Regenerative Protocol for Defects of the Plantar Fascia: A Case Series
Robert Parker DPM, FACFAS, FAENS1, John Shou2, Crislyn Woods2, Naomi Lambert2*, Tyler Barrett2
1Parker Foot and Ankle, Houston, TX, USA
2Regenative Labs, 1700 W Main St, Pensacola, FL 32502, USA
*Corresponding author: Naomi Lambert, Regenative Labs, 1700 W Main St, Pensacola, FL 32502, USA.
Received: 03 October 2025; Accepted: 10 October 2025; Published: 21 October 2025
Article Information
Citation: Robert Parker, John Shou, Crislyn Woods, Naomi Lambert, Tyler Barrett. Regenerative Protocol for Ligamentous Defects in Patients with Sinus Tarsitis: A Case Series. Archives of Clinical and Biomedical Research. 9 (2025): 424-429.
View / Download Pdf Share at FacebookAbstract
Foot pain related to the plantar fascia is common in adults regardless of athletic background. Many pathologies can lead to the diagnosis of plantar enthesopathy, but the most relevant to this case series is related to structural defects within the plantar fascia. Due to the variety and sometimes unclear etiology of plantar fasciosis, no single standard of care treatment protocol is recognized. Often, patients receive NSAIDs, night splints, taping, physical therapy, foot orthosis, and extracorporeal shock wave therapies. This study presents Wharton Jelly (WJ) tissue allografts as an additional intervention in a regenerative protocol for patients who fail standard-care treatments. WJ allografts are applied to supplement connective tissue defects directly, unlike other therapies, which aim to reduce swelling and symptomatic pain. This observational study includes seven patients who have previously failed standard-of-care treatment. Each received extracorporeal pulse-activated therapy (EPAT), WJ, class four laser therapy, and an orthotic. Patient progress was tracked using a visual analog scale, 0-10 scoring, reported by the patient at the initial visit and approximately thirteen weeks following, reporting an average improvement of 50% with significant differences in initial and final scores. The study's limitations include a small cohort size and a nonblinded observational design. These promising results provide evidence for a more extensive, randomized study to define dosage protocols further and confirm safety and efficacy.
Keywords
<p>Plantar fasciosis; Enthesopathies; Wharton’s jelly; Class four laser therapy; EPAT; Regenerative Medicine</p>
Article Details
1. Introduction
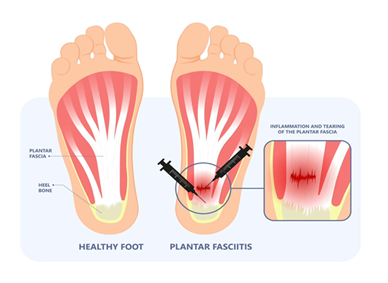
Nearly one in ten people will experience plantar fasciopathy in their lifetime [1]. Several diverse pathologies, including neurologic, arthritic, traumatic, neoplastic, infectious, or vascular, may lead to plantar enthesopathy [2]. Enthesopathy experienced in the plantar aspect of the foot may be Plantar Fasciosis (PF), a self-limiting condition often associated with chronic pain and tightness upon standing in the morning, and is exacerbated by long periods of standing or walking. A diagnosis is made based on patient history, risk factors, and physical examination. Risk factors often include excessive running, high arch, obesity, and a sedentary lifestyle [3]. A study by Rano [4] found that BMI (body mass index) plays a more significant role in plantar enthesopathy than foot structure [4]. Commonly associated with a high BMI are metabolic syndrome and diabetes, which complicates the diagnosis by adding compression of Baxter’s nerve, the first branch off of the lateral plantar nerve, and tarsal tunnel syndrome, mimicking fascial pain. Another leading factor in Plantar Fasciosis is defects in the collagen matrix within the plantar fascia. The body's most common protein is collagen, the main structural protein in connective tissues, including plantar fascia [5]. Micro and larger tears can occur in the plantar fascia band after prolonged strain or repetitive shocks, which, if the stress persists, can ultimately result in the inability of the body to repair itself naturally, causing irritation and inflammation.
Primarily due to the unclear etiology of plantar fasciosis, several standard-of-care treatments are available. Still, the most beneficial treatment can only be determined by accurate diagnosis with differentials such as nerve entrapments, micro tears, fibro-lipomas (frequently misdiagnosed as bursae), microfractures of the calcaneus, exostosis, enthesopathy, and systemic inflammatory disorders. Three categories exist in which standard, nonsurgical treatments are classified. Treatments are typically divided into reducing pain and inflammation, reducing tissue stress, and restoring muscle strength and flexibility of involved tissues [6]. Standard-of-care treatment options for plantar fasciosis often include corticosteroid injections, non-steroidal anti-inflammatory drugs (NSAIDs), night splints, taping, stretching, exercise, foot orthosis, and extracorporeal shock wave therapy [7]. Regarding pain treatment, a study by Nahin (2018) found that most patients use over-the-counter medications, such as NSAIDs, whereas approximately 40% of patients use prescription medications [8]. The number of PF cases has more than doubled from 2010 to 2018 and is likely to continue to increase, ultimately raising the annual economic burden of PF. Each year, nearly $600 is spent per person on NSAIDs alone. Including additional costs of standard treatment, the annual cost associated with PF is $284 million [9]. However, most PF studies follow patients up to less than one year. Given the chronic and relapsing nature of PF, more research needs to be conducted to understand the best treatment mode and better estimate the overall economic burden of PF [10]. If a patient has attempted standard care of treatment with no relief after 6 to 12 months, they may qualify for partial or complete plantar fasciotomy. Of the patients who have failed standard-of-care treatments and qualify for plantar fasciotomy, patients with no previous foot trauma and only unilateral symptoms attain the best results from an endoscopic plantar fascia release [11]. While surgical interventions have shown some success, the removal of greater than 40% of the plantar fascia may have detrimental effects on other ligamentous and bony structures in the foot [12,13]. Another study showed that during an open partial release of the plantar fascia, an increased risk of detrimental effects after surgery and a potential increase in pain, especially in the lateral column, there is a clear need for additional treatment options.
This study proposes using Wharton's Jelly (WJ) to supplement the damaged tissue and minimize the negative symptoms of plantar fasciosis, alongside non-surgical standard-of-care practices. Wharton's jelly contains collagen types I, III, and V and fibrous structures comparable to the extracellular matrices (ECM) of human articular cartilage, tendons, and dermal tissues [14]. WJ protects vessels in the umbilical cord from external forces. A recent study shows that when WJ is used, significant defects in the articular cartilage scaffold can be mitigated [15]. Individual regenerative therapies such as laser, light, and shockwave have also displayed beneficial outcomes for inflammation and pain in the plantar fascia. As there is a clear need for an alternative intervention for plantar enthesopathy, we present an observational analysis of a regenerative protocol including Wharton’s jelly allografts, shockwave therapy, and class IV lasers for defects in the plantar fascia.
2. Case Presentation Section
2.1. Materials and Methods
All methods complied with the FDA and American Association of Tissue Banks (AATB) standards. This study was conducted under an Institute of Regenerative and Cellular Medicine IRB-approved protocol (RL-UCT-001), and informed consent was obtained from the study participants. The Wharton’s jelly tissue allografts were processed and distributed by Regenative Labs. CryoText Plus is a minimally manipulated WJ allograft tissue product for homologous use only. The Wharton's jelly is aseptically dissociated from the rinsed umbilical cord. After dissociation, 50 mg of Wharton’s Jelly is suspended in approximately 1mL of sterile Sodium Chloride 0.9% solution (Regenative Labs, Pensacola, FL, USA). FDA guidelines specify minimal manipulation of tissue products, as human tissue products that are not combined with any article except saline and FDA-approved cryopreservatives. The regulations of the human cell and tissue product (HCT/P) require extensive testing to ensure that there are no clinical safety concerns for tissue products. The clinic purchased the allografts from Regenative Labs. Patient recruitment, allograft application, and patient tracking were performed at Parker Foot and Ankle.
2.2. Case Presentation
This study included seven consenting individuals who presented with either left or right plantar fasciosis. All individuals had previously exhausted standard-of-care treatment options. The study sample was 57% male and 43% female. The age of the sample ranged from 47 to 66 years old. Of the sample, five individuals received WJ to the defect site on their left foot, leaving the two remaining individuals to receive WJ to the defect on their right foot. Each individual received a single application of 1cc CryoTextPlus, class IV laser therapy, extracorporeal pulse-activated therapy (EPAT), and a pneumatic boot by Dr. Parker at his clinic, Parker Foot and Ankle, in Houston, Texas. All patients were prescribed optional medication to help combat discomfort. After the initial application, all individuals were assessed at a follow-up visit approximately 11 weeks later to evaluate pain improvement and to ensure no adverse side effects. This series aims to present improvements in patient-reported pain scales after the application of WJ to the site of tissue defect, including laser therapy, EPAT, and a pneumatic boot (Table 1).
|
Patient Number |
Gender |
Age |
Affected Foot |
|
1 |
Female |
47 |
left |
|
2 |
Male |
50 |
left |
|
3 |
Male |
50 |
right |
|
4 |
Female |
57 |
right |
|
5 |
Female |
64 |
left |
|
6 |
Male |
63 |
left |
|
7 |
Male |
66 |
left |
Table 1: Case presentation.
2.3. Patient Care Procedures
The regenerative-based protocol includes EPAT, applying a Wharton’s Jelly tissue allograft, class IV laser therapy, and a walking boot. The lower extremity was prepped and draped using the standard sterile technique. Before applying the tissue allograft, most patients received EPAT at 11 Hz, 3.0 bars, and 3231 to 3432 pulses to the affected tissue. One patient received EPAT at 11 Hz, 1.4 bars, and 3532 pulses according to the patient's tolerance. The WJ product used in this study was 1cc of CryoTextPlus, a minimally manipulated tissue allograft. While the patient received EPAT, CryoTextPlus was thawed slowly in a 35-degree bath per laboratory guidelines. The allograft was transplanted along the plantar medial origin of the plantar fascia throughout the inflamed tissues utilizing MyLab 15.0 MHz real-time diagnostic ultrasound guidance with a 4 cm transducer head (Figure 1). Further “needling” in a pin-cushion technique with a 22-gauge needle was performed to encourage neovascularization. At the end of the procedure, the patients received a prefabricated pneumatic boot that was examined for proper fitting. The patient was shown and instructed in detail on how to properly wear and care for the device, and demonstrate the ability to apply the device correctly and ambulate without distress. Six patients were prescribed acetaminophen, and one was prescribed hydrocodone for pain management. The patients received class IV laser treatments twice a week for two weeks. The patient's pain was determined using a visual analog scale (VAS), scoring numerically zero through ten at the initial visit and then again at an average of thirteen weeks after the start of care.
3. Results
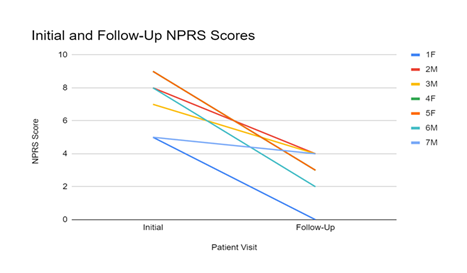
The sample's initial average VAS score was 6.88. With a 50% improvement, the final average VAS score was 3.44. When comparing gender improvement, females had an initial VAS of 7.66 and a final VAS of 2, which improved 74%. In comparison, males had an initial VAS of 7 and a final VAS of 3.5, improving by 50%. The wilcoxon signed rank test was used to determine statistical significance between initial and final scores (Table 2).
|
pre-post |
|
|
z |
-2.38 |
|
Asyp.sig. (2-tailed) |
0.017 |
|
r |
-0.636 |
Table 2: Test statistics for the Wilcoxon Signed Rank Test.
4. Discussion
The results show significant improvement in patient-reported pain relief after utilizing umbilical cord tissue allografts in combination with laser therapy, EPAT, and a pneumatic boot (Figure 2). The umbilical cord tissue allografts applied in a homologous fashion function as a scaffolding matrix to supply structural support to the damaged tissue. In the umbilical cord, Wharton's jelly provides structural support and cushioning against compressive forces to ensure the vessels it encases do not tear, stretch, or be subject to excess pressure. Components such as growth factors, cytokines, hyaluronic acid, and extracellular vesicles are found in WJ, contributing to WJ's regenerative effects [16]. The tissue allograft is minimally manipulated and immune-privileged, so when it is transplanted into the defect of tissue with the same basic function, it supplements the missing or damaged tissue without eliciting an immune response from the recipient.
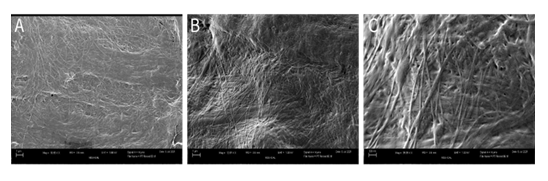
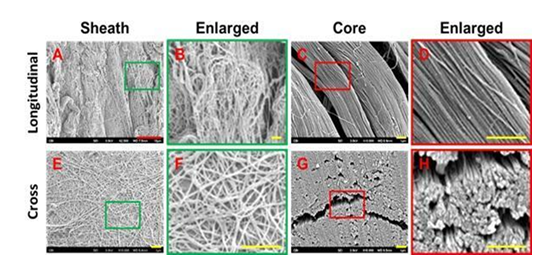
On a molecular level, the similarities in the structure of WJ and the plantar fascia allow for proper and effective supplementation of tissue. Scanning electron microscope (SEM) images of WJ tissue product's preserved collagen structure, compared to an SEM image analysis of healthy plantar fascia, reveal homologous crosslinked collagen structures (Figures 3,4) [17]. Plantar fascia and WJ are both primarily composed of type 1 collagen [16]. With structural similarity and makeup at a molecular level, the homologous implementation of WJ into damaged plantar fascia provides equivalent tissue for successful transplantation. SEM imaging of WJ tissue allografts showcases its ability to function as an architectural scaffold for ECM supplementation, not only in the fascia but in many other connective tissues around the body. When the tissue allograft is applied in a fanning technique, it is evenly distributed, allowing optimal coverage of the defective tissue and surrounding area. A pin-cushion technique was utilized to create micro-tears in the tendon, which sends messages to the body to excrete its own growth factors and cause neovascularization. New neovascularization accelerates the nitric oxide pathway, begins transcription, and helps with anti-inflammatory activity, which catalyzes the body's natural repair process.
Figure 4: Characterization of human PF tissue tested by Scanning Electron Microscope (SEM). A-D: Longitudinal tissue sections; E-H: Cross sections. SEM images show that human PF tissue has loose net-like mesh of sheath region outlined by a green box (A, B, E, F) and high-density collagen fiber bundles are found in core region outlined by a red box (C, D, G, H). The enlarged images of the sheath and core tissues show the diameter of collagen fibers in the sheath is thinner than that in the core tissues. Red bar: 10 mm; Yellow bars: 1 mm [17].
EPAT application before WJ injection functions as a modern physiotherapeutic method in musculoskeletal conditions [18]. While the exact mechanism is still debated in current literature, most agree that the acoustic waves create microtrauma to the tissue which elicits inflammatory reactions, initiating tissue pair processes and neovascularization [19]. A randomized placebo-controlled trial by Vahdatpour (2012) demonstrated safe, viable, and successful outcomes in addressing plantar fasiopathy utilizing EPAT as an independent treatment option [19]. Ultrasound imaging and significant reduction in patient pain scores compared to the placebo confirmed that EPAT can contribute to healing and pain reduction in fasiopathy [19]. No adverse reactions were reported within the study, further confirming EPAT as a safe, viable option in the treatment of musculoskeletal conditions.
Laser therapy provides photobiomodulation as a pain-reducing, anti-inflammatory, and tissue-improving modality. Class IV high-intensity laser therapy (HILT) decreases erythrocyte deformability and platelet coagulation, resulting in membrane revitalization, viscosity reduction, and erythrocyte stress adaptation [20]. With more efficient blood flow, the body's natural healing factors can be administered to the defective site quickly. High-power class IV laser therapy was used as a pain relief option for patients with oral mucositis [21]. The study reported an immediate decrease in pain after 94% of sessions, over 50% pain reduction in 61%, and complete elimination of initial pain in 35% of sessions. There were zero reports of increased pain following laser therapy. High-power laser therapy provides non-pharmacologic, patient-friendly, long-lasting, rapid pain relief [21]. Given the success of independent laser therapy, strong reasoning stands to utilize laser therapy in conjunction with other modalities to increase the success rate of treating plantar fasciosis.
The protocol's final component is a pneumatic walking boot. The function of the boot is to restrict and limit motion, provide stabilization, immobilize, and add compression to the affected area. Combining the application of WJ tissue allograft, EPAT, laser therapy, and boot application lays the foundation for a new, promising patient care protocol for plantar fasciosis. The results of this study provide data that suggest combining the four modalities improves the symptoms of plantar fasciosis. The 50% improvement in VAS scores reported in this study warrants further research with a larger cohort. The continuation of this research will include grading the thickness of the plantar fascia before and after the applications and additional follow-up visits at 30 days and 120 days post-care procedures with more specified lower extremity pain questionnaires.
5. Conclusions
In conclusion, the observational data obtained from the seven patients presenting with defects of the Plantar Fascia leading to Plantar Fasciosis reports WJ in combination with EPAT, laser therapy, and a pneumatic boot demonstrates statistically significant improvement in pain. The preliminary results presented in this study provide evidence of positive outcomes that should be confirmed with further research to compare this alternative protocol with the current non-surgical standard of care options. Future implications for the use of WJ in conjunction with standard care practices could significantly improve patient outcomes and potentially prevent or postpone invasive surgical procedures in many musculoskeletal defects.
Author Contributions:
Conceptualization, R.P., J.S. and T.B.; methodology, R.P.; software, T.Y.; validation, N.L., C.W. and T.Y.; formal analysis, N.L., C.W.; investigation, R.P.; resources, N.L., C.W.; data curation, R.P.; writing—original draft preparation, R.P., N.L., C.W.; writing—review and editing, R.P., N.L., C.W.; visualization, R.P., T.B.; supervision, J.S., T.B.; project administration, T.B.; funding acquisition, R.P., T.B. All authors have read and agreed to the published version of the manuscript.
Funding:
This research received no external funding.
Institutional Review Board Statement:
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Institute of Regenerative and Cellular Medicine (protocol code IRCM-2022-311 and approved on 12 January 2022).
Informed Consent Statement:
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement:
All relevant data in this study is reported within the manuscript.
Acknowledgements:
The authors would like to thank Stacy, Laura, and Greta at Parker Foot and Ankle for their contributions to data collection and filing.
Conflicts of Interest:
Regenerative Labs did not fund Dr.Parker or compensate him for doing this study. The research department of Regenative Labs worked on this publication along with Dr. Parker because he is a clinical site in the ongoing retrospective study backed by an IRB at the IRCM but does not receive any funding or payment from this research.
References
- Trojian T, Tucker AK. Plantar fasciitis. Am Fam Physician 99 (2019): 744-750.
- Thomas JL, Christensen JC, Kravitz SR, et al. The diagnosis and treatment of heel pain: a clinical practice guideline – revision 2010. J Foot Ankle Surg 49 (2010): S1-S19.
- Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician 84 (2011): 676-682.
- Rano JA, Fallat LM, Savoy-Moore RT. Correlation of heel pain with body mass index and other characteristics of heel pain. J Foot Ankle Surg 40 (2001): 351-356.
- Alabau-Dasi R, Nieto-Gil P, Ortega-Avila AB, et al. Variations in the thickness of the plantar fascia after training-based race: a pilot study. J Foot Ankle Surg 61 (2022): 1-6.
- Cornwall MW, McPoil TG. Plantar fasciitis: etiology and treatment. J Orthop Sports Phys Ther 29 (1999): 756-760.
- Heide M, Mørk M, Røe C, et al. The effectiveness of radial extracorporeal shock wave therapy, sham therapy, exercise programme, or usual care for patients with plantar fasciopathy: study protocol for a randomized trial. Trials 21 (2020): 589-595.
- Nahin RL. Prevalence and pharmaceutical treatment of plantar fasciitis in United States adults. J Pain 19 (2018): 885-896.
- Ahn J, Yeo J, Lee SH, et al. Healthcare usage and cost for plantar fasciitis: a retrospective analysis of national patient sample data. BMC Health Serv Res 23 (2023): 546-553.
- Rhim HC, Kwon J, Park J, et al. A systematic review of systematic reviews on the epidemiology, evaluation, and treatment of plantar fasciitis. Life (Basel) 11 (2021): 1287-1295.
- Hogan KA, Webb D, Shereff M. Endoscopic plantar fascia release. Foot Ankle Int 25 (2004): 875-881.
- Thompson J, Saini S, Reb C, et al. Diagnosis and management of plantar fasciitis. J Osteopath Med 114 (2014): 900-901.
- Brugh AM, Fallat LM, Savoy-Moore RT. Lateral column symptomatology following plantar fascial release: a prospective study. J Foot Ankle Surg 41 (2002): 365-371.
- Sobolewski K, Bańkowski E, Chyczewski L, et al. Collagen and glycosaminoglycans of Wharton’s jelly. Biol Neonate 71 (1997): 11-21.
- Davis JM, Sheinkop MB, Barrett TC. Evaluation of the efficacy of cryopreserved human umbilical cord tissue allografts to augment functional and pain outcomes in knee osteoarthritis: an observational study. Physiologia 2 (2022): 109-120.
- Gupta A, Maffulli N, Rodriguez HC, et al. Safety and efficacy of umbilical cord-derived Wharton’s jelly compared to hyaluronic acid and saline for knee osteoarthritis: study protocol for a randomized controlled trial. J Orthop Surg Res 16 (2021): 352-358.
- Zhang J, Yang Q. Research on plantar fasciitis. J Stem Cells Res Dev Ther 6 (2020): 1-8.
- Rajfur K, Rajfur J, Matusz T, et al. Efficacy of focused extracorporeal shock wave therapy in chronic low back pain: a randomized 3-month follow-up study. Med Sci Monit 28 (2022): e936614.
- Vahdatpour B, Sajadieh S, Bateni V, et al. Extracorporeal shock wave therapy in patients with plantar fasciitis: a randomized, placebo-controlled trial with ultrasonographic and subjective outcome assessments. J Res Med Sci 17 (2012): 834-838.
- Brandl A, Egner C, Reisser U, et al. Influence of high-energy laser therapy to the patellar tendon on its ligamentous microcirculation: an experimental intervention study. PLoS One 18 (2023): e0275883.
- Finfter O, Cohen R, Hanut A, et al. High-power laser photobiomodulation therapy for immediate pain relief of refractory oral mucositis. Oral Dis 30 (2023): 1-8.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 