Successful Utilization of the GLP-1 Receptor Agonist Semaglutide in Treating the Manifestations of Recalcitrant Celiac Disease
David S H Bell MB1*, Terri Jerkins MD2
1Southside Endocrinology, Crestwood Blvd, Irondale, Alabama, USA
2Lipscomb University, University Park Dr, Nashville, Tennessee, USA
*Corresponding author: David S H Bell MB, Southside Endocrinology, Crestwood Blvd, Irondale, Alabama, USA.
Received: 20August 2025; Accepted: 27 August 2025; Published: 29 September 2025
Article Information
Citation: David S H Bell, Terri Jerkins. Successful Utilization of the GLP-1 Receptor Agonist Semaglutide in Treating the Manifestations of Recalcitrant Celiac Disease. Archives of Clinical and Biomedical Research. 9 (2025): 392-395.
View / Download Pdf Share at FacebookAbstract
A refractory case of abdominal swelling (pseudoascites) due to celiac disease which did not respond to monotherapy with a gluten-free diet and for which treatment with traditional immunosuppressives was refused is described. Based on reports of the anti-inflammatory actions of the GLP-1 receptor agonist, semaglutide, weekly subcutaneous injections of semaglutide were added to the gluten-free diet with rapid and obvious success. Semaglutide and perhaps other GLP-1 receptor agonists should be considered for use as a largely safe anti-inflammatory drug in other autoimmunopathies and inflammatory states.
Keywords
<p>Celiac disease; Type 1 diabetes; Pseudoascites; Gluten free diet; Semaglutide</p>
Article Details
1. Introduction
We have previously described celiac disease in a type 1 diabetic subject causing severe abdominal swelling (pseudoascites) without evidence of malapsorbtion [1]. In this patient a gluten-free diet did not improve the abdominal distension. Because of this recalcitrance the use of an immunosuppressant drug such as azathioprine was proposed but was refused by the patient [2].
At around this time the GLP-1 receptor agonist semaglutide given subcutaneously in weekly injections had been shown in obese subjects to be associated with improvement in the symptoms of osteoarthritis of the knee [3]. One of the authors speculated that this improvement was not due to weight loss but due to the anti-inflammatory effects of semaglutide [4]. We also speculated that this anti-inflammatory effect accounted for semaglutide’s positive effect on diabetic nephropathy and coronary artery disease [5,6]. Based on the approval of semaglutide for therapy type 2 diabetes and obesity and its apparent safety, the patient agreed to an “off label” trial of semaglutide.
2. Case Report
A 64-year-old white male who had had type 1 diabetes (C-peptide negative) for 43 years presented with abdominal distension. His waist circumference had increased over the previous three months from 33 to 38 inches. Investigations for a cardiac, hepatic or renal cause were negative and abdominal ultrasound and MRI were normal. On attempted peritoneal aspiration no fluid was obtained but cytology was suggestive for celiac disease, the presence of which was confirmed by a positive duodenal biopsy and very positive serology (Table 1). However, there was no evidence of malabsorption [1].
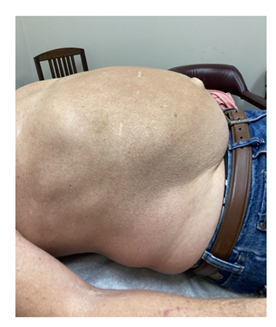
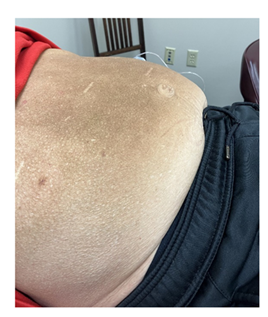
A gluten-free diet was initiated without any subjective or objective change. On adding semaglutide starting with 0.5 mg SC and advancing to 2 mg SC weekly over 3 months resulted in a decrease in the waist circumference from 38” to 34” (Figure 1 and 2). His weight decreased from 236 lbs to his preabdominal swelling weight of 195 lbs (BMI from 32.9 to 27.1 over 6 months) (Table 2). In addition, the positive serological marker for celiac disease declined or normalized (Table 1).
|
Test |
Pre-Semaglutide Result |
Post- Semaglutide Result |
Normal Range |
|
Endomysial Antibody IgA Titer |
1.160 |
1.10 |
Less than 1:10 |
|
Tissue Transgluminase Antibody IgA to Endomysial Tissue Transglutinase Antibody |
100.0 AU/ml |
36.0 AU/ml |
0-89 Au/ml |
|
Deamidated Gliadin Peptide (DGP) Antibody IgA |
43.6 AU/ml |
13.1 AU/ml |
0-7.9 |
|
Deamidated Gladin Peptide (DGP) Ab1IgG |
8.9 AU/ml |
43.6 AU/ml |
0-9.9 AU/ml |
|
Tissue Transglutaminase Antibody IgG |
4.2 AU/ml |
4.4 AU/ml |
0-19.9 AU/ml |
Table 1: Change in immunological markers of celiac disease with semaglutide.
|
Duration in Days |
Weight in lbs |
BMI |
% Lost |
|
Pre-pseudoascites |
196-206 |
29 |
- |
|
0 |
236 |
32.9 |
_ |
|
30 |
22 |
31.2 |
5.1 |
|
60 |
209 |
29.1 |
9.8 |
|
90 |
198 |
27.6 |
16.1 |
|
120 |
195 |
27.1 |
18.3 |
Table 2: Weight gain with pseudoascites and weight loss with semaglutide.
3. Discussion
In this report we describe for the first time the effect of semaglutide on the inflammation of bowel and the contents of the peritoneal cavity caused by celiac disease.
Celiac disease, also known as gluten-sensitive enteropathy or non-tropical sprue, is caused by an immune reaction to gluten and gluten related proteins in genetically prone individuals [7].
Celiac disease mostly effects the small bowel but multiple organs may be involved so that the presentations of celiac disease can be protean [7]. Being an immune-related enteropathy, celiac disease is strongly associated with other autoimmune diseases such as Hashimoto’s thyroiditis, Sjrogren’s syndrome and type 1 diabetes [8]. However, the incidence of celiac disease is not increased with type 2 diabetes [9]. In the general population celiac disease is estimated to occur in 0.5-2.0% but with type 1 diabetes the prevalence is approximately 5% [9,10]. Therefore, it is important that all type 1 diabetic patients be screened for celiac disease since, because in patients with celiac disease 40% to 60% have mild or no symptoms and less than 10% of patients with both type 1 diabetes and celiac disease are symptomatic [11,12]. It also should be stressed that celiac disease like other autoimmune diseases are more likely to occur in those patients with late-onset type 1 diabetes [13].
Semaglutide has been shown to have anti-inflammatory effects which are not fully understood [14]. Semaglutide probably reduces inflammatory cytokine levels, reduces immune cell recruitment into tissues and through decreasing inflammation may decrease thrombosis and atherogenesis and even induce angiogenesis [14]. Therefore, it is not surprising that semaglutide will also decelerate renal decompensation and improve cardiac outcomes [5,6]. It is also easily understood why semaglutide decreases pain in an osteoarthritic joints and improves the manifestations of celiac disease [3-6].
4. Conclusion
In conclusion, we have shown that the anti-inflammatory effects of semaglutide have in a single patient improved the manifestations of celiac disease. This report should not only lead to consideration of a placebo-controlled trial of semaglutide in celiac disease but also lead to trials in other inflammatory diseases as well as other autoimmunopathies.
5. Acknowledgements
Author Contribution: Dr. David Bell and Dr. Terri Jerkins were both fully involved in the writing of this manuscript. Additionally, both authors discussed the results and contributed to the final manuscript.
Funding: No funding or sponsorship was received for this study or publication of this article.
Ethics Approval: Written and informed consent to publish was obtained from the patient in this case report.
Conflict of Interest: David S. H. Bell is an Editorial Board member of Diabetes Therapy. David S. H. Bell was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Dr. Terri Jerkins does not have any conflict of interests.
References
- Bell DS, Jerkins TJ. Pseudoascites due to celiac disease in a patient with type 1 diabetes. Diabetes Therapy (2025).
- Iqbal U, Chaudhary A, Karim MA, et al. Refractory Celiac Disease Successfully Treated with Azathioprine. Gastroenterology Res 10 (2017): 199-201.
- Bliddal H, Bays H, Czernichow S, et al. Once-Weekly Semaglutide in Persons with Obesity and Knee Osteoarthritis. N Engl J Med 391 (2024): 1573-1583.
- Bell DSH. ACP Journal Club Editorial Team at McMaster University. In adults with obesity and knee OA, adding weekly semaglutide to diet and activity counseling reduced weight and knee pain at 68 wk. Ann Intern Med 178 (2025): JC20.
- Perkovic V, Tuttle KR, Rossing P, et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N Engl J Med 391 (2024): 109-121.
- Lingvay I, Deanfield J, Kahn SE, et al. Semaglutide and Cardiovascular Outcomes by Baseline HbA1c and Change in HbA1c in People with Overweight or Obesity but Without Diabetes in SELECT. Diabetes Care 47 (2024): 1360-1369.
- Farrell RJ, Kelly CP. Celiac Sprue. N Engl J Med 346 (2002): 180-188.
- Lauret E, Rodrigo L. Celiac disease and autoimmune-associated conditions. Biomed Res Int (2013): 127589.
- Kylökäs A, Kaukinen K, Huhtala H, et al. Type 1 and type 2 diabetes in celiac disease: prevalence and effect on clinical and histological presentation. BMC Gastroenterol 16 (2016): 76.
- Sategna-Guidetti C, Grosso S, Pulitanó R, et al. Celiac disease and insulin-dependent diabetes mellitus. Screening in an adult population. Dig Dis Sci 39 (1994): 1633-1637.
- Porter JA, MacKenzie K, Darlow B, et al. Looking for coeliac disease in children with type 1 diabetes mellitus. J Paediatr Child Health 50 (2014): 811-816.
- Eland I, Klieverik L, Mansour AA, et al. Gluten-Free Diet in Co-Existent Celiac Disease and Type 1 Diabetes Mellitus: Is It Detrimental or Beneficial to Glycemic Control, Vascular Complications, and Quality of Life? Nutrients 15 (2022): 199.
- Hughes JW, Riddlesworth TD, DiMeglio LA, et al. T1D Exchange Clinic Network. Autoimmune Diseases in Children and Adults with Type 1 Diabetes from the T1D Exchange Clinic Registry. J Clin Endocrinol Metab 101 (2016): 4931-4937.
- Yaribeygi H, Maleki M, Jamialahmadi T, et al. Anti-inflammatory benefits of semaglutide: State of the art J Clin Transl Endocrinol 36 (2024): 100340.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 