The Prognostic and Therapeutic Significance of T-Helper-1 Cell Response in Patients with Severe Systemic Inflammatory Response or Sepsis
Neslihan Cabioglu MD, PhD1*, Esin Çetin Aktaş PhD2, Sema Bilgiç Gazioğlu PhD2, Bayram Kiran PhD3*, Kayhan Günay MD1, Cemalettin Ertekin MD1, Recep Güloğlu MD1, Muzaffer Akıncı MD4, Günnur Deniz PhD1
1Department of Surgery, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Türkiye
2Department of Immunology, Aziz Sancar Institute of Experimental Medicine, Istanbul University, Istanbul, Türkiye
3Department of Genetics and Bioengineering, Kastamonu University, Kastamonu, Türkiye
4Department of General Surgery, Haseki Training and Research Hospital, Health Sciences University, Istanbul, Türkiye
*Corresponding author:Neslihan Cabioglu, Department of Surgery, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Türkiye.
*He was affiliated with “the Department of Microbiology, Istanbul Medical Faculty, Istanbul University, Istanbul, Türkiye” during the study period.
Received: July 23, 2025; Accepted: July 07, 2025; Published: October 13, 2025
Article Information
Citation: Neslihan Cabioglu, Esin Çetin Aktaş, Sema Bilgiç Gazioğlu, Bayram Kiran, Kayhan Günay, Cemalettin Ertekin, Recep Güloğlu, Muzaffer Akıncı, Günnur Deniz. The Prognostic and Therapeutic Significance of T-Helper-1 Cell Response in Patients with Severe Systemic Inflammatory Response or Sepsis. Archives of Clinical and Biomedical Research. 9 (2025): 403-411.
View / Download Pdf Share at FacebookAbstract
Background/Objectives: Severe sepsis/multiorgan failure is the cause of 50 to 80% of all deaths in surgical intensive care units. The aim of the present study is to study peripheral lymphocyte subsets and identify the immunomodulatory role of in vitro effect of IL-12 on interferon-gamma secreting lymphocytes in severe surgical sepsis who were treated in intensive care unit (ICU).
Methods: Patients with either severe systemic inflammatory response (SIRS) or surgical sepsis who were treated at the ICU, were included into the study. Blood samples were analyzed to estimate peripheral blood leucocyte (PBL) and T-helper lymphocyte subsets by flow cytometry. The in vitro effect of IL-12 on IFN-gamma secreting PBLs and cytokine levels were studied using specific kits.
Results: The 28-day survival rate was 50% (7/14), whereas the overall survival rate was 28.5% (4/14). The median APACHE II, SOFA and MARSHALL MOD scores were 23 (range, 14-27), 7 (2-12) and 7 (2-11), respectively. In 28-day survival analysis, CD56+ cell populations were decreased in nonsurvivors (p=0.05), whereas T-gamma delta+ and CD3+HLADR+ cells were statistically diminished in patients with higher SOFA scores (p=0.032, and p=0.019, respectively) associated with poor outcome. In correlation analyses, CD3+ cell ratios were positively correlated with APACHE II and MOD scores (p=0.025 and p=0.047), whereas CD3+HLADR+ cells and T-gamma delta+ cells have shown negative correlations with SOFA scores (p=0.03 and p=0.01, respectively). In vitro culture assays with IL-12 stimulation of PBLs have shown that the total IFN-gamma secreting cells and their subsets could be significantly modulated upon peptide stimulation according to their median values. Furthermore, IL-12 enhanced IFN-gamma and IL-12 levels in either stimulated or unstimulated culture supernatants (p=0.025 and p=0.046 for IFN-gamma, p=0.028 and p=0.007 for IL-12).”
Conclusions: Our results suggest that IL-12 modulates antigen spesific IFNgamma secreting cells and IFN-gamma levels in patients with severe sepsis or severe SIRS in ICU. The prognostic significance of CD56+ and T-gamma delta+ cells in sepsis and whether IL-12 could be used for therapeutical purposes in severe septic patients should be studied in future clinical studies.
Keywords
Sepsis; Systemic inflammatory response; IL-12, IL-10; Immunology; Surgical sepsis
Sepsis articles; Systemic inflammatory response articles; IL-12 articles, IL-10 articles; Immunology articles; Surgical sepsis articles
Article Details
Introduction
Severe sepsis/multiorgan failure is the cause of 50 to 80% of all deaths in surgical intensive care units (ICU) [1]. Critically ill patients are usually admitted in ICU due to severe multiple trauma, hemorrhagic shock, severe pancreatitis, or intraabdominal sepsis followed by abdominal surgery including mesenteric ischemia, leakage after intestinal anastomosis at the emergency surgery clinics. All these conditions cause either systemic inflammatory response syndrome (SIRS), or compensatory anti-inflammatory response syndrome (CARS), or sepsis in the presence of bacteremia [1,2]. Severe injury including trauma and burn has been shown to be associated with decreased T helper-1 (TH1) lymphocyte function with preservation of T helper-2 (TH2) function, as indicated by cytokine production [3- 13]. Similarly, we previously demonstrated diminished lymphocyte- and monocyte-associated proinflammatory and anti-inflammatory cytokine levels associated with worse prognosis in patients with severe sepsis [6]. In animal models of injury and sepsis, therapeutic interventions including anti- IL-10 antibody or IL-12 administration to restore the TH1 function was associated with improved survival [14-17]. In contrast to these studies, anti-IFN-gamma or anti-interleukin 12 (IL-12) therapy was shown to reduce the mortality induced by endotoxin in mice that might occur due to the unopposed autocrine TH1 response [18,19]. Therefore, the exact role of IL-12 is still controversial in surgical trauma and sepsis.
In clinical studies, monocytes from patients with SIRS or sepsis have been mostly studied as leucocyte subset, whereas there have been few studies regarding the role of natural killer (NK) cells in patients with severe SIRS or sepsis [20, 21]. Murine experimental studies have demonstrated a deleterious proinflammatory effect of NK cells as a major source of interferon (IFN)-gamma [22-28]. The role of NK cells in human sepsis is to be studied more detailed [23-33].
The aim of the present study is to identify the role of in vitro effect of IL-12 on the subgroups of the peripheral blood interferon-gamma secreting lymphocytes including NK cells, and T 1 and T 2 cytokines like IL-2 and IL-4 and interferon-gamma inducing cytokines such as IL-12 and IL-18. Furthermore, peripheral blood leucocyte analysis including the percentage of T 1 to T 2 cells by measurements of intracytoplasmic IL-4 and interferon-gamma expression, and T lymphocyte and monocyte cultures were also carried out for assessment of monocyte and T lymphocyte functions by analyzing the culture supernatant cytokine levels.
Material and Methods
Patients with either severe SIRS (severe trauma, haemorrhagic shock, intraabdominal sepsis, severe pancreatitis, major abdominal operation), or severe sepsis were eligible at the Emergency Surgical Intensive Care Unit of the Department of Surgery, Istanbul Faculty of Medicine, Istanbul University. Ethical approval was obtained from the Ethical Board at the Istanbul Faculty of Medicine, Istanbul University. The Acute Physiology and Chronic Health Evaluation (APACHE II), Sepsis-related Organ Failure Assessment (SOFA) and multi-organ dysfunction (MOD) scores were used to assess the severity of disease in critically ill patients [34-36].
Blood samples were analyzed to estimate peripheral blood leucocyte (PBL) and T-helper lymphocyte subsets by flow cytometry. The in vitro effect of IL-12 on IFN-gamma secreting PBLs and cytokine levels were studied using specific kits.
PBL subset analysis and assessment of TH1 and TH2 cells
Blood samples were studied to analyze PBL subgroups using spesific monoclonal antibodies for CD3+HLADR+ (Becton Dickinson, UK), CD14+HLA-DR+ (Becton Dickinson, UK), CD56 (Serotec, UK), and T-gamma delta+ cells (Caltag, USA) using spesific monoclonal antibodies by flowcytometry. Plasma samples were stored for cytokine analyses by specific ELISA kits for IL-12 and IL-18. Furthermore, the T-helper lymphocyte subset analysis was performed by estimating the intracytoplasmic IL-4 and IFN- gamma secretions by using a “Permiabilization Kit”.
Induction and assessment of TH1 and TH2 cells
For intracellular cytokine staining, whole blood samples (1x106/mL mononuclear cells) were stimulated with Poly- myristate-acetate (PMA) plus ionomycin for 16 hours at room temperature, then 2.5 µl monensin (2mmol) was added and incubated for 2 hours in dark at room temperature. The intracytoplasmic cytokine kit “Leucoperm (Serotec, BUF09) was used for fixation and permeabilization of cells for immunofluorescent staining of intracytoplasmic cytokines.
Cells then were stained with 5 µl fluorescein isothiocyanate (FITC)-anti CD2 (Coulter, USA) and 5 µl FITC-mouse IgG1 (0.5 mg/ml) isotype-matched antibodies (Pharmingen, San Diego, USA) for 30 minutes at dark. Erythrocytes were eliminated using lysing solution (Becton-Dickinson, San Jose, USA) and BD FACS Flow solution (Becton-Dickinson, San Jose, USA) thoroughly was used for fixation and permeabilization of the cells for immunofluorescent staining of intracytoplasmic cytokines.
The resuspended fixed/permeabilized cells were further stained with 5 µl phycoerythrin(PE)-mouse anti-IL-4 (0.2 mg/ml, Pharmingen, San Diego, USA) or 5 µl mouse anti- IFN-gamma (0.2 mg/ml, Pharmingen, San Diego, USA) or 5 µl PE-mouse IgG1 isotype-matched control antibodies (0.5 mg/ml, Pharmingen, San Diego, USA) for 30 minutes. Cells were washed two times with 1X Perm/Wash buffer resuspended with BD FACS Flow solution as final volume of a 500 µl sample and analyzed on a FACS Calibur (Becton Dickinson, San Jose, USA). The supernatants obtained from the 16 h cultures were studied for analysis of IL-2, IL-4 and IFN-gamma cytokines.
Analysis of IFN-gamma secreting cells
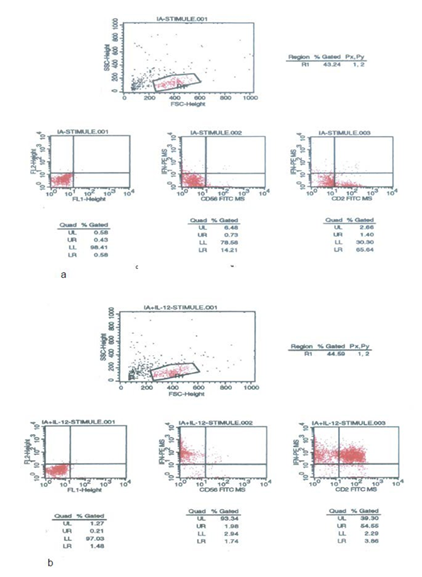
The effect of IL-12 on IFN-gamma secreting cells stimulated with Influenza A (IA) was evaluated using “IFN- gamma Secretion Assay-Cell Enrichment and Detection Kit” (Miltenyi Biotec) using specific immunomagmetic beads to collect the IFN-gamma secreting cells based on a positive selection method by using magnetic activated cell sorting (MACS) colones. IA (16 h), phosphate buffered saline (PBS) (24 h) and phytohaemagglutinin (PHA) (72 h) stimulated PBLs were studied for analyzing the total PBL levels along with CD2+ (CD2+FITC, Coulter) and CD56+ (CD56+FITC,
Serotec) cell subsets as IFN-gamma secreting cells followed by incubation with “IFN-gamma Catch Reagent” and “IFN- gamma detection antibody (IFN-gamma PE)”. The culture supernatants were assessed for IL-2, IL-4, IL-12, IL-18, IFN-gamma levels and plasma samples were evaluated to determine IL-12 and IL-18 levels using specific ELISA kits. Furthermore, IL-2, IL-4 and IFN-gamma were studied in supernatants obtained from PHA or lipopolisaccharide (LPS) stimulated (72 h) PBL cultures. Specific ELISA kits were used for determination of IL-2, IL-12 and IL-18 cytokines (Diaclone Research, USA), IL-4 and IFN-gamma (Endogen, USA).
Statistical analysis
All immunological findings were tested in terms of APACHE II, SOFA and MOD scores that were associated with the severity of sepsis in patients. High APACHE II and SOFA scores were defined as values above the median values. Paired parameters were analyzed with Wilcoxon test, and independent parameters were evaluated with Mann Whitney-U test. Spearman correlation test was used for defining the relationship between different parameters.
Results
The median age was 57 (range, 19-70). Clinical and demographic characteristics of patients (n=14) who were treated at the ICU due to severe surgical sepsis or SIRS were presented in Table 1. The 28-day survival rate was 50% (7/14), whereas the overall survival rate was 28.5% (4/14). The median APACHE II, SOFA and MARSHALL MOD scores were 23 (range, 14-27), 7 (2-12) and 7 (2-11), respectively.
Correlations between immunological parameters were shown in Table 2. Briefly, CD3+ cell ratios were found to be positively correlated with APACHE II and MOD scores (p=0.025 and p=0.047, respectively), while CD3+HLADR+ cell ratios were negatively correlated with SOFA scores (p=0.03). The T-gamma delta+ cell ratios also have shown negative correlations with SOFA and MOD scores (p=0.01 and p=0.05, respectively). The CD14+HLADR+ ratios had positive correlations with IL-18 levels of LPS-stimulated PBL culture supernatants (p=0.024).
Table 1: Clinical and demographic characteristics of patients who were treated at the ICU due to severe surgical sepsis or systemic inflammatory response syndrome (SIRS).
|
Patient code |
Age |
Sex |
Diagnosis |
APACHE II |
SOFA |
MOD |
Outcome |
|
1 |
67 |
Male |
Necrotizing pancreatitis |
27 |
7 |
6 |
Ex (49th day) |
|
2 |
51 |
Female |
Mesentery ischemia |
23 |
10 |
8 |
Ex (24th day) |
|
3 |
19 |
Male |
Thoracic&abdominal gunshot injury (liver+spleen+duodenum+colon injury) |
20 |
24 |
5 |
Ex (19th day) |
|
4 |
20 |
Female |
Blunt abdominal injury (Grade V liver injury) due to car accident |
22 |
24 |
5 |
Discharged (80th day) |
|
5 |
62 |
Female |
Severe multiple injury due to the car accident (frontal contusion+blunt thoracic injury+renal contusion+Grade I liver injury) |
14 |
9 |
7 |
Discharged (44th day) |
|
6 |
63 |
Female |
Mesenteric emboli |
22 |
3 |
2 |
Ex (12th day) |
|
7 |
52 |
Female |
Multiple small intestinal perforation |
24 |
12 |
10 |
Ex (49th day) |
|
8 |
70 |
Male |
peptic ulcer bleeding |
23 |
11 |
11 |
Ex (10th day) |
|
9 |
65 |
Male |
Mesenteric emboli |
24 |
7 |
7 |
Ex (22nd day) |
|
10 |
60 |
Female |
Acute mechanical intestinal obstruction due to sigmoid tumor |
23 |
12 |
9 |
Ex (10th day) |
|
11 |
51 |
Female |
Severe multiple injury due to the car accident (intraserebral contusion&blunt thoracic injury&bilateral radius fracture) |
18 |
4 |
2 |
Discharged (121st day) |
|
12 |
46 |
Female |
Necrotizing pancreatitis |
27 |
5 |
7 |
Ex (38th day) |
|
13 |
54 |
Female |
Necrotizing pancreatitis |
20 |
4 |
2 |
Discharged (127th day) |
|
14 |
62 |
Male |
ileum necrosis |
20 |
4 |
3 |
Ex (24th day) |
Table 2: Correlation analyses between immunological parameters obtained from PBL analyses.
|
Parameters |
P-value |
Correlation Coefficient |
|
CD3+ ratio vs APACHE II |
0.025 |
0.73 |
|
CD3+ ratio vs MOD |
0.047 |
0.67 |
|
CD3+ HLADR+ ratio vs SOFA |
0.03 |
-0.68 |
|
T-gamma delta+ cell ratio vs SOFA |
0.01 |
-0.80 |
|
T-gamma delta+ cell ratio vs MOD |
0.05 |
-0.66 |
|
CD3+ratio vs CD2-IL4+ |
0.067 |
0.63 |
|
CD3+ ratio vs CD2-IL4/CD2-IFN-gamma |
0.05 |
0.67 |
|
CD14+HLADR+ ratio vs IL-18LPS-stimulated |
0.024 |
0.70 |
|
CD2-IL4+ratio vs IL-18LPS-stimulated |
0.005 |
-0.75 |
|
CD56+ ratio vs *Total PBL-IFN-gamma+ ratio stimulated |
0.028 |
0.76 |
|
CD56+ ratio vs *CD2-IFN-gamma+stimulated |
0.028 |
0.76 |
|
CD56+ ratio vs plasma IL-18 |
0.047 |
-0.71 |
|
*CD56-IFN-gamma+stimulated vs IL-18 |
0.03 |
-0.68 |
|
IFN-gamma PHA-stimulated vs IL-12 LPS-stimulated |
0.001 |
0.84 |
|
*stimulated with Influenza A peptide |
||
Similarly, T-gamma delta+ and CD3+HLADR+ cell ratios were found to be statistically decreased in patients with high SOFA scores compared to those with lower SOFA scores (p=0.032, and p=0.019, respectively). In 28-day survival analysis, CD56+ cell populations were found to be decreased in nonsurvivors (p=0.05). No statistically significant changes were encountered in other parameters.
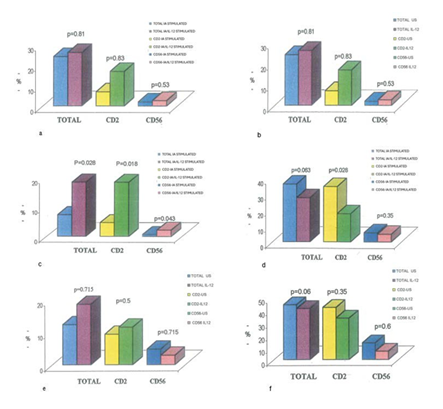
In flowcytometric analysis, no significant changes were found in both IA-stimulated (p=0.96, p=0.80, and p=0.44), and IA-unstimulated with/without IL-12 (p=0.81, p=0.83, p=0.53) total, CD2+ and CD56+IFN-gamma secreting cells, respectively. However, when the median value of total IFN-gamma secreting cell ratio as 24% (0.83%-63.5%) and CD2+IFN-gamma+ cell ratio as 9% (0.5%-57.1%) were taken as cut-off value into consideration, IL-12 administration increased the total and CD2+IFN-gamma secreting lymphocyte ratios under these values, but conversely decreased the ratios above these values in IA-stimulated cultures (p=0.028 and 0.063 for total lymphocytes, p=0.018 and p=0.028 for CD2+ cells, respectively). Furthermore, CD56+IFN-gamma+ lymphocyte ratios under the median value 2% (0.4-15.4%) were also found to be increased (p=0.043), while no changes were observed in CD56+IFN- gamma+ cell ratios above this value in IA-stimulated cultures
Figure 1: a) Effect of IL-12 on IFN-gamma secreting lymphocytes (shown as median values) in Influenza A (IA)-stimulated cultures, b) Effect of IL-12 on IFN-gamma secreting lymphocytes (shown as median values) in IA-unstimulated (US) cultures, c) Effect of IL-12 on low IFN- gamma secreting lymphocytes (shown as median values) in IA-stimulated cultures, d) Effect of IL-12 on high IFN-gamma secreting lymphocytes (shown as median values) in IA-stimulated cultures, e) Effect of IL-12 on low IFN-gamma secreting lymphocytes (shown as median values) in unstimulated cultures, f) Effect of IL-12 on high IFN-gamma secreting lymphocytes (shown as median values) in unstimulated cultures.
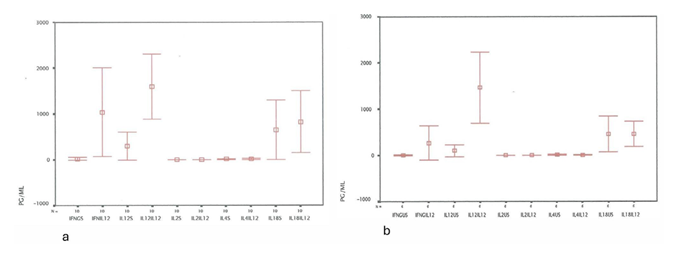
Figure 3: Effect of IL-12 on cytokine expression in either Influenza A(IA)-stimulated (Figure 3a), or unstimulated (US) cultures (Figure 3b). IL-12 enhanced the IFN-gamma and IL-12 levels in either stimulated or unstimulated culture supernatants (p=0.025 and p=0.046 for IFN- gamma, p=0.028 and p=0.007 for IL-12). Contrarly, no significant differences were estimated in IL-2, IL-4 and IL-18 levels.
following IL-12 administration (Figure 1a-f, Figure 2a, and Figure 2b). Nevertheless, IL-12 enhanced the IFN-gamma and IL-12 levels in either stimulated or unstimulated culture supernatants (p=0.025 and p=0.046 for IFN-gamma, p=0.028 and p=0.007 for IL-12). Contrarly, no significant differences were estimated in IL-2, IL-4 and IL-18 levels (Figure 3a and Figure3b) .
Discussion
Mortality from severe SIRS, sepsis and septic shock is still dramatically high, in spite of major advances in critical care medicine. The patient cohort studied in the present study suffers from either severe SIRS or sepsis with either high APACHE II or SOFA or MOD scores, and therefore had a high mortality rate as a 28-day survival rate of 50%, and an overall survival rate of 28.5%. We report here that CD3+HLADR+ and T-gamma delta+ cell ratios have shown negative correlations with the severity of sepsis and multiorgan dysfunction. Sáenz et al. [37] have reported decreased monocyte count, CD13+, CD14+, CD13+HLADR+ and CD14+HLADR+ ratios in septic patients who were nonsurvivors, whereas no difference could be found in CD3+ and CD3+HLADR+ levels. However, Ditschkowski et al. [38] have found decreased HLA-DR expression on T cells in patients with severe sepsis associated with major trauma compared to those with minor trauma or major trauma without sepsis in concordance with our findings [38]. Therefore, more data are needed on the role of CD3+HLADR+ cells in sepsis. Interestingly, IL-18 levels of LPS-stimulated PBL cultures correlated with CD14+HLADR+ ratios indicating IL-18 may play an important role in monocyte and T cell activation similar to prior studies [39,40].
Furthermore, Yang et al. [41] have demonstrated impaired antigen-presenting function of T-gamma delta+ cells in patients with sepsis [41]. The T-gamma delta+ cells obtained from septic patients responded poorly to 4-hydroxy- 3-methyl-2-butenyl pyrophosphate (HMBPP) stimulation, characterized by the deactivation of these antigen presenting cell (APC) markers including HLA-DR, CD27, CD80, and CCR7 with inhibition of adherence to E.coli and impaired proliferation in co-culture assays compared to those obtained from healthy individuals. Similarly, a progressive decrease in T cells, being much more intense in T-gamma delta+ cells was observed in septic patients (n=92) correlated with the severity of the septic process that might occur due to the apoptotic processes during severe sepsis [42]. Apoptosis of CD3+CD56+ and T-gamma delta+ cells has been shown to be increased associated with the severity of sepsis, especially in non-survivors [42,43]. Briefly, mortality was associated with a significant decrease in T-gamma delta+ cells.
Natural killer cells indicated by CD56+ phenotype has been demonstrated to play an important role in sepsis [21-33]. In the present study, we have found decreased CD56+ NK cell ratios in nonsurvivors in addition to CD14+HLADR+ ratios and T-gamma delta+ cells. Forel et al. [32] have demonstrated that the absolute number of PBL CD3–CD56+ NK cells was reduced in all groups of ICU patients, but with a normal percentage of NK cells [32]. Of note, decreased IFN-gamma production by NK cells obtained from PBL of septic patients was observed in cocultures with K562 cell line supernatants compared to healthy controls (6.2[2.2-9.9] % vs 10.2[6.3- 13.1] %, p<0.01), especially in patients with septic shock. In contrast, patients with SIRS exhibited increased IFN- gamma production (42.9[30.1-54.7]% vs 18.4[11.7-35.7]%, p<0.01) compared to sepsis-group, respectively. Roquilly et al. [33] similarly studied NK cells in patients (n=32) with severe traumatic brain injury (TBI) comparing with cardiac surgery patients (n=11), or healthy controls (n=29). Briefly, NK cells of TBI patients were found to have a differentiated phenotype expressing KIR and CD57 markers. A decreased NK-cell response was obtained by lower degranulation and lower IFN-gamma production after stimulation with HLA class I deficient cell line, whereas the NK-cell ADCC was not changed. Interestingly, both IFN-gamma production and the cytotoxicity capacities of NK cells could be restored by addition of IL-12 to the culture. These results suggest that NK-cell function was found to be decreased in critically-ill septic patients due to the impaired IFN-gamma production. Similarly, Coakley et al. [44] have found significantly (p = 0.036) fewer NK cells expressing IFN-gamma in septic patients (n=42) than in both the control group (n =30) and the infection group (n=30) [44].
In the current study, we further analyzed IFN-gamma secreting lymphocyte subpopulations in both unstimulated, and IA-peptide stimulated cultures with/without IL-12. Briefly, we were not able to detect any changes in total or other subpopulations including CD2+ and CD56+ lymphocytes. However, when the median values were considered as cut-off values, IL-12 administration restored the total, CD2+ and CD56+ IFN-gamma secreting lymphocyte ratios of those patients with relatively low values that were estimated below the cut-off values in peptide-stimulated cultures. Interestingly, IL-12 either modulated the total and CD2+ ratios above these values to the median values, while no changes have been obtained in CD56+ ratios in peptide- stimulated cultures. Overall, increased IFN-gamma and IL- 12 levels were estimated in either unstimulated or stimulated culture supernatants with IL-12 administration despite no changes in IL-2, IL-4 and IL-18 levels in patients with severe sepsis or SIRS.
We first demonstrate here, IL-12 has enhanced IFN- gamma levels in in vitro peptid-stimulated peripheral blood mononuclear cell culture supernatants, whereas it has modulated antigen spesific IFN-gamma secreting cells. We recently have reported that IL-12-administration improved the survival rate of septic mice with bacterial peritonitis which was induced by cecal ligation and puncture by restoring the number of TH1 and TH2 cells, while IL-10 administration alone resulted in lower survival rate compared to sham- operated mice [45]. In sepsis biology, massive activation of mononuclear phagocytes by bacterial components and release of proinflammatory cytokines are rapidly compensated by an anti-inflammatory response, defined as immunoparalysis or CARS, which can cause secondary nosocomial infections increasing mortality. Patients may also exhibit both hyper- inflammation and immune paralysis concomitantly, with one being transiently dominant over the other [1,2]. All these complex conditions may partly explain the failure of anti-inflammatory drugs in management of severe sepsis in ICU. Thus, only better characterization of the nature of immune responses during severe sepsis and septic shock can enable design of appropriate immuno-interventions. Therefore, clinical studies should focus on agents with an immunomodulatory effect in the treatment of severe sepsis or SIRS [46,47].
In conclusion, IL-12 might be a good candidate for therapeutical use in severe septic patients with either immunological anergy or SIRS. Furthermore, the prognostic significance of CD56+ and T-gamma delta+ cells in sepsis should be studied in future studies.
Conflict of Interest:
None of the authors have conflict of interest.
Funding:
This study is supported by the Research Fund of the Istanbul University (Project Numbers: G-1384/081299 and T-920/06112000).
Author contributions:
“Conceptualization, NC and GD; methodology, NC, EA, SB,BK, GD; data curation and manuscript writing; NC; review and editing: EA, SB, BK, KG, CE, RG, MA, GD. All authors have read and agreed to the published version of the manuscript.
References
- Bone RC, Balk RA, Cerra FB, et American College of Chest Physician’s/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organe failure and guidelines for the use of innovative therapies in sepsis. Chest 101 (1992): 1644-1655.
- Bone RC: Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med 7 (1996): 1125-1128.
- O'Sullivan ST, Lederer JA, Horgan AF, et Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg 222 (1995): 482-492.
- Puyana JC, Pellegrini JD, Kumar De A, et Both T-helper-1 and T-helper-2 type lymphokines are depressed in posttrauma anergy. J Trauma 6 (1998): 1037-1046.
- Spolarics Z, Siddiqi M, Siegel JH, et Depressed interleukin-12-producing activity by monocytes correlates with adverse clinical course and a shift toward Th2-type lymphocyte pattern in severely injured male trauma patients. Crit Care Med 31 (2003): 1722-1729.
- Cabioglu N, Bilgic S, Deniz G, et al. Decreased cytokine expression in peripheral blood leukocytes of patients with severe Arch Surg 137 (2002): 1037-1043.
- Ono S, Uenoa C, Aosasaa S, et Severe sepsis induces deficient interferon-gamma and interleukin-12 production, but interleukin-12 therapy improves survival in peritonitis. Am J Surg 182 (2001): 491-449.
- Wood JJ, Rodrick ML, O’Mahony JB, et al. Inadequate interleukin 2 production: a fundamental immunological deficiency in patients with major burns. Ann Surg 200 (1984): 311-320.
- Decker D, Schondorf M, Bidlingmaier F, et al. Surgical stress induces a shift in the type-1/type-2 T helper cell balance, suggesting down-regulation of cell mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 119 (1996): 316-325.
- Mokart D, Capo C, Blache JL, et al. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with Br J Surg 89 (2002): 1450- 1456.
- Mack VE, McCarter MD, Naama HA, et al. Dominance of T-helper 2-type cytokines after severe injury. Arch. Surg 131 (1996): 1303-1309.
- Sherry RM, Cue JI, Goddard JK, et Interleukin-10 is associated with the development of sepsis in trauma patients. J Trauma 40 (1996): 613-616.
- Lyons A, Kelly JL, Rodrick ML, et Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann. Surg 4 (1997): 450-460.
- Kelly JL, Lyons A, Soberg CC, et Anti-IL-10 antibody restores burn induced defects in T cell function. Surgery 122 (1997): 246-252.
- Zisman DA, Kunkel SL, Strieter RM, et Anti- interleukin-12 therapy protects mice in lethal endotoxemia but impairs bacterial clearance in murine Escherichia coli peritoneal sepsis. Shock 8 (1997): 349-356.
- Göebel A, Kavanagh E, Lyons A, et al. Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann Surg 231 (2000): 253-261.
- O’Suilleabhain C, O’Sullivan ST, Kelly JL, et Interleukin-12 treatment restores normal resistance to bacterial challenge after burn injury. Surgery 120 (1996): 290-296.
- Silva AT, Cohen Role of interferon-gamma in experimental gram-negative sepsis. J Infect Dis 166 (1992): 331-335.
- Zisman DA, Kunkel SL, Strieter RM, et Anti- interleukin-12 therapy protects mice in lethal endotoxemia but impairs bacterial clearance in murine Escherichia coli peritoneal sepsis. Shock 8 (1997): 349-356.
- Docke W, Randow F, Syrbe U, et Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nature Med 3 (1997): 678-681.
- Müller-Heck RM, Bösken B, Michiels I, et al. Major Surgical Trauma Impairs the Function of Natural Killer Cells but Does Not Affect Monocyte Cytokine Life (Basel) 12 (2021): 13.
- Emoto M, Miyamoto M, Yoshizawa I, et Critical role of NK cells rather than V alpha 14(+) NKT cells in lipopolysac charide-induced lethal shock in mice. J Immunol 169 (2002): 1426-1432.
- Carson WE, Yu H, Dierksheide J, et al. A fatal cytokine- induced systemic inflammatory response reveals a critical role for NK J Immunol 162 (1999): 4943-4951.
- Kerr AR, Kirkham LA, Kadioglu A, et al. Identification of a detrimental role for NK cells in pneumococcal pneumonia and sepsis in immunocompromised Microbes Infect 7 (2005): 845-852.
- Badgwell B, Parihar R, Magro C, et al. Natural killer cells contribute to the lethality of a murine model of Escherichia coli Surgery 132 (2002): 205-212.
- Etogo AO, Nunez J, Lin CY, et al. NK but not CD1- restricted NKT cells facilitate systemic inflammation during polymicrobial intra-abdominal sepsis. J Immunol 180 (2008): 6334-6345.
- Chiche L, Forel JM, Thomas G, et al. The role of natural killer cells in J Biomed Biotechnol (2011): 986491.
- Heremans H, Dillen C, van Damme J, et Essential role for natural killer cells in the lethal lipopolysaccharide- induced Shwartzman-like reaction in mice. Eur J Immunol 24 (1994): 1155-1160.
- Souza-Fonseca-Guimaraes F, Parlato M, Philippart F, et al. Toll-like receptors expression and interferon-γ production by NK cells in human sepsis. Crit Care 16 (2012): R206.
- Hauser CJ, Joshi P, Jones Q, et al. Suppression of natural killer cell activity in patients with fracture/soft tissue Arch Surg 132 (1997): 1326-1330.
- Pan A, Deng Y, Yang T, et al. Phenotype and functions of natural killer cells in septic patients and its clinical Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 26 (2014): 827-31.
- Forel JM, Chiche L, Thomas G, et al. Phenotype and functions of natural killer cells in critically-ill septic PLoS One 7 (2012): e50446.
- Roquilly A, David G, Cinotti R, et al. Role of IL-12 in overcoming the low responsiveness of NK cells to missing self after traumatic brain injury Clin Immunol 177 (2017): 87-94.
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification Crit Care Med 13 (1995): 818-829.
- Marshall JC, Cook DJ, Christou NV, et Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 23 (1995): 1638-1652.
- Vincent JL, Moreno R, Takala J. The SOFA (Sepsis- related Organ Failure Assessment) score to describe organ dysfunction failure. Intensive Care Med 22 (1996): 707-710.
- Sáenz JJ, Izura JJ, Manrique A, et Early prognosis in severe sepsis via analyzing the monocyte immunophenotype. Intensive Care Med 27 (2001): 970-7.
- Ditschkowski M, Kreuzfelder E, Rebmann V, et HLA- DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg 229 (1999): 246-54.
- Munder M, Mallo M, Eichmann K, et Murine macrophages secrete interferon-gamma upon combined stimulation with interleukin-12 and interleukin-18: A novel pathway of autocrine macrophage activation. J Exp Med 187 (1998): 2103-2108.
- Yoshimoto T, Mizutani H, Tsutsui H, et IL-18 induction of IgE: dependence on CD4+T cells, IL-4 and STAT6. Nature Immunol 1 (2000): 132-137.
- Yang X-W, Li H, Feng T, et al. Impairment of antigen- presenting function of peripheral γδ T cells in patients with Clin Exp Immunol 207 (2022): 104-112.
- Andreu-Ballester JC, Tormo-Calandín C, Garcia- Ballesteros C, et Association of γδ T cells with disease severity and mortality in septic patients. Clin Vaccine Immunol 20 (2013): 738-46.
- Andreu-Ballester JC, Arribas MA, Rico M, et Changes of CD3+CD56+ γδ T cell number and apoptosis during hospital admission are related to mortality in septic patients. Clin Immun 236 (2022): 108956.
- Coakley JD, Breen EP, Moreno-Olivera A, et Innate lymphocyte Th1 and Th17 responses in elderly hospitalised patients with infection and sepsis. Vaccine (Basel) 8 (2020): 311.
- Kiran B, Zeybek Ü, Turna A, et Increased survival after cecal ligation and puncture in mice delivering interleukin 12: the role of Th1 and Th2 cell. Arch Clin Biomed Res 9 (2025): 177-182.
- Yang Q, Feng Z, Ding D, et al. CD3 and CD247 are the molecular targets of septic shock. Medicine (Baltimore) 102 (2023): e34295.
- Neurath New therapies for sepsis: focus on the interleukin (IL)12 family member IL27. Ann Rheum Dis 66 (2007): iii29-iii31.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 