Quantifying the Impact of Real-World Evidence: The Sacubitril/Valsartan Experience
Andrew J. Epstein, PhD MPP1, Morgan H. Persky1, Saif S. Rathore, MD PhD MPH2*, James L. Januzzi, MD3*
1Medicus Economics, Milton, Massachusetts, LLC, Milton, MA, USA
2Sandbar Life Sciences, Kankakee, Illinois, USA
3Baim Institute for Clinical Research, Boston, Massachusetts and Division of Cardiology, Massachusetts General Hospital, Boston, MA, USA
*Corresponding Author: Saif S. Rathore, Sandbar Life Sciences, Kankakee, Illinois, USA.
Received: 15 December 2025; Accepted: 17 December 2025; Published: 29 December 2025
Article Information
Citation: Andrew J Epstein, Morgan H Persky, Saif S Rathore, James L Januzzi. Quantifying the Impact of Real-World Evidence: The Sacubitril/ Valsartan Experience. Archives of Clinical and Biomedical Research. 9 (2025): 559-561.
View / Download Pdf Share at FacebookKeywords
<p>Sacubitril; Valsartan</p>
Article Details
Real-world evidence (RWE) can demonstrate safety, effectiveness, and value following a new therapy’s approval and launch [1]. The FDA has prioritized RWE programs to regulate approved therapies and products and has may expand approved indications based on RWE [2]. Recognizing RWE’s potential value, manufacturers, payers, and providers have invested ~$18B in RWE in 2024, with growth forecasted to increase 13% annually through 2032 [3]. Although publications of clinical trial results have been clearly linked to drug sales [4], the influence of RWE on the use of approved therapies is unknown. This is especially important for therapies with novel mechanisms of action in competitive therapeutic spaces.
We sought to assess the relationship between the cadence of RWE publications and subsequent sales of sacubitril/valsartan. As a first-in-class angiotensin receptor-neprilysin inhibitor approved for heart failure treatment, sacubitril/valsartan entered a therapeutic area with multiple established therapies. We evaluated overall drug sales following peer-reviewed RWE publications, including timing to and gain in sales following publication.
Publication data were derived from a PubMed search over 7/01/2015-3/31/2025 for English-language, human subjects’ studies with the drug name (Entresto® or sacubitril) and indication (heart failure) in the title or abstract. Studies without an abstract were excluded. Studies were classified as RWE-related if the abstract had at least one keyword from this list: “clinical,” patient,” “treatment,” “hospital,” “physician,” “visit,” and “admission,” and at least one keyword from this list: “real world,” “real-world,” “observation,” “cohort,” “practice,” “effectiveness,” “registry,” and “cost-effective.” Candidate abstracts were reviewed by an author (SSR) to confirm that they contained RWE as defined by the Food and Drug Administration: “clinical evidence regarding a medical product's use and potential benefits or risks derived from analysis of real-world data [5].”
Quarterly RWE publication data were combined with quarterly information on sacubitril/valsartan sales over the 39-quarter period spanning Q3 2015 through Q1 2025, inclusive. RWE publications were assigned to a calendar quarter based on their publication date and counted by quarter. Total quarterly sales were collected from Novartis’s US Securities and Exchange Commission [6] -K quarterly reports6 and inflated to 2025 USD using the US Bureau of Labor Statistics’ Producer Price Index for pharmaceutical manufacturing [7].
We estimated linear regression models of the quarter-to-quarter change in total sales as predicted by counts of previously published RWE-related studies. As it is unclear when RWE publications may influence sales (if at all), we examined unadjusted and adjusted associations between publication count in quarter q and three consecutive quarterly changes in future sales (q and q+1, q+1 and q+2, and q+2 and q+3 in separate models). To mitigate concerns of spurious correlation between simultaneously increasing trends in publications and sales, the adjusted models accounted for a linear time trend to control for any underlying trend in sales growth; the change in total sales from the quarter prior to publication to the quarter of publication (q-1 to q) to control for recent sales changes; and the cumulative count of RWE publications from Q3 2015 through the previous quarter (q-1) to control for the contribution of previous RWE publications to future sales. Standard errors were made robust to heteroskedasticity and autocorrelation for quarters q-1 through q+3 [8].
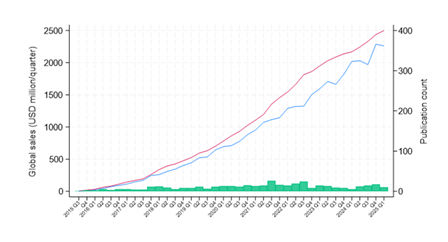
The PubMed search identified 1,048 studies; of these, 400 (38.2%) were classified as RWE-related. Mean quarterly publication count was 10.3 and ranged from 1 (Q3 2015) to 26 (Q3 2021). Total quarterly sales of sacubitril/valsartan increased steadily from $0 in Q3 2015 to $2.261B in Q1 2025; mean quarterly sales were $885M. The mean quarter-to-quarter change in sales was $60M and ranged from -$61M (Q3 2023) to $317M (Q1 2025) (Figure 1).
In the unadjusted models, an additional RWE-related publication was associated with an estimated mean increase in quarterly sales of $2.0M (from quarter q to q+1 following publication), $4.4M (from q+1 to q+2), and $2.2M (from q+2 to q+3). In adjusted models accounting for a secular trend in sales, the number of earlier RWE publications, and change in sales in the prior quarter, an additional RWE publication was associated with a mean gain in sales from quarter q+1 to q+2 of $2.3M (95% CI -$1.1M to $5.47). RWE publications were not associated with an increase in sales in the quarter following publication, and the association was attenuated by third quarter following publication (Table 1).
|
Change in sales from quarters q to q+1 |
Change in sales from quarters q+1 to q+2 |
Change in sales from quarters q+2 to q+3 |
||||
|
|
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|
Number of clinical pubs in quarter q |
||||||
|
Coefficient |
2.0 |
-2.3 |
4.4 |
2.3 |
2.2 |
1.5 |
|
95% confidence interval |
[-0.7, 4.8] |
[-5.6, -0.9] |
[2.5, 6.3] |
[-1.1, 5.7] |
[-1.7, 6.1] |
[-3.7, 6.7] |
|
Sample size (quarters) |
37 |
37 |
36 |
36 |
35 |
35 |
Table 1: Impact of RWE publication and subsequent change in sacubitril/valsartan sales.
Notes:
Outcome is measured in 2025 US dollars.
Adjusted models control for a linear time trend, the change in sales from quarters q-1 to q, and the cumulative count of RWE publications through quarter q-1.
Linear regression models used Newey-West heteroskedasticity and autocorrelation consistent standard errors (up to lag 4)
In the 8 years following approval of sacubitril/valsartan, publication of RWE studies was associated with sales growth 2 quarters later, with each extra publication associated with an extra $2.3M in revenue after accounting for other factors. In response to documented challenges achieving goals of guideline-directed medical therapy in heart failure [9], our study suggests continued RWE generation may be helpful in increasing use of newer therapies.
Our study has limitations. While our study cannot demonstrate a true causal relationship, we believe it does provide suggestive evidence to support the value of RWE generation. These findings are limited to a single therapy and may not generalize to other products. Our analysis was constructed to assess the average impact across a series of publications and does not quantify the total impact of a publication on lifetime sales, which would require more data and complex analysis. Moreover, our data do not disentangle how RWE publication leads to more sales, including through any professional or commercial efforts that might have contributed to medication use. Lastly, although this analysis provides average potential impact of each RWE publication, the effect will vary depending on the specific publications. Nonetheless, the results provide important insights to the potential impact of RWE.
Publication of RWE studies was associated with more use of sacubitril/valsartan following its introduction. As such, this analysis provides novel preliminary evidence of RWE’s ability to support therapy sales and thus adoption.
Acknowledment:
Dr. Januzzi has received research grants from Abbott Diagnostics, Applied Therapeutics, AstraZeneca, HeartFlow, and Novartis; he has received consulting fees/honorarium from Abbott, AstraZeneca, Beckman-Coulter, Boehringer-Ingelheim, Bristol-Myers, Intellia, Jana Care, Novartis, Pfizer, Merck, Roche Diagnostics and Siemens; He participates on data safety monitoring boards or endpoint committees for Abbott, AbbVie, Bayer, CVRx, Pfizer, Roche Diagnostics and Takeda and reports equity holdings in Imbria Pharmaceuticals and Jana Care.
References
- Sherman RE, Anderson SA, Dal Pan, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med 375 (2016): 2293.
- Purpura CA, Garry EM, Honig N. The role of real-world evidence in FDA-approved new drug and biologics license applications. Clin Pharmacol Ther 111 (2022): 135-144.
- Real word evidence solutions market size, share, and industry analysis. https://www.fortunebusinessinsights.com/real-world-evidence-solutions-market-107676, accessed September 20, 2024.
- Azoulay P. Do pharmaceutical sales respond to scientific evidence? Journal of Economics & Management Strategy 11 (2002): 551-94.
- Real-world evidence. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence, accessed September 20 (2024).
- Electronic data gathering, analysis, and retrieval systems (EDGAR). https://www.sec.gov/search-filings, accessed September 20 (2024).
- Producer price index by industry: pharmaceutical preparation manufacturing. https://fred.stlouisfed.org/series/PCU325412325412, accessed September 20 (2024).
- Newey WK, West KD. A simple, positive semi-definite, heteroskedasticity and autocorrelation consistent covariance matrix. Econometrica, 55 (1987): 703-8
- Shahid I, Khan MS, Fonarow GC, et al. Bridging gaps and optimizing implementation of guideline-directed medical therapy for heart failure. Prog Cardiovasc Dis 82 (2024): 61-69.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 