Serum Beta- 2 Microglobulin is a Reliable Biomarker to Predict Diabetic Nephropathy
Maliha Kamal1*, Sheam Ahmed Apu2, Auni kamal3, Iftequar Alam4, Asu- Ma kamal5, A.K.M Shahidur Rahman6
1Clinical pathologist, Department of Pathology, National Institute of Cardio Vascular Diseases (NICVD), Dhaka, Bangladesh
2M.Phil Student (Physiology) Department of Physiology, Dhaka Medical College (DMC), Dhaka, Bangladesh
3Associate Professor (cc), Department of Anatomy, International Medical College, Tongi, Gajipur, Bangladesh
4Junior Consultant, Department of Cardiology, National Institute of Cardio Vascular Diseases (NICVD), Dhaka, Bangladesh
5MD Student (Cardiology), Department of Cardiology, Mymensingh Medical College Hospital (MMCH), Mymensingh, Bangladesh
6Medical Officer, Department of Nephrology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh
*Corresponding author: Dr. Maliha Kamal, Clinical pathologist, Department of Pathology, National Institute of Cardio Vascular Diseases (NICVD), Dhaka, Bangladesh
Received: 09 September 2021; Accepted: 16 September 2021; Published: 21 September 2021
Article Information
Citation: Kamal M, Apu SA, kamal A, Alam I, kamal AM and Rahman AKMS. Serum Beta- 2 Microglobulin is a Reliable Biomarker to Predict Diabetic Nephropathy. Archives of Clinical and Biomedical Research 5 (2021): 724-736.
View / Download Pdf Share at FacebookAbstract
Background: Diabetic nephropathy (DN) is the major microvascular complication and leading cause of chronic kidney disease (CKD) globally. Early detection of diabetic nephropathy is necessary. Objective: To evaluate the serum beta-2 (β2) microglobulin as a biomarker to predict diabetic nephropathy.
Methods: This study was conducted from March 2020 to February 2021 at Department of Laboratory Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh. A total of 60 patients with type-2 diabetes mellitus (DM) were enrolled purposively. Among them, 30 patients had diabetic nephropathy (group I) and 30 were without diabetic nephropathy (group II). Their random blood sugar (RBS), serum creatinine, estimated glomerular filtration rate (e-GFR), serum beta- 2 (β2) microglobulin and urinary microalbumin levels were measured. Data were analyzed and compared by statistical tests.
Results: Most of the patients in nephropathy group were male who belonged to 51 - 60 years age group and their mean e-GFR was significantly decreased (p<0.001). The mean serum β2 microglobulin level was significantly higher in patients with diabetic nephropathy (6.72 ± 2.032 μg/ml versus 3.44 ± 1.12 μg/ml, p<0.001). There was significant positive correlation between serum β2 microglobulin with serum creatinine (r=+0.549, p=0.002) and with urinary microalbumin (r=0.755, p<0.001), but a significant negative correlation with e-GFR (r=-0.627, p<0.001) in group I. The best cut-off point of serum β2 microglobulin for diabetic nephropathy was 4.35 μg/ml with 93.3% sensitivity and 80.0% specificity.
Conclusions: Serum beta-2 (β2) microglobulin level is significantly high in diabetic nephropathy. Serum β2 microglobulin may be used as a reliable biomarker to predict diabetic nephropathy.
Keywords
<p>Chronic Kidney Disease (CKD); Diabetes Mellitus (DM); Diabetic Nephropathy (DN); Serum Beta 2 (β2) Microglobulin</p>
Article Details
1. Introduction
Diabetes mellitus (DM) is a group of metabolic disorders characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both [1]. Diabetes mellitus causes long term damage, dysfunction and failure of different organs especially the eyes, kidneys, heart, and blood vessels [1]. Diabetes mellitus is associated with both microvascular and macrovascular complications [1]. Microvascular complications of diabetes mellitus include- diabetic nephropathy (DN), retinopathy, neuropathy, peripheral vascular disease etc. Among them diabetic nephropathy (DN) is the major microvascular complication and the leading cause of chronic kidney disease (CKD) as well as end stage renal disease (ESRD) globally [2-4]. DN is characterized by elevated urine albumin excretion or reduces glomerular filtration rate (GFR) or both in diabetic patients [5]. On the basis of urinary albumin excretion diabetic nephropathy is categorized in to microalbuminuria (urinary albumin excretion rate, 20-200 μg/minutes or (30-300 mg/day) and macroa-lbuminuria (urinary albumin excretion rate ≥200 μg/minutes) [5]. Measurement of urinary albumin excretion is the gold standard for the diagnosis of diabetic nephropathy [6]. But urinary albumin excretion has some limitation to predict the early stages of nephropathy in diabetic individuals [7]. Moreover, urinary albumin excretion rate could be altered by blood pressure, exercise and intra-individual variability [8]. On the other hand, estimated glomerular filtration rate (e-GFR) is more accurate and useful than the serum concentrations of filtration markers [9]. In clinical practice e-GFR is the calculated equation of glomerular filtration rate by using age, sex and body weight of the individual [5]. Changes in glomerular filtration rate provide a valuable indicator for the progression of diabetic nephropathy [4]. For the assessment of glomerular filtration rate, serum creatinine is the most widely used index [8]. Despite its specificity serum creatinine demonstrates an inadequate sensitivity particularly in the early stage of renal impairment [8]. Creatinine is not an inert substance, which is secreted by the proximal tubule and its level is affected by other factors like- diet, exercise, hydration etc [8, 9]. Therefore, novel biomarkers are needed to identify the diabetic nephropathy at an early stage in patients who are at risk of developing chronic kidney disease (CKD) [10]. Measurement of various low molecular weight proteins has been proposed as a useful tool to evaluate the impairment of glomerular filtration rate [8-10]. Among them, serum β2 microglobulin has been suggested as a better marker of glomerular filtration rate than serum creatinine [11]. Serum β2 microglobulin is the light chain in the major histocompatibility complex (MHC) class I molecule [12]. It is widely distributed in all nucleated cells in the body [12, 13]. Under normal physiologic conditions, β2 microglobulinis produced at a constant rate and normal β2 microglobulin concentration is 1.5-3 mg/L [12-14]. Liabeuf et al. found an association between serum β2 microglobulin with different stages of CKD [14]. While, Inker et al. established that serum β2 microglobulin is a novel endogenous filtration marker and developed a glomerular filtration rate estimating equation by using serum β2 microglobulin [9]. Recently it was reported that serum β2 microglobulin is an early predictor of renal function [9, 15]. Serum β2 microglobulin exclusively eliminated by glomerular filtration and has been used to determine the estimated glomerular filtration rate (e-GFR) [9, 15, 16]. It was reported that, high level of serum β2 microglobulin has the higher prevalence of diabetic nephropathy compared with low level of β2 microglobulin with normal renal function [16]. Increase of serum β2 microglobulin found to be highly specific for diabetic nephropathy in contrast to other biomarkers; moreover it is not influenced by gender, muscle mass and drugs [8, 16, 17]. In this context, the aim of this study was to evaluate the serum β2 microglobulin as a biomarker to predict diabetic nephropathy.
2. Materials and Methods
This cross sectional study was conducted in the Department of Laboratory Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh from March 2020 to February 2021. The study was approved by the Ethical Review Committee, BSMMU, Dhaka, Bangladesh. A total of sixty (60) diabetic patients were selected by purposive sampling technique following selection criteria. According to American Diabetes Association (ADA) [1] adult type 2 diabetic patients (age ≥18 years) of both sexes with or without nephropathy were included in this study. Among them, 30 patients had type 2 diabetes mellitus with nephropathy (group I) and 30 patients were type 2 diabetes mellitus without nephropathy (group II). Patients with hypertension, urinary tract infection, leukemia, multiple myeloma, collagen diseases, viral disease and pregnant subjects were excluded from the study. Informed written consent was obtained from each study subjects prior to enrollment. Then relevant investigations of the study subjects like- urine for routine microscopic examination (R/M/E), random blood sugar (RBS), serum creatinine, serum β2 microglobulin and urinary microalbumin were measured accordingly. The estimated glomerular filtration rate (e-GFR) of each patient was calculated by Cockcoft-Gault (CG) formula [18]. Each patient’s body mass index (BMI) was estimated as weight (in kilograms)/height square (in meters). BMI was categoried according to the World Health Organization (WHO) Asia-Pacific guidelines [19].
2.1 Analysis of blood samples
About 6.0 ml venous blood from each study subject was collected into a plane test tube following standard procedure. Tube was labeled with the patient’s identification number and kept in a vertical position at room temperature (22°C - 24°C) for 30 minutes. Then blood was centrifuged at 3000 rpm/minutes in room temperature (22°C - 24°C) for 15 minutes. Serum was separated by micro-pipette and collected to appendrope, then preserved at −20°C until further analysis. The random plasma glucose was assessed by automated biochemistry analyzer on the principle of photometric technique, serum creatinine was measured by kinetic method and serum β2 microglobulin was assessed by ELISA method.
2.2 Analysis of urine samples
About 5.0 ml of urine was collected into a plastic tube. Tube was labeled with patient’s identification number. Urine for routine microscopic examination (R/M/E) was assessed by automated analyzer, recheck manually with light microscope. Urinary microalbumin rate was assessed by automated analyzer on the principle of immunoturbidimetric assay.
2.3 Statistical analysis
Data analysis was performed using the Statistical Package for Social Science (SPSS) software version- 26. Quantitative data were expressed as mean with standard deviation (±SD), while qualitative data were expressed as frequency and percentage. The statistics used to analyze the data was descriptive statistics. Chi-square test, unpaired student’s t-test, Pearson’s correlation test and Receiver operator characteristic curve (ROC) were used to analysis the data. A p value <0.05 was considered as statistically significant.
3. Results and Observations
This cross-sectional study was carried out to explore the association of serum β2 microglobulin with diabetic nephropathy. A total of sixty (60) adult diabetic patients were enrolled. Among them, 30 patients were type-2 diabetes mellitus with nephropathy (group I) and 30 patients were type 2 diabetes mellitus without nephropathy (group II). Both groups were selected according to clinical history and laboratory findings.
Table 1 shows the age distribution of the study subjects. It was observed that, age group of 51-60 years had highest percentage of nephropathy patients 9(30.0%) and 31-40 years of age group had highest percentage of without nephropathy patients 13(43.3%).
The mean (±SD) age was 50.5 ± 11.71 years in group I and 45.53 ± 9.97 years in group II. The age difference was not significant between the groups (p=0.082).
|
Age (years) |
Group I (n=30) |
Group II (n=30) |
p value |
|
|
n (%) |
n (%) |
|
|
31-40 |
7 (23.3) |
13 (43.3) |
- |
|
41-50 |
8 (26.7) |
11 (36.7) |
- |
|
51-60 |
9 (30.0) |
3 (10.0) |
- |
|
60-70 |
6 (20.0) |
3 (10.0) |
0.082ns |
|
Mean±SD |
50.5 ± 11.71 |
45.53 ± 9.97 |
- |
|
Range (minimum to maximum) |
30 – 70 |
30 – 70 |
- |
p value reached from Unpaired t-test; ns=not significant
Group I =Type 2 Diabetes mellitus patients with nephropathy
Group II=Type 2 Diabetes mellitus patients without nephropathy
Table 1: Age distribution of the study subjects (N=60).
Table 2 shows the gender distribution of the study subjects. It was found that 20(66.7%) were male patient and 10(33.3%) were female patient in group I. While in group II, 13(43.3%) were male and 17(56.7%) were female. Male patients were predominant in group I but females were predominant in group II. The gender difference was not significant in the groups (p=0.069).
|
Gender |
Group I (n=30) |
Group II (n=30) |
p value |
|
|
n (%) |
n (%) |
|
|
Male |
20 (66.7) |
13 (43.3) |
- |
|
Female |
10 (33.3) |
17 (56.7) |
.069ns |
p value reached from Chi-square test; ns =not significant
Table 2: Gender distribution of the study subjects (N=60).
Data analysis showed that; 1(3.3%) subject in group-I and 5(16.7%) subjects in group-II were within normal weight, 14(46.7%) subjects in group-I and 12(40.0%) subjects in group-II were overweight, While 15(50.0%) subjects in group-I and 13(43.3%) subjects in group-II were obese. The mean (±SD) body mass index (BMI) was 30.1 ± 43.72 kg/m2 in group I and 28.9 ± 4.65 kg/m2 in group II. The body mass index (BMI) was not significantly different between the groups (p=0.256) (Table 3).
|
Body Mass Index (BMI*) categories |
Group I (n=30) |
Group II (n=30) |
p value |
|
|
n (%) |
n (%) |
|
|
Normal weight (19.0-24.99 kg/m2) |
1 (3.3) |
5 (16.7) |
- |
|
Overweight (25.0-29.9 kg/m2) |
14 (46.7) |
12 (40.0) |
- |
|
Obese (>30.0kg/m2) |
15 (50.0) |
13 (43.3) |
- |
|
Mean±SD Range (minimum to maximum) |
30.1 ± 3.72 21.5 – 38.8 |
28.9 ± 4.65 19.4 – 41.0 |
0.256ns |
p value reached from Unpaired t-test; ns = not significant, *BMI categories according to the World Health Organization (WHO) Asia-Pacific guidelines [19]
Table 3: Distribution of the study subjects by different body mass index (BMI) categories (N=60).
Table 4 shows comparison of different biochemical parameters of the study groups. It was found that mean (±SD) urinary microalbumin and serum creatinine levels were significantly high (p<0.001) but estimated glomerular filtration rate (e-GFR) was significantly low (p<0.001) in group I.
|
Biochemical parameters
|
Group I (n=30) Mean±SD |
Group II (n=30) Mean±SD |
p-value
|
|
Urinary microalbumin (mg/24-hour) |
580.87 ± 478.85 |
9.83 ± 7.46 |
<0.001s |
|
S. creatinine (mg/dl) |
2.51 ± 1.50 |
0.91 ± 0.32 |
<0.001s |
|
*e-GFR (ml/min) |
47.56 ± 35.48 |
93.75 ± 31.29 |
<0.001s |
p value reached from Unpaired t-test, s = significant, *e-GFR was calculated by Cockcoft-Gault (CG) formula [18]
Table 4: Comparison of different biochemical parameters of the study groups (N=60).
Table 5 shows comparison of serum β2 microglobulin level between two groups. It was observed that mean (±SD) serum β2 microglobulin level was significantly high (p<0.001) among patients with diabetic nephropathy (group I).
|
Variable |
Group I (n=30) |
Group II (n=30) |
p- value |
|
Serum β2 microglobulin (µg/ml) |
6.72 ± 2.03 |
3.44 ± 1.12 |
<0.001s |
p value reached from Unpaired t-test, s = significant
Table 5: Comparison of serum β2 microglobulin level between two groups (N=60).
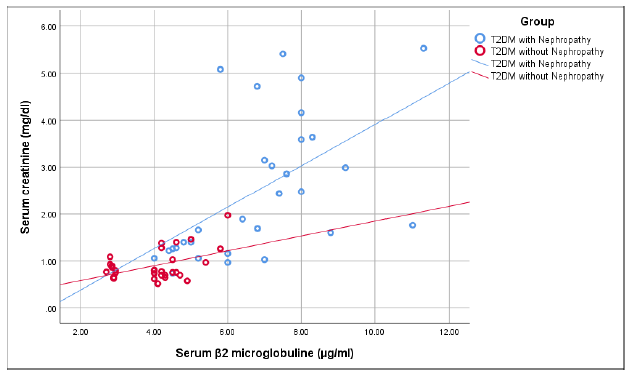
Figure 1 shows that there was a significant positive correlation of serum β2 microglobulin with serum creatinine (r=+0.549, p=0.002) in group I. But in group II, serum β2 microglobulin had weak positive correlation with serum creatinine which was not statistically significant (r=+0.349, p=0.063).
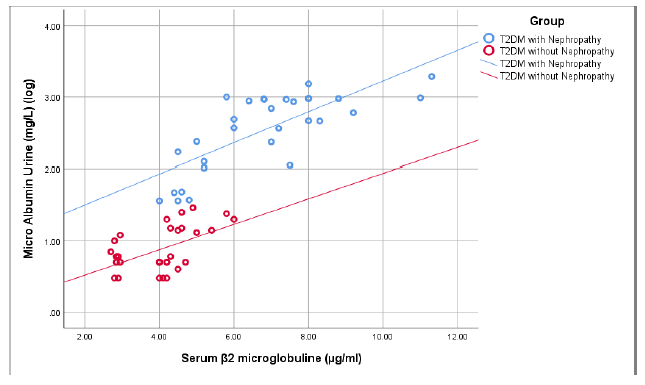
Figure 2 shows that there was a significant positive correlation between serum β2 microglobulin and urinary microalbumin (r=0.755, p<0.001) in group I. On the other hand in group II, serum β2 microglobulin had a positive correlation with urinary microalbumin but that was not statistically significant ((r=0.528, p=0.078).
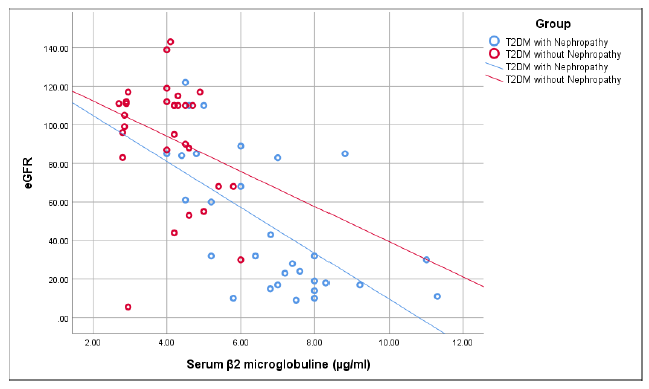
Figure 3 shows that there was a significant negative correlation of serum β2 microglobuliin with estimated glomerular filtration rate (e-GFR) in group I (r=-0.627, p<0.001). While in group II, serum β2 microglobulin had weak negative correlation with e-GFR which was not statistically significant (r=-0.270, p=0.149).
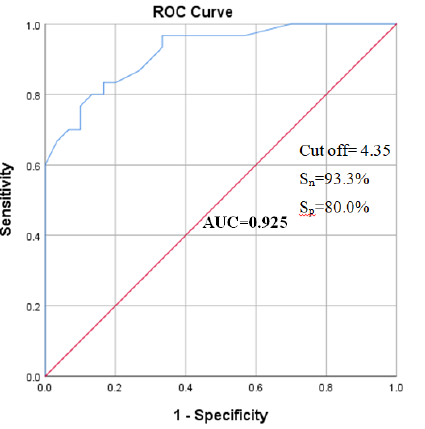
Receiver operating characteristic (ROC) curve was constructed for serum β2 microglobulin to predict diabetic nephropathy. The best cut off point of β2 microglobulin (blue color) was found 4.35 µg/ml [with 93.3% sensitivity (Sn) and 80.0% specificity (Sp)] (Figure- 4).
The analysis of receiver operating characteristic (ROC) curve of serum β2 microglobulin to predict diabetic nephropathy is displaying in table- VI. It was found that, best cut off point of β2 microglobulin for prediction of diabetic nephropathy was 4.35 µg/ml. That was the highest sensitivity (93.3%) and specificity (80.0%) with an area under the curve (AUC) of 0.925 [95% confidence interval (CI): 0.861-0.989, (p <0.001)].
|
Variable |
Cut off value |
Sensitivity |
Specificity |
AUC |
p-value |
95% CI |
|
β2 microglobulin |
4.35 |
93.3 |
80.0 |
0.925 |
<0.001 |
0.861-0.989 |
Table 6: The analysis of receiver operating characteristic (ROC) curve of serum β2 microglobulin to predict diabetic nephropathy.
4. Discussion
Diabetic nephropathy (DN) is a serious long-term major microvascular complication of diabetes mellitus. It was recorded in approximately one third of patients with diabetes [1]. Diabetic nephropathy is the main cause of end stage renal disease (ESRD) and 30-50% of ESRD developed from type 2 diabetes mellitus [2]. Several biomarkers associated with diabetic nephropathy have been the subject of research in last few decades [10]. This study intended to evaluate the association of serum β2 microglobulin in type 2 diabetes mellitus patients with diabetes nephropathy. A total of 60 clinically diagnosed type 2 diabetes mellitus patients were enrolled in this study; among them, 30 patients were with nephropathy (group I) and 30 patients were without nephropathy (group II). It was observed that majority [9 (30%)] patients with diabetes nephropathy (group I) were belonged to 51 - 60 years and majority 13(43.3%) patients without diabetes nephropathy (group II) were belonged to 31 - 40 years. The mean (±SD) age of the study subjects was 50.5 ± 11.71 years in group I and 45.53±9.97 years in group II. No significant age difference was observed between two groups (p=0.082). These findings were consistent with a couple of similar previous studies [8, 16, 20].
In this study, it was found that out of total 60 patients, majority 20(66.7%) of the study participants were male in group I, while majority 17(56.7%) of study population were female in group II. In accordance a previous study found that prevalence of diabetic nephropathy was higher among male compared to female [21]. However, a couple of related study observed female predominance in diabetic nephropathy [8, 22]. It may be due to different lifestyle, several environmental factors and ethnic diversity.
In this current study, half of the [15(50.0%)] nephropathy subjects were obese (BMI > 25 kg/m2) in group I and 13(43.3%) subjects were obese (BMI >25 kg/m2) in group II. The mean (±SD) body mass index (BMI) of nephropathy group was comparatively higher than without nephropathy group (30.1 ± 3.72 kg/m2 versus 28.9 ± 4.65 kg/m2, p=0.256). In a similar study Kim et al. found the mean body mass index (BMI) of diabetic nephropathy and without diabetic nephropathy respondents were 25.0 ± 3.5 kg/m2 and 24.6 ± 3.6 kg/m2 respectively (p=0.260) [16]. This result was comparable with a similar previous study [16].
In this study, urinary microalbumin (mg/24h) and serum creatinine levels were significantly elevated in group I than group II (p<0.001). Similar findings were observed in a related previous study and reported that serum creatinine and urinary microalbumin levels were significantly elevated in type 2 diabetes mellitus patient with nephropathy group compared to the control group (p<0.01) [20]. Another study observed significantly increased mean values of serum creatinine and urinary microalbumin level in nephropathy group than control group [8]. Kim et al. found that, patient with diabetic nephropathy group had significantly low estimated glomerular filtration rate (e-GFR) compared to control group [16]. On the other hand Bianchi et al. documented that, decreasing e-GFR with increasing serum β2 microglobulin among patient with diabetic nephropathy [11]. In these contexts, the current study was supported by previous studies [8, 11, 16, 20]. In this current study, the mean serum β2 microglobulin level was significantly high in group I than group II (6.72 ± 2.032 µg/ml versus 3.44 ± 1.12 µg/ml, p<0.001). This finding was an agreement of similar previous studies [8, 16, 17, 20].
In this present study Pearson’s correlation coefficient (r) test was performed to assess the correlation of serum beta-2 (β2) macroglobulin with serum creatinine, urinary microalbumin and estimated glomerular filtration rate (e-GFR). A significant positive correlation of serum β2 microglobulin with serum creatinine was observed in diabetic nephropathy group (r=+0.549, p=0.002). Similar correlation was reported in a couple of previous studies [16, 20, 23]. In this study a strong positive correlation of serum β2 microglobulin with urinary microalbumin was found among patients with diabetic nephropathy (r=+.755, p<0.05). This finding was well accordance with the findings of related previous studies [8, 20]. Serum β2 macroglobulin had shown significant negative correlation with e-GFR in diabetic nephropathy group of this study (r=-0.627, p<0.001), that finding was supported by similar previous studies [8, 24].
The receiver-operating characteristics (ROC) curve of serum β2 microglobulin to predict diabetic nephropathy was depicted in this study. The area under the curve (AUC) for evaluating the diagnostic efficacy of serum β2 microglobulin was found 0.925 (0.861 to 0.989). The best cut-off point of serum β2 microglobulin for diabetic nephropathy was found 4.35 µg/ml. At this cut off point, the sensitivity of serum β2 microglobulin was 93.3% and specificity was 80.0%. This observation was consistent with similar previous studies [22, 24]. However, Kim et al. found that the best cut-off point of serum β2 microglobulin for diabetic nephropathy was 1.81 mg/L with sensitivity 57.3% and specificity 61.7% [16]. This result was not consistent with this current study probably due to sample size variation and they enrolled study participant with normal renal function.
The current study demonstrated a strong and positive association between serum β2 microglobulin and diabetic nephropathy. Several study reported that serum creatinine, e-GFR, urinary albumin excretion rate altered by some independent variables. But serum β2 microglobulin was not affected by any independent variables. It showed significant sensitivity and specificity which might be useful for the physicians as an additional tool for diagnosis of diabetic nephropathy more accurately. Further studies are warranted to confirm the issue.
5. Conclusion
Serum β2 microglobulin level was significantly higher in patients with diabetic nephropathy than without nephropathy. There was a significant positive correlation of serum β2 microglobulin with serum creatinine and urinary microalbumin excretion rate but significant negative correlation of serum β2 microglobulin with e-GFR in patients with diabetic nephropathy. Because of significant sensitivity and specificity, serum β2 microglobulin may be used as a reliable biomarker to predict diabetic nephropathy.
Limitations of the Study
It was a single center study with a relatively small sample size.
Recommendations
Multicenter broad-based prospective cohort study is required to confirm the findings of this present study.
Competing Interests
The authors have declared that no competing interests exist.
References
- Chamberlain JJ, Rhinehart AS, Shaefer Jr CF, Neuman A. Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Annals of internal medicine 164 (2016): 542-552.
- Levin A, Rocco M. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. American Journal of Kidney Diseases 49 (2007): S10-S79.
- Islam SM, Islam MS, Rawal LB, Mainuddin AK, Wahiduzzaman M, et al. Clinical profile of patients with diabetic nephropathy in a tertiary level hospital in Dhaka, Bangladesh. Archives of Medicine and Health Sciences 3 (2015): 191.
- Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: worldwide difference of prevalence and risk factors. Journal of nephropharmacology 5 (2016): 49.
- Gross JL, De Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes care 28 (2005): 164-176.
- Uwaezuoke SN. The role of novel biomarkers in predicting diabetic nephropathy: a review. International journal of nephrology and renovascular disease 10 (2017): 221.
- Konen JC, Shihabi ZK. Microalbuminuria and diabetes mellitus. American family physician 48 (1993): 1421-1428.
- Aksun SA, Özmen D, Özmen B, Parildar Z, Mutaf I, et al. β2-microglobulin and cystatin C in type 2 diabetes: assessment of diabetic nephropathy. Experimental and clinical endocrinology and diabetes 112 (2004): 195-200.
- Inker LA, Tighiouart H, Coresh J, Foster MC, Anderson AH, et al. GFR estimation using β-trace protein and β2-microglobulin in CKD. American journal of kidney diseases 67 (2016): 40-48.
- Sulaiman MK. Diabetic nephropathy: recent advances in pathophysiology and challenges in dietary management. Diabetology and metabolic syndrome 11 (2019): 1-5.
- Bianchi C, Donadio C, Tramonti G, Consani C, Lorusso P, et al. Reappraisal of serum β2-microglobulin as marker of GFR. Renal failure 23 (2001): 419-429.
- Saper MA, Bjorkman P, Wiley DC. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 Å resolution. Journal of molecular biology 219 (1991): 277-319.
- Shinkai S, Chaves PH, Fujiwara Y, Watanabe S, Shibata H, et al. β2-microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C-reactive protein. Archives of internal medicine 168 (2008): 200-206.
- Liabeuf S, Lenglet A, Desjardins L, Neirynck N, Glorieux G, et al. European Uremic Toxin Work Group (EUTox. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney international 82 (2012): 1297-1303.
- Argyropoulos CP, Chen SS, Ng YH, Roumelioti ME, Shaffi K, et al. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Frontiers in medicine 4 (2017): 73.
- Kim MK, Yun KJ, Chun HJ, Jang EH, Han KD, et al. Clinical utility of serum beta-2-microglobulin as a predictor of diabetic complications in patients with type 2 diabetes without renal impairment. Diabetes and metabolism 40 (2014): 459-465.
- Latha T, Jaganmohan P, Subramanyam P. Evaluation of Diabetic Nephropathy using selected biochemical markers in settiyar (Vysya) community of Nellore and Prakasam Districts of Andhra Pradesh. Advan Bio l Res 6 (2012): 81-86.
- Santo NG, Cirillo M. Estimating glomerular filtration rate: Cockcroft–Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clinical Journal of the American Society of Nephrology 4 (2009): 899-906.
- Consultation WE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England) 363 (2004): 157-163.
- Elsebai AA, Saad WE, Mahdy MM. Serum chemerin and beta 2-microglobulin in type 2 diabetes: assessment of diabetic nephropathy. Life science journal 11 (2014): 992-1000.
- Mohiuddin AK. Diabetes Fact: Bangladesh Perspective. International Journal of Molecular Biotechnology 4 (2018): 1-9.
- Foster MC, Inker LA, Hsu CY, Eckfeldt JH, Levey AS, et al. Filtration markers as predictors of ESRD and mortality in Southwestern American Indians with type 2 diabetes. American Journal of Kidney Diseases 66 (2015): 75-83.
- Sheen YJ, Sheu WH. Risks of rapid decline renal function in patients with type 2 diabetes. World journal of diabetes 5 (2014): 835.
- Eguvbe AO, Nwagu MU, Idogun ES, Akande AA. The role of urine albumin creatinine ratio and serum β2 microglobulin as biomarkers of chronic kidney disease. Universa Medicina 38 (2019): 172-17178.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 