The Emergency Use of the oXiris Device with Continuous Kidney Replacement Therapy in COVID-19 Patients with Acute Kidney Injury
Rupesh Raina1, 2*, Isabelle Mawby1, 3, Nikhil Datla3, Ronith Chakraborty1, 2, Kabir Gulati1, Siddhartha Singh1, 2, Nirav Agrawal1, 4, Alexander Botsch5, Jeffrey Welko5 and Javier Neyra6
1Department of Nephrology, Akron Nephrology Associates/ Cleveland Clinic Akron General Medical Center, OH, USA
2Department of Nephrology, Akron Children’s Hospital, OH, USA
3Department of Medicine, Northeast Ohio Medical University, OH, USA
4Feinstein Institute for Medical Research, Northwell Health, NY, USA
5Summa Akron City Hospital, OH, USA
6Division of Nephrology, Department of Internal Medicine, Bone and Mineral Metabolism, University of Kentucky, KY, USA
*Corresponding author: Rupesh Raina, Consultant Nephrologist, Adult-Pediatric Kidney Disease/Hypertension,
Department of Nephrology, Cleveland Clinic Akron General and Akron Children's Hospital, Akron, Ohio, USA, Tel: 330-543-8950; Fax: 330-543-3980
Received: 19 July 2021; Accepted: 27 July 2021; Published: 10 August 2021
Article Information
Citation: Rupesh Raina, Isabelle Mawby, Nikhil Datla, Ronith Chakraborty, Kabir Mehra, Siddhartha Singh, Nirav Agrawal, Alexander Botsch, Jeffrey Welko, Javier Neyra. The Emergency Use of the oXiris Device with Continuous Kidney Replacement Therapy in COVID-19 Patients with Acute Kidney Injury. Archives of Clinical and Biomedical Research 5 (2021): 629-639.
View / Download Pdf Share at FacebookAbstract
Throughout the pandemic, presentations of the Coronavirus disease-2019 (COVID-19) infection have varied. Upon entering the body, SARS-CoV-2 causes a rapid release of pro-inflammatory cytokines and endotoxins, leading to multi-organ failure in intensive care unit (ICU) patients. In our case report, we present a 51-year-old male with severe COVID-19 infection in the ICU needing mechanical ventilation. He developed oliguric acute kidney injury (AKI) with acute respiratory distress syndrome and fluid overload >20% and was started on high flow continuous venovenous hemodiafiltration with oXiris filter via Prismaflex® (HF-CVVHDF). The patient received oXiris filter-based HF-CVVHDF for 72 hours and was switched to conventional CVVHDF. The patient gradually recovered with supportive care and was discharged. Through this case, we wish to highlight the significance of the use of an extracorporeal blood purification technology in COVID-19 with an oXiris filter to prevent morbidity and mortality among severe COVID-19 patients admitted to the ICU with AKI.
Keywords
Acute kidney injury; CKRT; COVID-19; Oxiris
Acute kidney injury articles; CKRT articles; COVID-19 articles; Oxiris articles
Article Details
1. Introduction
COVID-19 may be asymptomatic or present with potentially life threatening symptoms. When the virus enters the body, massive epithelial and endothelial cell death and vascular leakage may initiate the rapid production of pro-inflammatory cytokines and chemokines. The overwhelming systemic inflammation is known as cytokine storm syndrome. The presence of cytokine storm syndrome among COVID-19 patients is strongly associated with disease severity, including an increased risk for kidney failure, and mortality. We present the case of a 51-year-old male with severe COVID-19 who developed oliguric acute kidney injury (AKI) with ARDS and fluid overload >20%. He received high flow continuous venovenous hemodiafiltration (HF-CVVHDF) with oXiris filter to enhance hemoadsorption for 72 hours. Our report provides a useful and detailed guide stating the current modalities available for COVID-19 patients with AKI, including the oXiris filter's role and its effectiveness in reducing cytokine storm.
2. Case Report
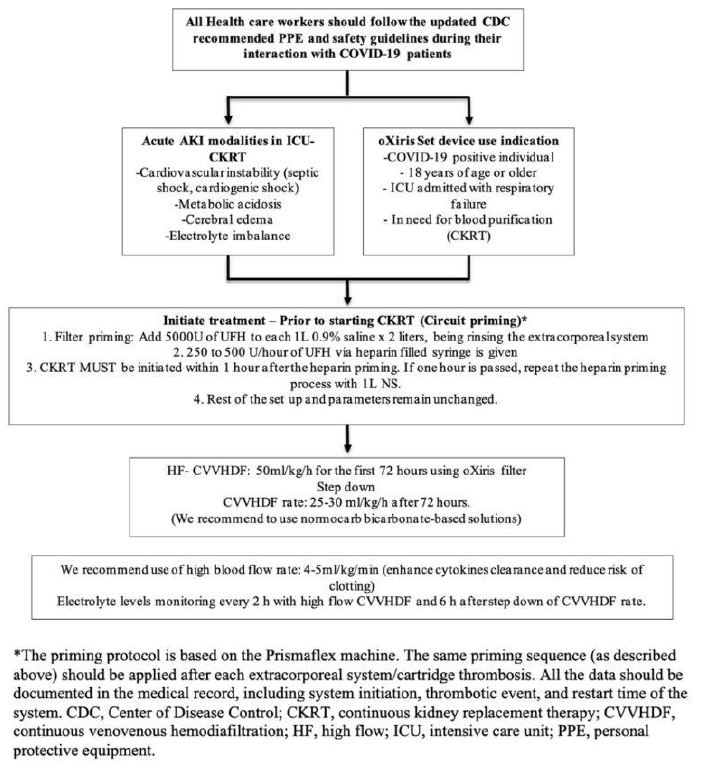
A 51-year-old obese male with a past medical history of type 2 diabetes mellitus came to the emergency department with a one-week history of progressively worsening shortness of breath and cough. The patient tested positive for COVID-19 as an outpatient. Physical exam showed labored breathing with diminished breath sounds bilaterally. Initial vitals showed a temperature of 37°C, heart rate of 116 beats per minute, respiratory rate of 20 per min, oxygen saturation of 30% on room air, and blood pressure of 78/52 mmHg. He continued to deteriorate, requiring intubation, and was transferred to the intensive care unit (ICU). Subsequently, he developed septic shock and was started on vasopressor and COVID-related medications: remdesivir, dexamethasone, and tocilizumab. After 48 hours of admission, his serum creatinine (sCr) peaked from 1.2 mg/dl to 4.62 mg/dl. The nephrology team was consulted for oliguric AKI (AKIN Class III/KDIGO stage III) and the management of fluid overload (FO) >20% in the context of ARDS. HF-CVVHDF was started with the oXiris filter via PrismaFlex® for 72 hours without anticoagulant. The total effluent dose was 50ml/kg/hr and was divided into 3 parts: 1/3 of the total effluent dose was used as pre-filter fluid replacement, another 1/3 was used post-filter, and the remaining effluent fluid was used as dialysate. We were able to achieve a total of 40-45 ml/kg/hr total effluent doses despite investigation and down times. The isolated ultrafiltration (IUF) cumulative as negative 50ml/hr at start then escalated to 200ml/hr with initial day cumulative Input:Output (I:O) were equal and the total FO (20%) was removed over the duration of four days with the help of pressors and intermittent albumin (25 gm/Q 6hrs). Total circuit time was 12 hours, the two oXiris filters were used with heparin prime which lasted for 15 hrs. After 48 hours, we switched to HF14000 with HF-CVVHDF. The patient received 12 sessions of CVVHDF which lasted for 7 days and showed significant recovery.
Based on the laboratory findings, there was a decreasing trend noted for D-Dimer (DD) from 17.34 mg/l to 6.42 mg/l, CRP levels from 43.5 mg/l to 40.3 mg/l and interleukin (IL)-6 levels from 34.5 to 8.6 pg/ml post therapy. On further AKI evaluation, kidney biopsy showed degenerative changes and tubular epithelium necrosis, suggesting acute tubular necrosis. The patient gradually improved clinically and was weaned of vasopressors and extubated before getting discharged. He was followed up by a nephrologist and received three weeks of HD in the outpatient setting leading to complete recovery of kidney function and liberation from HD.
3. Discussion
The symptoms of COVID-19 range from mild to severe, including pneumonia, ARDS, MODS, and multisystem organ failure (MSOF) [1, 2].AKI is common (20–40%) in severe COVID-19 requiring ventilatory support. In a systematic review by Robbins-Juarez et al., 77% of AKI patients experienced severe COVID-19 infection with a mortality rate of up to 52% (OD: 15.27) and with severe COVID-19 without AKI patients had approximately 11% mortality rate [3]. A similar outcome was noted in several other studies [4, 5]. The exact mechanisms of kidney involvement in COVID-19 patients are still unclear. Nonetheless, one of the proposed hypotheses includes massive epithelial and endothelial cell death, and subsequent vascular leakage, which promotes rapid production of pro-inflammatory cytokines and chemokines (cytokine storm), leading to virus-induced cytopathy of kidney tissue and sepsis. Unlike classic SARS and MERS infections of the past, Covid-19 appears to have an increased level of the Th2 family of cytokines secreted which include IL-4 and IL-10 [6]. Interferons that are very keen in the body’s immune defense against viruses have been shown to have a delayed rsponse to Covid-19 as the virus delays the release of interferons casuing the body’s immune respsonse to be subdued initially [7]. Alternatively, AKI may occur after kidney inflammation, volume depletion, and cardiomyopathy [8, 9].
The exaggerated cytokine release due to COVID-19 virus infection may worsen AKI and emerge to MODS or MOF. The elevated pro- and anti-inflammatory circulating biomarkers and cytokine levels, including tumor necrosis factor, IL-1, IL-6, and IL-8, are prognosed to increase mortality [10]. Therefore, timely initiation of kidney replacement therapy (KRT) with extracorporeal organ support (ECOS) has shown to be beneficial in removing immunomodulatory mediators and mitigating organ failure in AKI patients with COVID-19 [11]. The ECOS incorporates CKRT, extracorporeal membrane oxygenation (ECMO), and extracorporeal carbon dioxide removal (ECCO2R). The utility of ECOS in restoring a balanced systemic immune response involves strategies such as hemoperfusion, coupled filtration adsorption, high cut-off (HCO), and membranes with enhanced adsorptive profiles [10]. The modalities listed above are not exhaustive, but focus on the advances in technology and the different therapeutic targets. When comparing the three main adsorption filters, oXiris managed to remove both cytokine and endotoxins more effectively (68 ± 4.4%) than Toraymyxin (83.4 ± 3.8%) and CytoSorb (6.3 ± 4.9%; p<0.05) [12] (Table S1).
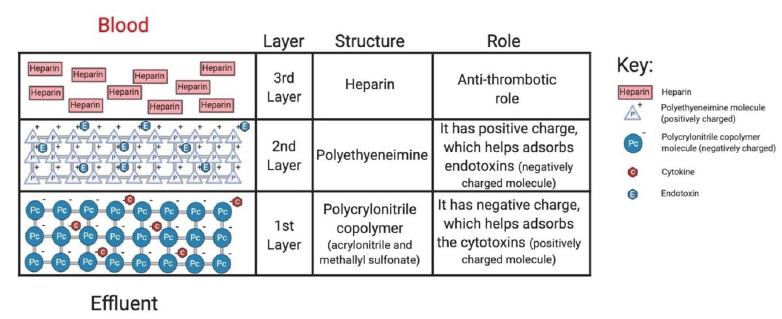
The oXiris filter works by reducing cytokine levels and inflammatory mediators due to its enhanced hemoadsorptive capability. Additionally, the filter material contains a layer of anticoagulant (heparin), making it advantageous for patients with high clotting risk or those who are at risk of citrate accumulation (Figure 1) [13]. Based on a retrospective study (n= 37), significant decrease in IL-6 was noted within 72 hours of treatment initiation (p=0.001), with a decrease in SOFA scores (p<0.001) suggesting the efficacy of oXiris in mitigating the inflammatory markers in severe COVID-19 AKI patients [14]. Similarly, Zhang et al. presented four cases of septic AKI patients in China who all benefited from using the oXiris filter early in their treatment. Additionally, patients with a high risk of bleeding successfully used oXiris without additional anticoagulation for up to 36 hours without any adverse effects [13, 15] (Table S2). There is limited data available suggesting the use of highly adsorptive hemofilter with KRT in COVID-19 AKI patients, especially when complicated with ARDS and FO; thus, the management became more challenging in our patient. Ideally, patients with COVID-19 symptoms with AKI and other complications should receive CKRT with anticoagulation. However, citrate was not used as an anticoagulant as the patient also suffered from MSOF and MODS, including acute liver failure.
One of the biggest challenges faced by our team during dialysis was the priming of the filter. To address these issues, we used oXiris for 72 hours for the speculated role of chemokine burst after COVID-19 with ARDS. We modified our HF-CVVHDF concept with total effluent of 50ml/kg/hr and used 1/3 of the total effluents as replacement fluid pre-filter. Another 1/3 was provided post-filter while the remaining effluent fluid was utilized as dialysate (Figure 2). With constant close monitoring in the ICU, our patient showed significant improvement in the symptoms with our developed protocol. However, intervention with HF-CVVHDF is not without risk as there is always a risk of non-specific removal of both pro- and anti-inflammatory markers via convection, adsorption, and/or dispersion. Thus, a multidisciplinary team should be considered in making all treatment decisions.
4. Conclusion
In conclusion, we report the case of a COVID-19 critically ill patient complicated with AKIN class III, ARDS, and FO admitted to the ICU. The oXiris filter use with HF-CVVHDF showed significant improve-ment in our patient. However, the oXiris filter use with extracorporeal blood purification therapies in COVID-19 patients is still a new and debateable therapy and more detailed multicentered trials are required to validate its effect on clinical outcomes.
Acknowledgement
None
Conflict of Interest
The authors have no conflict of interest to report.
Funding
There is no funding to report.
References
- WHO coronavirus disease (COVID-19) dashboard. Geneva: World Health Organization (2020).
- Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili SM, Bahreini E. A comprehensive review of COVID-19 characteristics. Biol Proced Online 22 (2020): 19.
- Robbins-Juarez S Y, Qian L, King K L, Stevens J S, Husain S A, Radhakrishnan J, et al. Outcomes for Patients With COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Kidney international reports 5 (2020): 1149-1160.
- Raina R, Mahajan Z A, Vasistha P, Chakraborty R, Mukunda K, Tibrewal A, et al. Incidence and Outcomes of Acute Kidney Injury COVID-19: A Systematic Review. Blood Purif (2020).
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 76 (2012): 16-32.
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect 80 (2020): 607-613.
- Sophie Trouillet-Assant, Sebastien Viel, Alexandre Gaymard, Sylvie Pons, Jean-Christophe Richard, Magali Perret, et al. Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol 146 (2020): 206-208.e2.
- Raina R, Chakraborty R, Sethi SK, Bunchman T. Kidney Replacement Therapy in COVID-19 Induced Kidney Failure and Septic Shock: A Pediatric Continuous Renal Replacement Therapy [PCRRT] Position on Emergency Preparedness With Resource Allocation. Front Pediatr 8 (2020): 413.
- Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol 16 (2020): 308-310.
- Lee D W, Faubel S, Edelstein C L. Cytokines in acute kidney injury (AKI). Clinical nephrology, 76 (2011): 165-173.
- Chen G, Zhou Y, Ma J, Xia P, Qin Y, Li X. Is there a role for blood purification therapies targeting cytokine storm syndrome in critically severe COVID-19 patients?. Ren Fail 42 (2020): 483-488.
- Malard B, Lambert C, Kellum J A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive care medicine experimental 6 (2018): 12.
- Padala SA, Vakiti A, White JJ, Mulloy L, Mohammed A. First Reported Use of Highly Adsorptive Hemofilter in Critically Ill COVID-19 Patients in the USA. J Clin Med Res 12 (2020): 454-457.
- Villa G, Romagnoli S, De Rosa S, Greco M, Resta M, Pomarè Montin D, et al. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID-19: a pilot study. Critical care (London, England) 24 (2020): 605.
- Zhang H, Zhu G, Yan L, Lu Y, Fang Q, Shao F. The absorbing filter Oxiris in severe coronavirus disease 2019 patients: A case series. Artif Organs (2020): 1-7.
Supplymentary Tables
Table S1: Various extracorporeal hemoadsorption filters and their properties.
Table S2: Characteristics of oXiris filter set and CKRT.
|
oXiris filter set [10] |
||
|
About |
The oXiris set device was authorized for emergency use to treat COVID-19 patients in April of 2020 by the FDA [11].Prior to the authorization of oXiris, there was a lack of lifesaving treatment options for COVID-19 patients in the ICU with AKI. |
|
|
Target population |
Any COVID-19 positive patients 18 years or older, admitted in the ICU with confirmed or impending respiratory failure in need of blood purification, including use in CKRT, per standard indications. |
|
|
Indication |
The oXiris Set device is specifically authorized to be used in: 1. COVID-19 positive individuals 2. 18 years of age or above 3. ICU with confirmed Respiratory Failure 4. In need of blood purification, including CKRT. Furthermore, the patient should have any one of the following conditions: a. Early acute lung injury (ALI)/early acute respiratory distress syndrome (ARDS). b. Severe respiratory distress: dyspnea, respiratory rate ≥ 30/min, oxygen saturation ≤93%, the partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, and/or lung infiltrates >50% within 24 to 48 hours c. Life-threatening disease: respiratory failure, septic shock, and/or MOD. |
|
|
Dialysis Device |
Set device only with the Prismaflex control unit or with the PrisMax control unit. |
|
|
Technical information (Filter composition) |
The oXiris filter set can be used with both Prismaflex and Prismax CKRT machines. The blood volume in the set is 193 ml.The filter material consists ofacrylonitrile and sodium methallyl sulfonate copolymer + polyethyleneimine (surface treatment agent) + heparin grafted (4500+/-1500 IU/m2). |
|
|
Benefits |
1. Reducing circulating inflammatory mediators, 2. Adsorption of both endotoxins and cytokines, 3. Respiratory response, 4. Stabilization of hemodynamic status, 5. Staged improvement of organ function while providing renal support through CKRT |
|
|
Use duration |
72-hour use, then switch to regular filter (m100 filter). |
|
|
Side effects [12, 21] |
1. Hemodynamic compromise (such as hypotension, increased vasopressor requirement, reduced cardiac perfusion) 2. Thrombocytopenia/leukopenia 3. Allergic reaction to device materials 4. Removal of other blood substances (e.g., infection, blood loss, thrombosis, tissue/organ injury) |
5. Risks related to anticoagulation (e.g., blood loss, allergic reaction) 6. Arrhythmia 7. Blood loss 8. Thrombosis 9. Air embolism 10. Infection 11. Hemolysis 12. Electrolyte imbalance 13. Particle embolism |
|
Absolute Contraindications |
1. History of Heparin allergy (HIT) 2. AN-69 filter allergies 3. ACE inhibitors use while on CKRT |
|
|
Relative Contraindication |
Projected use of CKRT for ≤ 72 hours. |
|
|
Precaution |
· The recommendation is to wear full PPE when caring for COVID-19 positive or suspected patients during high-risk prevalence. However, the recommendation changes rapidly; the doctors and staff must stay updated with the recent recommendation and trainings. · Use appropriate personal protective equipment when caring for individuals suspected of having COVID-19 as outlined in the CDC Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings or on the CDC webpage on Infection Control. |
|
|
CKRT |
||
|
Disinfection |
It is crucial to clean and disinfect and dispose of the dialysis equipment as per the manufacturer/local hospital policy/CDC recommendation before moving the equipment from the isolation room. |
|
|
CVVHDF composition Challenges to initiate CKRT therapy |
Replacement fluid composition using Standard Prismasol replacement solution (Gambro-Baxter International, Inc.) was used For CVVHDF with the following composition: NaCl 140 mEq/L, NaHCO3 32 mEq/L, KCl 2 or 4 mEq/L, MgSO4 1.0 mEq/L, Dextrose 100 mg/dL, Calcium 2.5 mEq/L. For CVVHD, standard Nx- Stage dialysate (NxStage, Inc., Lawrence, MA, USA) was used with the following composition: NaCl 140 mEq/L, NaHCO3 35 mEq/L, KCl 2 or 4 mEq/L, MgSO4 1 mEq/L, Dextrose 100 mg/dL, Calcium 3.0 mEq/L. The oXiris filter set has been approved to treat patients who have confirmed COVID-19 and have been admitted to the ICU with confirmed or imminent respiratory failure in need of blood purification therapy to reduce pro-inflammatory cytokine levels, including use in CKRT. The indications for CKRT are the same as in non-COVID-19 patients, including electrolyte imbalance, fluid overload, and acid-base disturbances. However, prior to the CKRT therapy initiation, it is vital to stabilize patients with severe volume depletion due to fever, reduced oral intake, or diuretic therapy. As the pandemic escalates, CKRT therapy among COVID-19 patients presents unique challenges, including a shortage of staff and machines and the risk of disease transmission to healthcare workers [12]. |
|
- Ling Zhang, Gloria Kai Yan Tang, Songqiao Liu, Jing Cai, Wai Ming Chan, Yi Yang, et al. Hemofilter with adsorptive capacities: case report series. Blood Purif 47 (2019): 1-6.
- Franco Turani, Riccardo Barchetta, Mauro Falco, Silvia Busatti, Luca Weltert. Continuous renal replacement therapy with the adsorbing filter oXiris in septic patients: a case series. Blood Purif 47 (2019): 1-5.
- Gianluca Villa, Stefano Romagnoli, Silvia De Rosa, Massimiliano Greco, Marco Resta, Diego Pomarè Montin, et al. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID-19: a pilot study. Crit Care 24 (2020): 605.
- Alharthy A, Faqihi F, Memish Z A, Balhamar A, Nasim N, Shahzad A, et al. Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: A case-series. Artificial organs (2020).
- Katagiri D, Ishikane M, Asai Y, Izumi S, Takasaki J, Katsuoka H, et al. Direct hemoperfusion using a polymyxin B-immobilized polystyrene column for COVID-19. Journal of clinical apheresis (2020).
- Ala-Kokko T I, Laurila J, Koskenkari J. A new endotoxin adsorber in septic shock: observational case series. Blood purification 32 (2011): 303-309.
- Adamik B, Zielinski S, Smiechowicz J, Kübler A. Endotoxin Elimination in Patients with Septic Shock: An Observation Study. Archivum immunologiae et therapiae experimentalis 63 (2015): 475-483.
- Yaroustovsky M, Abramyan M, Popok Z, Nazarova E, Stupchenko O, Popov D, et al. Preliminary report regarding the use of selective sorbents in complex cardiac surgery patients with extensive sepsis and prolonged intensive care stay. Blood purification, 28 (2009): 227-233.
- Huang Z, Wang S R, Yang Z L, Liu J Y. Effect on extrapulmonary sepsis-induced acute lung injury by hemoperfusion with neutral microporous resin column. Therapeutic apheresis and dialysis: official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy 17 (2013): 454–461.
- Yaroustovsky M, Abramyan M, Popok Z, Nazarova E, Stupchenko O, Popov D, et al. Preliminary report regarding the use of selective sorbents in complex cardiac surgery patients with extensive sepsis and prolonged intensive care stay. Blood purification 28 (2009): 227-233.
- Padala SA, Vakiti A, White JJ, Mulloy L, Mohammed A. First Reported Use of Highly Adsorptive Hemofilter in Critically Ill COVID-19 Patients in the USA. J Clin Med Res 12 (2020): 454-457.
- Victor Schwindenhammer, Thibaut Girardot, Kevin Chaulier, Arnaud Grégoire, Céline Monard, Laetitia Huriaux, et al. oXiris(R) use in septic shock: experience of two french centres. Blood Purif 47 (2019): 1-7.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 