due to severe bone marrow failure. Features of infantile osteopetrosis include dense and deformed bones, growth failure, anemia, hypoplastic dentition, chronic infection, splenomegaly, and neurological impairment [25]. Adult type of Osteopetrosis has an autosomal dominant inheritance pattern and approximately one-half of the patients are asymptomatic, and the diagnosis is made incidentally or is based on family history. Autosomal recessive osteopetrosis may have less severe manifestations and is known as “intermediate” autosomal recessive osteopetrosis (in which bony changes are there but bone marrow failure is not prominent and it has poor prognosis). Recently a case has been described with abnormal bone modeling, increased bone density, and histological features of osteopetrosis in a 12-year-old boy with a clinical picture acquired osteopetrosis with symptomatic treatment. We present a case of autosomal recessive osteopetrosis which is probably of intermediate severity as she is a 10-year-old girl and has presented with bony changes and anemia but there is no history of transfusion in the past. X-ray shows sclerotic changes but there was still some residual marrow seen on bone marrow. Her two siblings however died at the age of 3 and 3.5 years with the same disease, probably having a severe form of malignant osteopetrosis. Osteopetrosis is a rare cause of anemia both in infantile and adult varieties. In a study done in Pakistan on clinical and laboratory features of 6 patients of infantile osteopetrosis in 5 years duration, increasing pallor and listlessness were the most common initial symptoms. Other features included abdominal distension, fever, frontal bossing, nasal block, anemia, and thrombocytopenia [26]. The risk is always there in developing hematological impairment in the first year of life. Infantile osteopetrosis requires treatment due to the adverse outcome associated with the disease. Calcitriol may help by stimulating dormant osteoclasts and, thus, stimulating bone resorption. Erythropoietin can be used to correct anemia. Children with infantile osteopetrosis have disease-related complications that affect nutritional status. Good nutritional support can provide nutrients needed for improved growth and response to treatment in these patients. Recently, the use of interferon-gamma has been found to decrease the rate of infection and transfusion requirements after 24 months of therapy. Bone marrow transplant is the only treatment that can be offered to cure the disease and has remarkably shown improvement in some cases of osteopetrosis [27]. However, in adult OP which has relatively normal bone marrow function no specific medical treatment exists. Though rare, AR osteopetrosis should be considered in the differential diagnosis of an infant or young child presenting with anemia with or without hepatosplenomegaly. An accurate diagnosis is essential given the availability of curative treatment and genetic counseling.
9. Conclusion
Osteopetrosis is a group of rare bone disorders characterized by reduced osteoclastic bone resorption that results in a high bone mass. The overly dense bone builds a structural brittleness that is prone to fracture. The disruption of normal bone modeling and remodeling can give rise to skeletal deformity and dental abnormalities and can interfere with mineral homeostasis. Expansion of bone into marrow cavities and cranial nerve foramina can compromise hematologic and neurologic function, the former may manifest as profound anemia, bleeding, frequent infections, and hepatosplenomegaly from extramedullary hematopoiesis. This can cause blindness, deafness, and nerve palsies. Not every patient is affected in the same way. Osteopetrosis comprises a clinical spectrum ranging from very mild to severe disease. Therefore, the objectives of these consensus guidelines are to summarize the collective experience of our panel of experts and provide a basis for standardizing the diagnostic approach to medical management of patients with noninfantile forms of osteopetrosis. Healing was not achieved. This Literature review shows that patients with osteopetrosis with even severe complications can be treated if the original disease is successfully cured by HSCT. The rehabilitation must be performed cautiously and gradually, it should not disqualify the individual patient. The literature concerning patients diagnosed with osteopetrosis is scarce. Other important factors for the long-term success of the treatment were frequent professional oral hygiene maintenance, sustained patient cooperation, and annual prosthetic follow-ups with a re-examination of marginal bone height and checking for signs of inflammation. The results from the three-year follow-up support this strategy. We recommend rehabilitation of patients with osteopetrosis or conditions with persistent sequelae from the osteopetrosis diagnosis should be planned and treated by a team that includes several.
10. Ethics Declarations
Ethics approval and consent to participate. The patient gave permission to publish the medical history, clinical photos, and radiographs, all documented in his medical chart. This case report was conducted in accordance with the Declaration of Helsinki regarding medical protocol and ethics. Consent for publication. Informed consent was obtained from the patient's parents/legal guardian for publication of this case report.
Limitations: As the evidence identified by the author is of limited quality.
Author Contributions: All authors contributed equally.
Conflict of Interests: No conflict of interests were disclosed.
Grant Information: The authors declared that no grants were.
References
- Albers-Schönberg. Rontgenbilder einer seltenen Knockenerkrankung, Munch Med Wochenschr 5 (1904): 365-368.
- Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med 351 (2004): 2839-2849.
- Albers-Schonberg. Rontgenbilder einer seltenen Knockenerkrankung. Munch Med Wochenschr 5 (1904): 365-368.
- Shapiro F. Osteopetrosis: current clinical considerations. Clin Orthop Relat Res 294 (1993): 34-44.
- Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis 4 (2009).
- Charoenngam N, Nasr A, Shirvani A, et al. Hereditary metabolic bone diseases: a review of pathogenesis, diagnosis and management. Genes (Basel) 13 (2022): 1880.
- Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis 4 (2009): 5.
- Balasubramanian D, et al. Advances in the molecular diagnosis and treatment of osteopetrosis. Current Osteoporosis Reports 18 (2020): 143-157.
- Penna S, et al. Role of CLCN7 and TCIRG1 mutations in autosomal recessive osteopetrosis. Bone 125 ((2019)): 98-108.
- Teti A, et al. Osteopetrosis: A pathophysiological and clinical perspective. Nature Reviews Endocrinology 13 (2017): 100-118.
- Arruda M, Coelho MCA, Moraes AB, et al. Bone mineral density and microarchitecture in patients with autosomal dominant osteopetrosis: a report of two cases.J Bone Miner Res 31 (2016): 657-662.
- Campos-Xavier AB, Casanova J-L, Doumaz Y, et al. Intrafamilial phenotypic variability of osteopetrosis due to chloride channel 7 (CLCN7) mutations. Am J Med Genet A 133A (2005): 216-218.
- Landa J, Margolis N, Di Cesare P. Orthopaedic management of the patient with osteopetrosis. J Am Acad Orthop Surg 15 (2007): 654-662.
- Ross AC, Taylor CL, Yaktine AL, et al. 2011 DRI dietary reference intakes for calcium and vitamin D. Institute of Medicine (2011)
- Orchard PJ, Fasth AL, Le Rademacher J, et al. Hematopoietic stem cell transplantation for infantile osteopetrosis. Blood 126 (2015): 270-276.
- Ul Haq F, Amin S, Yunus H, et al. Diagnosis of Primary Hepatic Lymphoma in a 55-Year-Old Male Patient Presented with Pain in the Right Hypochondrium: A Very Rare Case. Cureus 14 (2022): e25547.
- Martinez C, Polgreen LE, DeFor TE, et al. Characterization and management of hypercalcemia following transplantation for osteopetrosis. Bone Marrow Transplant 45 (2010): 939-944.
- Dlouhy BJ, Menezes AH. Osteopetrosis with Chiari I malformation: presentation and surgical management. J Neurosurg Pediatr 7 (2011): 369-374.
- Sekerci AE, Sisman Y, Ertas ET, et al. Infantile malignant osteopetrosis: report of 2 cases with osteomyelitis of the jaws. J Dent Child (Chic) 79 (2012): 93-99.
- Pangrazio A, et al. RANK-dependent autosomal recessive osteopetrosis: characterization of five new cases with novel mutations. J Bone Miner Res 27 (2012): 342-351.
- Palagano E, Slatter MA, Uva P, et al. Hematopoietic stem cell transplantation corrects osteopetrosis in a child carrying a novel homozygous mutation in the FERMT3 gene. Bone 97 (2017): 126-9.
- Iida A, Xing W, Docx MK, et al. Identi Fi cation of Biallelic LRRK1 mutations in osteosclerotic metaphyseal dysplasia and evidence for locus heterogeneity. J Med Genet 53 (2016): 568-74.
- Haider I, Ullah H, Fatima M, et al. Tissue characterization of benign cardiac tumors by cardiac magnetic resonance imaging, a review of core imaging protocol and benign cardiac tumors. Frontiers in Cardiovascular Medicine 10 (2023).
- Lu SY, Li M, Lin YL. Mitf regulates osteoclastogenesis by modulating NFATc1 activity. Exp Cell Res 328 (2014): 32-43.
- George A, Zand DJ, Hufnagel RB, et al. Biallelic mutations in MITF cause coloboma, osteopetrosis, microphthalmia, macrocephaly, albinism, and deafness. Am J Human Genet 99 (2016): 1388-94.
- Haq F, Siraj A, Ameer M, et al. Comparative Review of Drugs Used in Diabetes Mellitus—New and Old. Journal of Diabetes Mellitus 11 (2021): 115-131.
- Rehman Z, Hamid M, Khan A, et al. Frequency Of Clinical Syndromes in Children with Congenital Heart Defects Admitted at Tertiary Care Hospital. Biological and Clinical Sciences Research Journal 2024 (2024) : 1026.


 Impact Factor: * 5.3
Impact Factor: * 5.3 Acceptance Rate: 73.64%
Acceptance Rate: 73.64%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 