SAA and lipid metabolism in IPF
Lucia Vietri1*, Miriana d’Alessandro2, Paolo Casolari1, Andrea Lo Monaco3, Mariano Reginato1, Irene Marchi1, Ilaria Gatti1, Aldo Carnevale4, Ludovica Rizzo1, Laura Bergantini2, Sara Gangi2, Paolo Cameli2, Alberto Papi1, Elena Bargagli2
1Research Centre on Asthma and COPD, University Hospital St. Anna - Ferrara, Italy
2Respiratory Diseases and Lung Transplantation Unit, Department of Medical and Surgical Sciences and Neurosciences, University of Siena, Italy
3Rheumatology Unit, Department of Medical Sciences, University Hospital St. Anna - Ferrara, Italy
4 Radiology Unit, Radiology Department, University Hospital St. Anna - Ferrara, Italy
*Corresponding author: Lucia Vietri, Research Centre on Asthma and COPD, University Hospital St. Anna - Ferrara, Italy.
Received: 16 March 2025; Accepted: 24 March 2025; Published: 31 March 2025
Article Information
Citation: Lucia Vietri, Miriana d’Alessandro, Paolo Casolari, Andrea Lo Monaco, Mariano Reginato, Irene Marchi, Ilaria Gatti, Aldo Carnevale, Ludovica Rizzo, Laura Bergantini, Sara Gangi, Paolo Cameli, Alberto Papi, Elena Bargagli. SAA and lipid metabolism in IPF. Archives of Clinical and Biomedical Research 9 (2025): 242-254.
View / Download Pdf Share at FacebookAbstract
Lipid metabolism has been demonstrated altered in different interstitial lung diseases (ILDs) including idiopathic pulmonary fibrosis (IPF); it can influence the pathogenesis of fibrotic lung disorders. Serum amyloid A (SAA) is an acute phase protein mainly produced by the liver in response to proinflammatory cytokines. Our study compared SAA serum levels in IPF patients to other ILD groups, to explore its potential use as a clinical biomarker. We enrolled 168 patients (40 stable IPF, 8 IPF on acute exacerbation, 30 sarcoidosis, 30 chronic HP, 17 PLCH and LAM, 6 NSIP, 9 UIP-non IPF, 16 SSc-ILD, 6 COPD, 6 other-ILDs, 17 healthy controls), monitored at Siena Regional Centre for ILDs and Saint Anna Hospital at University of Ferrara. IPF patients had higher SAA levels than healthy controls and other patients. We found a cut-off value of SAA (48.84 mcg/ml) which differentiate stable IPF from AE-IPF group. IPF patients have significantly higher SAA serum levels than SSc-ILD with a progressive fibrosis (PPF), indicating that a patient with a progressive fibrosis with serum SAA levels above that cutoff (45,21 mcg/ml) may help to discriminate IPF from other PPFs. ROC curve analysis of SAA levels discriminated cases of IPF from other ILDs, providing evidence of its specificity. SAA is a potential specific biomarker for IPF that can predict clinical course and prognosis of patients. SAA could discriminate an inflammatory ILD from a IPF. SAA could become a potential target for IPF treatment, including via apolipoproteins.
Keywords
<p>IPF; SAA; Systemic sclerosis; Lipid metabolism; HDL; Pulmonary fibrosis</p>
Article Details
Abbreviations: HP: Hypersensitivity Pneumonia; PLCH: Pulmonary Langerhans Cell Hystiocytosis; LAM: Lymphangioleiomyomatosis; NSIP: Non Specific Interstital Pneumonia; Ssc-ILD: Interstitial Lung Disease Associated To Systemic Sclerosis; COPD: Chronic Obstructive Pulmonary Disease; Other-Ilds: Pleuroparenchymal Fibroelastosis and Diffuse Pulmonary Ossification.
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive interstitial lung disease (ILD) of unknown cause that occurs predominantly in adult males and is limited to the lung [1]. This disease is characterized by a progressive functional worsening. The median survival from diagnosis is about 3-5 years, but the natural history of the disease is variable from patient to patient and for this reason the prognosis is relatively unpredictable [2]. Serum amyloid A (SAA) is an acute phase protein (apolipoprotein) mainly produced by the liver in response to proinflammatory cytokines from activated monocytes [3]. Literature data reported the influence of lipid metabolism in the development of inflammatory and fibrotic lung disorders [4-7]. The physiological role of SAA is still unclear, although it has been involved in acute-phase response control and/or propagation, cell communication and many inflammatory/immune responses, such as granuloma formation and carcinogenesis [4]. SAA was investigated in different biological fluids for its potential as a biomarker of many inflammatory lung diseases such as sarcoidosis, obstructive lung diseases (chronic obstructive pulmonary disease, asthma), obstructive sleep apnea and lung cancer [8,9]. Elevated concentrations of SAA were associated with progressive systemic sclerosis (SSc) characterized by severe skin thickening and poor five-year survival [5,6]. Serum SAA levels were correlated with lung involvement in SSc-ILD patients and prognosis: they were inversely correlated with DLCO rates, suggesting that SAA may be a biomarker of interstitial lung involvement in SSc-ILD patients [6]. Veltkamp et al recently observed higher SAA concentrations in fibrotic sarcoidosis patients, together with increased SAA levels in IPF patients, suggesting that SAA may also be reflective of fibrogenesis in addition to granulomatous processes [10]. It is important for further studies to clarify the exact role of SAA in fibrosis and to determine the underlying mechanisms. Our group of researchers already analyzed SAA concentrations to definite the potential value of such protein as a biomarker of fibrosis and its specificity in IPF [11].
The aim of the present study was to evaluate SAA concentrations in an IPF cohort compared with patients with other ILDs, to definite the potential value of this protein as a biomarker of fibrosis and its specificity in IPF. Our aim is to demonstrate the role of the lipid metabolism in the fibrotic process, especially the role of the apolipoprotein SAA as a biomarker of IPF that can predict clinical course, prognosis and survival of IPF patients.
2. Materials and Methods
2.1 Study population
A total of 168 (mean age ± standard deviation, 65 ± 23.4) patients followed at Regional Reference Center for Sarcoidosis and other Interstitial Lung Disease of Siena University and the Respiratory Department of the Saint Anna Hospital of Ferrara University were consecutively enrolled in the study. A multidisciplinary discussion confirmed the diagnosis of stable IPF in 40 patients (32 males, 12 former smokers; mean age 73,32 ± 6,25 years), 8 acute exacerbation (AE)-IPF (6 males, 8 former smokers; mean age 69,38 ± 7,90 years), 30 fibrotic HP patients (18 males, 16 former smokers; mean age 66,72 ± 8,44 years), 6 non-specific interstitial pneumonia (NSIP) (0 males, 2 former smokers; mean age 66 ± 12,09 years) and 17 cystic lung diseases (11 Pulmonary Langerhans cell Histiocytosis (PLCH) and 6 patients with Lymphangioleyomiomatosis (LAM) (3 males, 7 former smokers, mean age 50,94 ± 12,08 years) according to the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines [12,13]. Thirty patients (6 males, 2 former smokers; mean age 59,27 ± 11,35 years) were diagnosed as having sarcoidosis according to international criteria based on clinical signs, chest radiography findings and were confirmed through multidisciplinary evaluation as well. Nine patients had radiological UIP pattern (non-IPF) (7 males, 7 former smokers, mean age 78 ± 5,61 years). According to international criteria, sixteen patients had a diagnosis of Systemic sclerosis associated with ILD (SSc-ILD) (0 males, 4 former smokers; mean age 65,25±10,35 years) where ILD diagnosis was made according to ATS/ERS guidelines. Six patients had acute exacerbation of COPD in pulmonary emphysema (4 males, 4 former smokers, 1 current smoker; mean age 69,8 ± 6,3 years) and 6 had other-ILD (3 patients affected by diffuse pulmonary ossification and 3 patients with pleuroparenchymal fibroelastosis; 3 males, 3 former smokers, mean age 74,4± 8,01 years). We excluded patients with significant hypercholesterolemia or those undergoing statin treatment, to avoid assessment bias.
Serum samples were further collected from 17 healthy controls younger than fibrotic patients (HC-Y) (7 males, 8 former smokers; mean age 43,35 ± 13,03 years). To obtain data with greater statistical validity, we have added a group of 17 healthy controls older than HC-Y group (HC-O: 10 males, never smokers; mean age 64,70 ± 9,49), with an age range similar to that of the fibrotic patients (mainly IPF), to make the data more homogeneous and less influenced by age. However, we would like to point out that it was quite challenging to identify healthy individuals, non-smokers and those not affected by cardiovascular diseases in the 50-80 age range (Table1). At the time of SAA sampling, patients and controls had been fasting for at least 6 hours. Blood samples were centrifuged at 1500g for 10 minutes and serum samples were stored at -80°C degrees until the analysis.
|
n° pts |
males |
former smoker |
age (years) |
|
|
IPF |
40 |
32 |
12 |
73,32 ± 6,25 |
|
AE-IPF |
8 |
6 |
8 |
69,38 ± 7,90 |
|
Sarcoidosis |
30 |
6 |
2 |
59,27 ± 11,35 |
|
fHP |
30 |
18 |
16 |
66,72 ± 8,44 |
|
PLCH + LAM |
11 + 6 |
3 |
7 |
50,94 ± 12,08 |
|
NSIP |
6 |
0 |
2 |
66 ± 12,09 |
|
UIP non IPF |
9 |
7 |
7 |
78 ± 5,61 |
|
SSc-ILD |
16 |
0 |
4 |
65,25 ± 10,35 |
|
COPD |
6 |
4 |
4 |
69,8 ± 6,3 |
|
Other ILD |
3 DPO + 3 PPFE |
3 |
3 |
74,4 ± 8,01 |
|
HC-Y |
17 |
7 |
8 |
43,35 ± 13,03 |
|
HC-O |
17 |
10 |
0 |
64,70 ± 9,49 |
IPF, AE-IPF, UIP-non IPF, emphysema/COPD and other-ILDs patients were predominantly males and older than the other groups of patients (over 65 years of age).
IPF=idiopathic pulmonary fibrosis; AE-IPF= acute exacerbation of IPF; fHP= fibrotic hypersensitivity pneumonia; PLCH= Pulmonary Langerhans cell Histiocytosis; LAM= Lymphangioleyomiomatosis; NSIP= non-specific interstitial pneumonia; UIP non IPF: RA (rheumatoid arthritis), IPAF (interstitial pneumonia with autoimmune features ANA 1:320 + Raynaud phenomenon); SSc-ILD= Systemic sclerosis associated with ILD; COPD=chronic obstructive pulmonary disease; Other ILD: DPO (diffuse pulmonary ossification), PPFE (pleuroparenchymal fibroelastosis); HC-Y= younger healthy controls; HC-O= older healthy controls.
Table 1: Demographics characteristics of the study population.
Pulmonary function tests (PFTs) included forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), forced expiratory volume in 1 second/vital capacity (Tiffenau index), intrathoracic gas volume, total lung capacity (TLC), residual volume, diffusing capacity of the lung for carbon monoxide (DLCO) and transfer coefficient of the lung for carbon monoxide (absolute values expressed in milliliters and as a percentage of predicted) [14]. All patients underwent clinical and functional follow-up with PFTs at 6-months intervals in accordance with our protocol. Patients with relevant cardiovascular comorbidities, on statin therapy or with ongoing cancer were excluded, potentially responsible for the increased SAA levels. All these patients underwent bronchoscopy for diagnostic reasons and bacterial, viral and tuberculosis infections were excluded. Comorbidities were also recorded for the entire population, with particular attention to: hypertension, hypercholesterolemia, ischemic heart disease, multi-district atherosclerosis (ATS), hepatic steatosis, mild OSAS, diabetes mellitus type II, atrial fibrillation, visceral obesity, bronchial asthma, gastro-esophageal reflux (GER).
Clinical, functional, radiological and immunological data were collected from all patients. Demographic and clinical data, including comorbidities, family history, lung function parameters and radiological features were obtained from the medical records and entered an electronic database for statistical analysis.
The study was approved by the ethics committee (C.E.A.V.S.E. approval number 180712) of University of Siena and by the ethics committee (Lung-Biomarkers protocol, CE: 303/2023/Oss/AOUFE) of University of Ferrara. All patients provided written informed consent prior to participating in the study.
2.2 SAA assay
The SAA assay was performed using a commercially available enzyme-linked immunosorbent assay kit (Human Invitrogen KHA0011) in accordance with the manufacturer's instructions [15]. The microplate wells were coated with a highly purified monoclonal antibody specific for Human SAA. During the first incubation, standards with known SAA contents, controls and samples were pipetted into the coated wells, followed by the addition of a second biotinylated monoclonal antibody. After washing, the enzyme Streptavidin-Peroxidase was added; the latter, by binding to the biotinylated antibody, completed the characteristic sandwich of the ELISA method. Subsequently to a second incubation and a second washing (necessary to remove the unbound enzyme) a solution was added, so as to be welcomed by the bound enzyme to produce color. The intensity of the resulting coloration was considered directly proportional to the concentration of Human SAA present in the analyzed samples.
2.3 Statistical analysis
Results were expressed as means and standard deviations. Since the data were not normally distributed, Kruskal-Wallis one-way analysis of variance and Dunn test were used for multiple comparisons. The Mann-Whitney test was used for pairwise comparison of variables. Areas under receiver operating characteristic (ROC) curves were assessed and Youden's index was used to obtain cut-off values with the best sensitivity and specificity.
The Spearman test was used to look for correlations between variables. A P-value less than 0.05 was considered statistically significant. Statistical analysis and graphic representation of the data were performed by GraphPad Prism 9.0 software.
3. Results
Significant differences in gender and smoking habits were observed between patient groups. Considering patients affected by interstitial lung diseases, in the groups IPF, AE-IPF, UIP-non IPF, emphysema/COPD and other-ILDs patients were predominantly males and older than the other groups of patients (over 65 years of age). The main radiological patterns divided according to diagnosis were reported in Table s1. All IPF patients showed a UIP radiological pattern (25 UIP definite and 15 UIP probable), while 5 and 11 SSc-ILD patients had UIP and NSIP patterns, respectively, 2 HP patients had UIP definite pattern.
In the UIP-non IPF group 7 patients with rheumatoid arthritis (RA) had a UIP pattern (5 UIP definite, 2 UIP probable). Half of sarcoidosis patients had stage II, 23% stage III and 10% stage IV. The incidence of comorbidities in each group of patients was reported in Table s2. All patients affected by emphysema (with a definite diagnosis of COPD) showed hypertension as well as 50% of IPF, 55% of UIP non-IPF and 83% of other ILD patients.
Ischemic heart disease was in 40% of IPF patients and 50% of pulmonary emphysema. GER was in 75% of SSc-ILD, 20% of IPF and 33% of NSIP patients.
The functional worsening of the study population is described in Table s3. The decline of FVC and DLCO percentage in each group of fibrotic patients and sarcoidosis group is illustrated in Figure s1. The graphical representation shows how, especially in the IPF group, there was no serious functional worsening over the years.
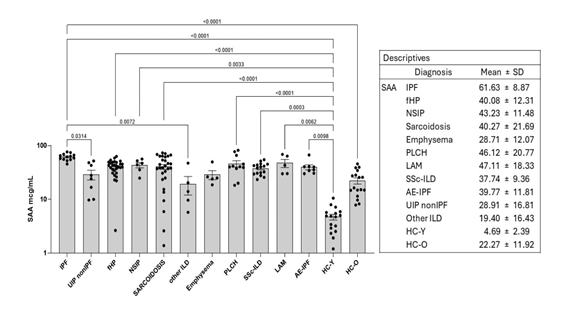
SAA serum concentrations were reported in Figure 1. IPF patients have significantly higher SAA serum levels than the other ILDs, mainly IPF versus UIP-non IPF (P=0.0155), IPF versus fHP (P=0.0003), IPF versus SSc-ILD (P=0.0009). Statistically significant difference between SAA serum levels in the IPF group vs healthy controls (P <0.0002). Comparative analysis showed statistically significant differences between IPF and UIP non IPF (P=0.0314), IPF and other ILD (P=0.0072). All patients had higher SAA concentrations than HC-Y group (P <0.05), moreover IPF had higher SAA concentrations than HC-O (P <0.0001).
IPF=idiopathic pulmonary fibrosis; AE-IPF= acute exacerbation of IPF; fHP= fibrotic hypersensitivity pneumonia; PLCH= Pulmonary Langerhans cell Histiocytosis; LAM= Lymphangioleyomiomatosis; NSIP= non-specific interstitial pneumonia; UIP non IPF: RA (rheumatoid arthritis), IPAF (interstitial pneumonia with autoimmune features ANA 1:320 + Raynaud phenomenon); SSc-ILD= Systemic sclerosis associated with ILD; COPD=chronic obstructive pulmonary disease; Other ILD: DPO (diffuse pulmonary ossification), PPFE (pleuroparenchymal fibroelastosis); HC-Y= younger healthy controls; HC-O= older healthy controls; SAA= serum amyloid A.
IPF patients have significantly higher SAA serum levels than the other ILDs, mainly IPF versus UIP-non IPF (P=0.0155), IPF versus fHP (P=0.0003), IPF versus SSc-ILD (P=0.0009). Statistically significant difference between SAA serum levels in the IPF group vs healthy controls (P <0.0002). All patients had higher SAA concentrations than HC-Y group (P <0.05), moreover IPF had higher SAA concentrations than HC-O (P <0.0001).
ROC curve analysis (Table 2) distinguished diseases groups according to cut-off values of serum concentrations of SAA as well as diseases vs HC.
|
ROC of IPF vs |
AUC |
P values |
Cut-off |
Sensitivity % |
Specificity % |
|
UIP-nonIPF |
98.1 |
0.0003 |
46.73 |
75 |
92.31 |
|
fHP |
94.7 |
<0.0001 |
45.28 |
68.97 |
100 |
|
NSIP |
92.3 |
0.0038 |
56.83 |
100 |
69.23 |
|
SARCOIDOSIS |
79.31 |
0.0026 |
54.36 |
72.41 |
84.62 |
|
OTHER ILD |
98.5 |
0.0019 |
49.73 |
100 |
92.31 |
|
EMPHYSEMA |
98.5 |
0.0019 |
51 |
100 |
92.31 |
|
SSc-ILD |
97.6 |
<0.0001 |
45.21 |
81.25 |
100 |
|
PLCH |
74.8 |
0.0397 |
48.86 |
72.73 |
92.31 |
|
AE-IPF |
90.4 |
0.0024 |
48.84 |
87.5 |
92.31 |
|
ROC of HC-O vs |
|||||
|
IPF |
99.5 |
<0.0001 |
42.45 |
100 |
94.12 |
|
fHP |
84.99 |
<0.0001 |
35.18 |
72.31 |
82.35 |
|
NSIP |
88.24 |
0.0063 |
41.75 |
66.67 |
94.12 |
|
SARCOIDOSIS |
73.83 |
0.0075 |
35.76 |
65.52 |
82.35 |
|
PLCH |
86.63 |
0.0013 |
39.83 |
63.64 |
94.12 |
|
AE-IPF |
86.76 |
0.0036 |
34.91 |
62.5 |
82.35 |
|
ROC of HC-Y vs |
|||||
|
IPF |
100 |
<0.0001 |
9.25 |
100 |
94.12 |
|
UIP nonIPF |
98.53 |
0.0001 |
8.84 |
100 |
94.12 |
|
fHP |
97.36 |
<0.0001 |
9.25 |
96.55 |
94.12 |
|
NSIP |
100 |
0.0004 |
9.25 |
100 |
94.12 |
|
SARCOIDOSIS |
93.31 |
<0.0001 |
8.7 |
89.66 |
94.12 |
|
Other ILD |
94.12 |
0.0033 |
8.81 |
80 |
94.12 |
|
Emphysema |
100 |
0.0009 |
9.25 |
100 |
94.12 |
|
PLCH |
100 |
<0.0001 |
9.25 |
100 |
94.12 |
|
SSc-ILD |
100 |
<0.0001 |
9.25 |
100 |
94.12 |
|
LAM |
100 |
0.0009 |
9.25 |
100 |
94.12 |
|
AE-IPF |
100 |
<0.0001 |
9.25 |
100 |
94.12 |
|
ROC of other ILD vs |
|||||
|
fHP |
82.76 |
0.0209 |
21.28 |
90 |
96.55 |
|
NSIP |
86.7 |
0.0446 |
22.64 |
80 |
100 |
ROC curve analysis distinguished diseases groups according to cut-off values of serum concentrations of SAA as well as diseases vs healthy controls (HC). SAA cut-off values >45 mcg/mL identified IPF patients in accordance with P < 0.0001 through ROC curve analysis. HC group were identified according to SAA concentrations of < 5 mcg/mL. With low serum SAA concentrations < 6 mcg/ml we can discriminate healthy subjects from inflammatory pathological status. Instead, if the SAA values are very high with a cut-off > 50 mcg/ml, we can suspect a progressive fibrosis lung disease, probable IPF.
IPF=idiopathic pulmonary fibrosis; AE-IPF= acute exacerbation of IPF; fHP= fibrotic hypersensitivity pneumonia; PLCH= Pulmonary Langerhans cell Histiocytosis; LAM= Lymphangioleyomiomatosis; NSIP= non-specific interstitial pneumonia; UIP non IPF: RA (rheumatoid arthritis), IPAF (interstitial pneumonia with autoimmune features ANA 1:320 + Raynaud phenomenon); SSc-ILD= Systemic sclerosis associated with ILD; COPD=chronic obstructive pulmonary disease; Other ILD: DPO (diffuse pulmonary ossification), PPFE (pleuroparenchymal fibroelastosis); HC-Y= younger healthy controls; HC-O= older healthy controls.
Table 2: ROC-curve analysis according to SAA serum levels in the study population.
SAA cut-off values >45 mcg/mL identified IPF patients in accordance with P < 0.0001 through ROC curve analysis. HC group were identified according to SAA concentrations of < 5 mcg/mL.
Direct correlation was between “Age” and “SAA” (rho 0.28, P <0.0004) in IPF patients, while inverse correlation was found between DLCO percentages at T0 and age (rho -0,25, P <0,009). Inverse correlation was found between peripheral neutrophilia (in percentage) and DLCO in percentage at T0 (rho -0.25, P = 0.04).
We also found that for the same UIP radiological pattern, IPF patients have higher absolute levels of monocytes in peripheral blood (P <0.006) than UIP non-IPF patients.
4. Discussion
In 2019 we evaluated the serum levels of SAA in IPF patients, publishing for the first time a study that demonstrated a statistically significant difference in SAA concentrations between healthy controls and IPF patients (stable phase), and a direct correlation between SAA values and functional decline in FVC [16]. High serum SAA values correlated with worse survival, suggesting a prognostic role of this biomarker [11]. The current study aims to validate our previous results on a larger population of IPF patients in order to evaluate the specificity of SAA in IPF compared to other ILDs and better define its role as a possible prognostic biomarker of the disease. It is not clear how an acute phase protein involved in inflammatory processes can be overexpressed at a peripheral level in a fibrotic disease [17–19]. Probably, SAA is produced following profibrotic and hypoxic stimuli. The low partial pressure of oxygen would induce the activation of many mediators including acute phase proteins [20]. Our results show a clear statistically significant difference between SAA serum levels in the IPF group vs healthy controls (P <0.0002), which indicates that SAA is a disease marker. Furthermore, IPF patients have higher SAA serum levels than the other ILD groups with a statistically significant difference, which would indicate how SAA is a disease marker that allows discriminating the IPF from others lung fibrosis. Our data show that IPF patients have statistical significance higher SAA serum levels than SSc-ILD with a progressive fibrosis. According to our results, with low serum SAA concentrations < 6 mcg/ml we can discriminate healthy subjects from inflammatory pathological status. Instead, if the SAA values are very high with a cut-off > 50 mcg/ml, we can suspect a progressive fibrosis lung disease, probable IPF. Our findings allow to hypothesize SAA as a possible specific biomarker for IPF patients. Moreover, the results of our study showed higher SAA values in IPF patients in stable phase compared to those with acute exacerbation (AE-IPF), although with the limitation of the small sample of AE-IPF patients (due to the fact that our patients often present exacerbated phases of the disease which lead them to be hospitalized in hospitals other than our center, making it difficult to obtain serum samples for our laboratory) [16]. This data suggests the role of SAA as a biomarker of fibrosis in IPF rather than as an indicator of inflammation (since in the exacerbated phase were inflammation prevails over fibrosis). It almost seems that this data indicates that AE-IPF is not always determined by a true inflammatory state, at least not systemic, as we detect a decrease in serum SAA levels in the acute phase compared to the stable phase of IPF (unlike as demonstrated in the literature where in systemic inflammation from chronic intestinal diseases or in septic states there is an increase in serum SAA resulting from the inflammatory state). Increase of SAA serum levels in IPF vs other ILDs, in a statistically significant manner, would indicate that upstream of the entire pathway of its release there is not exactly an inflammatory stimulus but rather a profibrotic stimulus. SAA levels, as known in the literature, are not modified by ongoing steroid or immunosuppressive therapy. In our study many patients with pulmonary fibrosis with a prevalent inflammatory pattern or with acute exacerbation of the disease were taking high-dose steroids or immunosuppressive drugs at the time of SAA sampling. In line with literature, our IPF patients were older than the other groups [21]. Our statistically significant positive correlation between “age” and “SAA” confirm the correlation of IPF with age. Considering the “age factor”, a statistical analysis with ANOVA test confirms the well-known data from the literature that patients suffering from IPF are older than other lung fibrosis and patients suffering from interstitial lung diseases in which an inflammatory state prevails. It also shows that patients with a radiological UIP pattern non IPF are older than other ILDs. This data could be related to what reveals the statistical analysis with Spearman correlation, which shows a direct correlation (rho 0.28, P <0.0004) between “Age” and “SAA”. This indicates that in older patients (as IPF), we have higher serum SAA levels. However, the same correlation did not appear in the group of patients with fibrosis showing a radiological pattern consistent with UIP but not idiopathic, nor in the HP group where patients have roughly the same age as those with IPF. In the population of our healthy controls HC-O (aged > 50 years), compared to the HC-Y group (aged < 50 years), we found a higher serum concentration of SAA in the HC-O group, although still lower than that observed in IPF patients of the same age and/or sex. This finding further supports our hypothesis of SAA as a specific marker for IPF (regardless of the fact that its serum concentrations increase with aging) since, for the same age and sex, the serum concentration of SAA in IPF patients is three times higher. It would be interesting in this context to understand whether the age-related alteration of lipid metabolism is somehow involved in the process of fibrogenesis. Genome-wide association studies have found several lipid-related variants to be associated with human aging. For example, the epsilon 2 and epsilon 4 alleles of apolipoprotein-E are associated with extreme longevity and late-onset neurodegenerative disease, respectively. In humans, blood triglyceride levels tend to increase, while blood lysophosphatidylcholine levels tend to decrease with age. Specific sphingolipid and phospholipid blood profiles have also been shown to change with age and are associated with exceptional human longevity (we remember that SAA is an apolipoprotein). These data suggest that lipid-related interventions may improve human healthspan and that blood lipids likely represent a rich source of human aging biomarkers [34]. Our results confirm what is being studied in the literature about neutrophils involvement in the fibrotic process. In our patients the high peripheral neutrophilia is associated to a functional worsening of gas exchange (DLCO). We know from literature that SAA-HDL complex chemoattracts inflammatory cells and it can interfere with the lipoxin signaling pathway, which can increase the survival time of neutrophils and aggravate the degree of inflammation. The neutrophil elastase is involved in extracellular matrix turnover, as well as the proliferation of lung fibroblasts and myofibroblast differentiation. Some studies demonstrated that blood neutrophilia is associated with a decline in FVC and mortality in IPF; it is also associated to a progression to IPF in patient with a radiological UIP indeterminate pattern [22]. In our population older patients (as IPF) already present at T0 (time at diagnosis) a more compromised functional state, with a high neutrophilia with a negative predictive value. This result demonstrates the role of innate immunity in the pathogenesis of IPF. Innate immunity can be a target for IPF treatment, including via neutrophils. We know that pulmonary fibrosis is the result of a persistent alveolitis with abnormal deposition of connective tissue. The alveolitis consists of infiltration by inflammatory cells including eosinophils, which release cytokines and stimulate the proliferation, migration and activation of mesenchymal cells increasing matrix synthesis [23,24]. IPF patients have, instead, higher absolute levels of monocytes in peripheral blood than UIP non-IPF patients. We know that in the process of pulmonary fibrosis, monocytes are recruited into the lung in response to tissue injury and differentiate into long-lived macrophages producing TGF-β, metalloproteinasis (MMPs), eventually leading to fibroblast activation, myofibroblast differentiation and extracellular matrix (ECM) remodeling [25]. Peripheral blood monocyte counts has recently emerged as a promising and easily measurable prognostic biomarker in IPF patients, with several studies showing that a monocyte count > 0.60 x 10^9 cell/µL-1 is strongly associated with disease progression; a monocyte count > 0.95 x 10^9 cell/µL-1 indicating a very high risk for poor outcomes [26–28]. Our data about SAA in ILDs support the hypothesis that lipid metabolism is involved in the fibrotic process. We know that phospholipids and sphingolipids are the structural components of the membranes and regulate intra and intercellular signaling. Aberrations in these phospholipids may have a role in the development of lung fibrosis [29]. SAA is an apolipoprotein that can replace apolipoprotein A1 (apoA1) as the major apolipoprotein of HDL. Some studies demonstrated that apoA1 BALF levels were lower in IPF patients compared to healthy controls [30]. This apolipoprotein is produced by the liver and has many anti-inflammatory mechanisms, including inhibition of TNFα, IL-6, IL-8 release [31]. ApoA1 is the major apolipoprotein of HDL, the anti-inflammatory effect of HDL may be caused by the action of ApoA1. We know that SAA binds HDL (isoform3) cholesterol, reducing HDL levels in blood stream and the Apo1 protection from acute lung damage [32]. The reduction of apoA1 makes the SAA-HDL complex acquire a profibrotic role through the activation of the lipoxin pathway, which increases the survival of neutrophils and through neutrophil elastase promotes the proliferation of myofibroblasts and the deposition of extracellular matrix [33]. Despite the contribution of these results to our understanding of the diagnostic and prognostic role of SAA in IPF patients, our study has some limitations. First, it would be worthwhile validating these results in other fibrotic ILD by means of multicentric prospective studies. Second, a long-term follow-up need to be evaluated for establishing the changes of PFT parameters according to SAA measurements.
5. Conclusions
Our findings about SAA support the hypothesis of such protein as a potential specific biomarker for IPF that can predict clinical course and prognosis of patients. SAA could discriminate an inflammatory ILD from a pulmonary progressive fibrosis mainly for IPF (which is a progressive fibrosis by definition). Monitoring serum levels could be useful to identify patients with rapidly progressive IPF disease phenotype or at risk of acute exacerbation, in order to direct such patients to antifibrotic therapies and lung transplantation earlier. Moreover, it strengthens the hypothesis about the role of lipid metabolism in the fibrotic process. SAA may play a crucial role in the regulation of lipid metabolism and production of MMPs in IPF. May be SAA could become a potential target for IPF treatment, including via apolipoproteins.
6. Declarations
Supplementary Materials: Table s1. Radiological findings and therapy were reported in different disease groups. Table s2: Main comorbidities in the study population. Table s3: Functional worsening in the study population at T0 (time at diagnosis and starting antifibrotic treatment), T6 (6 months after diagnosis and starting antifibrotic treatment), T12 (12 months after diagnosis and starting antifibrotic treatment), T24 (24 months after diagnosis and starting antifibrotic treatment), T36 (36 months after diagnosis and starting antifibrotic treatment). Figure s1: Functional decline of FVC and DLCO percentage at T0 (time at diagnosis and starting antifibrotic treatment), T6 (6 months after diagnosis), T12 (12 months after diagnosis), T24 (24 months after diagnosis), T36 (36 months after diagnosis) in the study population.
7. Acknowledgments
Guarantor statement: Lucia Vietri takes the responsibility for the content of the manuscript, including the data and analysis.
Author Contributions: Conceptualization, Lucia Vietri and Miriana d'Alessandro; Data curation, Andrea Lo Monaco, Aldo Carnevale, Sara Gangi, Alberto Papi and Elena Bargagli; Formal analysis, Miriana d'Alessandro; Investigation, Miriana d'Alessandro, Paolo Casolari, Ludovica Rizzo, Irene Marchi, Ilaria Gatti, Andrea Lo Monaco, Mariano Reginato, Aldo Carnevale, Laura Bergantini, Sara Gangi, Paolo Cameli, Alberto Papi and Elena Bargagli; Methodology, Paolo Casolari and Andrea Lo Monaco; Resources, Alberto Papi and Elena Bargagli; Validation, Lucia Vietri, Irene Marchi, Ilaria Gatti, Andrea Lo Monaco, Mariano Reginato, Ludovica Rizzo, Aldo Carnevale, Laura Bergantini, Sara Gangi, Paolo Cameli, Alberto Papi and Elena Bargagli; Writing – original draft, Lucia Vietri, Miriana d'Alessandro, Paolo Casolari, Ludovica Rizzo, Irene Marchi, Ilaria Gatti, Andrea Lo Monaco, Mariano Reginato, Aldo Carnevale, Laura Bergantini, Sara Gangi, Paolo Cameli, Alberto Papi and Elena Bargagli.
Data Availability Statement: The data presented in this study are available on request from the corresponding author.
Conflicts of Interest: The authors have declared that no conflict of interest exists.
Funding: This research received no external funding.
References
- Vancheri C, Cottin V, Kreuter M, et al. Comorbidities and Management Implications. Sarcoidosis Vasc Diffuse Lung Dis 32 (2015): 17-23.
- Ley B, Collard HR, King TE. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 183 (2011): 431-440.
- Sack GH. Serum Amyloid A (SAA) Proteins. Subcell Biochem 94 (2020): 421-436.
- Bargagli E, Magi B, Olivieri C, et al. Analysis of Serum Amyloid A in Sarcoidosis Patients. Respir Med 105 (2011): 775-780.
- Brandwein SR, Medsger TA, Skinner M, et al. Serum Amyloid A Protein Concentration in Progressive Systemic Sclerosis (Scleroderma). Ann Rheum Dis 43 (1984): 586-589.
- Lakota K, Carns M, Podlusky S, et al. Serum Amyloid A Is a Marker for Pulmonary Involvement in Systemic Sclerosis. PLoS One 10 (2015): e0110820.
- O’Rielly S. Serum Amyloid A (SAA) as a Universal Lung Disease Biomarker. Internal Medicine Journal 51 (2021): 1195-1196.
- Lai Y, Li Y, Gao L. Serum Amyloid A Protein in Cancer Prognosis: A Meta-Analysis and Systematic Review. Transl Cancer Res 10 (2021): 2255-2264.
- Zhou J, Sheng J, Fan Y, et al. Association between Serum Amyloid A Levels and Cancers: A Systematic Review and Meta-Analysis. Postgrad Med J 94 (2018): 499-507.
- Beijer E, Roodenburg-Benschop C, Schimmelpennink MC, et al. Elevated Serum Amyloid a Levels Are Not Specific for Sarcoidosis but Associate with a Fibrotic Pulmonary Phenotype. Cells 10 (2021): 585.
- Vietri L, d’Alessandro M, Bergantini L, et al. Specificity of Serum Amyloid A as a Biomarker of Idiopathic Pulmonary Fibrosis. Intern Med J 50 (2020): 1571-1574.
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 198 (2018): e44-e68.
- Costabel U, Hunninghake GW. ATS/ERS/WASOG Statement on Sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J 14 (1999): 735-737.
- Culver BH, Graham BL, Coates AL, et al. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med 196 (2017): 1463-1472.
- SAA Human ELISA Kit - Invitrogen Available online: https://www.thermofisher.com/elisa/product/SAA-Human-ELISA-Kit/KHA0011
- Vietri L, Bennett D, Cameli P, et al. Serum Amyloid A in Patients with Idiopathic Pulmonary Fibrosis. Respir Investig 57 (2019): 430-434.
- Meng K, Zhang B, Ma C, et al. Serum Amyloid A/Anti-CCL20 Induced the Rebalance of Th17/Regulatory T Cells in SodA-Induced Sarcoidosis. Int Immunopharmacol 109 (2022): 108784.
- Huho A, Foulke L, Jennings T, et al. The Role of Serum Amyloid A Staining of Granulomatous Tissues for the Diagnosis of Sarcoidosis. Respir Med 126 (2017): 1-8.
- Chen ES, Song Z, Willett MH, et al. Serum Amyloid A Regulates Granulomatous Inflammation in Sarcoidosis through Toll-like Receptor-2. Am J Respir Crit Care Med 181 (2010): 360-373.
- Zhao D, Abbasi A, Rossiter HB, et al. Serum Amyloid A in Stable COPD Patients Is Associated with the Frequent Exacerbator Phenotype. Int J Chron Obstruct Pulmon Dis 15 (2020): 2379-2388.
- Wakwaya Y, Brown KK. Idiopathic Pulmonary Fibrosis: Epidemiology, Diagnosis and Outcomes. Am J Med Sci 357 (2019): 359-369.
- Jegal Y. The Role of Neutrophils in the Pathogenesis of IPF. Korean J Intern Med 37 (2022): 945-946.
- Gharaee-Kermani M, Phan SH. The Role of Eosinophils in Pulmonary Fibrosis (Review). Int J Mol Med 1 (1998): 43-53.
- Peterson MW, Monick M, Hunninghake GW. Prognostic Role of Eosinophils in Pulmonary Fibrosis. Chest 92 (1987): 51-56.
- DeLeon-Pennell KY, Barker TH, Lindsey ML. Fibroblasts: The Arbiters of Extracellular Matrix Remodeling. Matrix Biol 91–92 (2020): 1-7.
- Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. N Engl J Med 384 (2021): 325-334.
- Kreuter M, Lee JS, Tzouvelekis A, et al. Monocyte Count as a Prognostic Biomarker in Patients with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 204 (2021): 74-81.
- Scott MKD, Quinn K, Li Q, et al. Increased Monocyte Count as a Cellular Biomarker for Poor Outcomes in Fibrotic Diseases: A Retrospective, Multicentre Cohort Study. Lancet Respir Med 7 (2019): 497-508.
- Suryadevara V, Ramchandran R, Kamp DW, et al. Lipid Mediators Regulate Pulmonary Fibrosis: Potential Mechanisms and Signaling Pathways. Int J Mol Sci 21 (2020): 4257.
- Magi B, Bargagli E, Bini L, et al. Proteome Analysis of Bronchoalveolar Lavage in Lung Diseases. Proteomics 6 (2006): 6354-6369.
- Mangaraj M, Nanda R, Panda S. Apolipoprotein A-I: A Molecule of Diverse Function. Indian J Clin Biochem 31 (2016): 253-259.
- Webb NR. High-Density Lipoproteins and Serum Amyloid A (SAA). Curr Atheroscler Rep 23 (2021): 7.
- Kim TH, Lee YH, Kim KH, et al. Role of Lung Apolipoprotein A-I in Idiopathic Pulmonary Fibrosis: Antiinflammatory and Antifibrotic Effect on Experimental Lung Injury and Fibrosis. Am J Respir Crit Care Med 182 (2010): 633-642.
- Johnson Adiv A, Stolzing A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging cell 18 (2019): e13048.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 