Uncommon Neonatal Hemi-Diaphragmatic Paralysis: Case Reports and Literature Review
Audrey Carlhan-Ledermann1†, Otis Olela1†, Riccardo E Pfister1, Sylviane Hanquinet2, Francisca Barcos Munoz1, *
1Department of Pediatrics, Division of Neonatology and Pediatric Intensive Care Unity, University Children’s Hospital of Geneva, University Geneva, Geneva, Switzerland
2Department of Radiology, Unit of Pediatric Radiology, University Children’s Hospital of Geneva, University of Geneva, Geneva, Switzerland
†These authors have contributed equally to this work and share first authorship
*Corresponding author: Francisca Barcos Munoz, Department of Neonatology and Pediatric Intensive Care University Hospitals of Geneva Rue Willy Donzé 6, 1205 Genève, Switzerland
Received: 28 December 2021; Accepted: 04 January 2022; Published: 14 February 2022
Article Information
Citation: Audrey Carlhan-Ledermann, Otis Olela, Riccardo E Pfister, Sylviane Hanquinet, Francisca Barcos Munoz. Uncommon Neonatal Hemi-Diaphragmatic Paralysis: Case Reports and Literature Review. Archives of Clinical and Biomedical Research 6 (2022): 228-239.
View / Download Pdf Share at FacebookAbstract
Background: Diaphragmatic paralysis (DP) is a rare cause of respiratory distress in newborns with potentially severe outcome. Two cases illustrate the clinical presentation, etiology, evaluation, treatment, and outcome of diaphragmatic paralysis in newborn.
Cases presentation: First, a 890 g male preterm of 26 weeks who developed a left DP after pleural drain placement and completely recovered within 6 to 9 months without surgery. The second case, a female neonate born at 38 1/7 weeks of gestation, developed a right DP secondary to obstetrical trauma with associated Erb’s palsy and completely recovered spontaneously within one week.
Conclusions: Diaphragmatic paralysis is a rare but potentially life-threatening condition in newborns. Indications and timing for surgical or conservative treatment remain controversial. DP management should be addressed in a multidisciplinary manner. With optimized respiratory and feeding management, spontaneous favorable outcomes may be expected, avoiding long-term complications of a surgical plication, but require concerted expectant management and considerable time. Our cases encourage supportive expectant management.
Keywords
<p>Neonatal Hemi-Diaphragmatic Paralysis</p>
Article Details
1. Background
The first case of unilateral diaphragmatic paralysis (DP) was reported in 1902 by Naunyn and published cases remain sporadic. DP is a rare cause of respiratory distress in neonates, mostly caused by ipsilateral phrenic nerve injury after cardiac surgery or obstetrical trauma [1-3]. More than in adults, breathing in infants strongly depend on their diaphragm. In addition, in the newborn poor compensatory respiratory mechanisms lead to serious complications with often prolonged ventilatory support, weaning failure, repeat infections, prolonged hospital stay and even death [1, 3]. Approximately 2-6% of newborns with shoulder dystocia related brachial plexus injury (BPI) develop DP with large differences in spontaneous recovery [3-5]. Pleural drain placement for pneumothorax is a common practice in neonatal intensive care units but occurrence of iatrogenic DP remains excessively rare, with only sparse literature reporting mostly favorable spontaneous recovery after prompt drain withdrawal [6].
Early diagnosis of DP allows planning of proper management, follow-up and surgery, when indicated. Careful ventilatory and feeding strategies are keystones of supportive approach. The choice between a conservative or surgical approach highly depends on the expected spontaneous recovery potential weighted against the clinical severity of the condition. Despite growing literature advocating favorable clinical outcomes and security profiles of early hemidiaphragmatic plication in newborns, clear prognostic tools for time-guided management are lacking[2, 3]. We report two exemplary cases of DP, one after pleural drain placement, the other secondary to obstetric trauma, and complete with a literature review.
2. Case description
2.1 Case 1
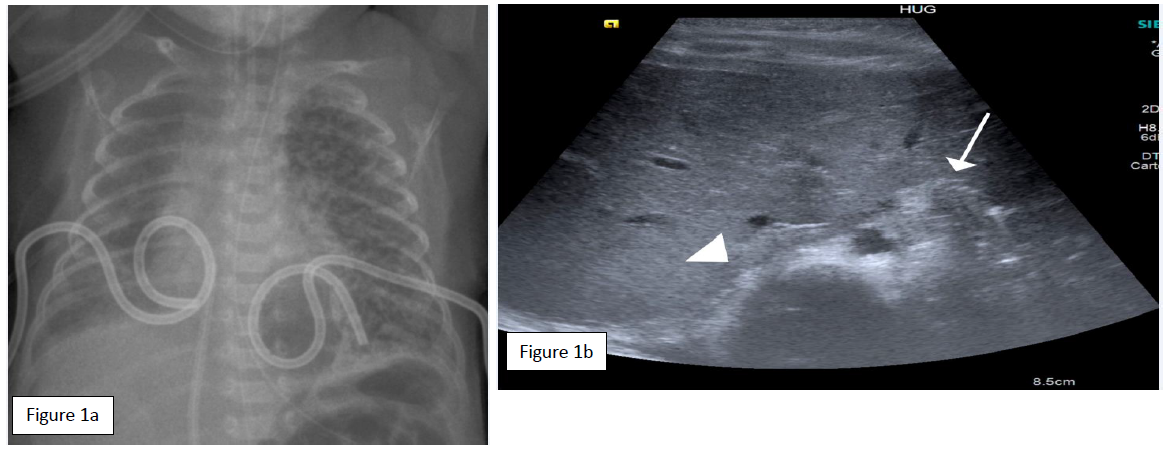
The 26 weeks’ gestation, 890 g (p25-50) male preterm, was born by emergency C-section from a 2P3G mother for placenta praevia hemorrhage, after completed pulmonary maturation. APGAR score was 8/9/10 at 1, 5 and 10 minutes, and continuous positive airway pressure (CPAP) with FiO2 0.3 was needed for RDS at birth. In the NICU he was intubated at 10 hours for increasing oxygen requirements and received two administrations of surfactant within the first 24 hours, the second rapidly followed by extubation. The chest x-ray follow-up showed a right pneumothorax and motivated a pleural drain placement (pigtail tube 5Fr) with successful air drainage. On 2 day of life (DOL), the patient developed a left pneumothorax with hemodynamic compromise (Fig 1a). After fentanyl and atropine, an emergency left pleural drain was placed in the 5th intercostal space (ICS) on the anterior axillary line with subsequent re-intubation for shallow breathing. The procedure was marked by a hemothorax (4 ml blood aspiration through the pleural drain) that required transfusion. No further bleeding occurred, and radiographic findings excluded a significant hemothorax. A favorable air-leak progression allowed right and left pleural drain withdrawal at day 3 and 5, respectively, followed by extubation to nasal CPAP.
At 7 DOL increasing respiratory compromise with hypercapnia (pH 7.13; PCO2 11.6 kPa) prompted a chest x-ray showing a left hemidiaphragmatic elevation. The oblique transverse diaphragmatic ultrasound in the subxiphoid plane confirmed the absence of left diaphragm movements compared to the contralateral hemidiaphragm (Figure 1b). A left DP was strongly suspected, either from phrenic nerve trauma or from irritation by the pleural hemorrhage during drain insertion. On frontal radiograph, the pleural tube position overlapped the diaphragmatic silhouette, with more than 2 cm distance from the tube tip to mediastinum and vertebrae.
After extubating the patient, non-invasive Neurally adjusted ventilatory assist (NAVA) was initially used with low benefits on CO2 washout, followed by nasal high frequency oscillatory ventilation (nHFOv) providing efficient support whilst still allowing permissive pCO2 levels (mean PCO2 11.7 kPa). Due to critically poor tolerance in right decubitus, the patient was nursed in alternating left-sided positions. Poor tolerance to nasogastric tube feeding with frequent feeding-associated episodes of apnea-bradycardia-desaturation and reduced postnatal growth led to continuous enteral feeding via a post-pyloric oro-duodenal tube from the 22nd DOL, undeniably followed by a nutritional improvement. Full oral feeds were reached at 34 DOL, with a satisfactory weight gain on a volume restricted (130 ml/k/d) high caloric diet (1 Cal/ml).
Progressive improvement allowed a relay from nHFOv to CPAP on day 31, high flow on day 51, and low flow on day 67. At 74 DOL, ultrasound demonstrated first small diaphragmatic movements with full ultrasonographic recovery at 9 months. At 36 weeks corrected gestational age (70 DOL), formal evaluation confirmed a moderate bronchopulmonary dysplasia (Walsh criteria) without retinopathy. Full weaning from oxygen was achieved at 99 DOL. A nocturnal oxycapnometry confirmed satisfactory ventilation and alveolar oxygenation and patient dismissal home at 112 DOL without any respiratory support, with a weight of 3900g (P50-75).
2.2 Case 2
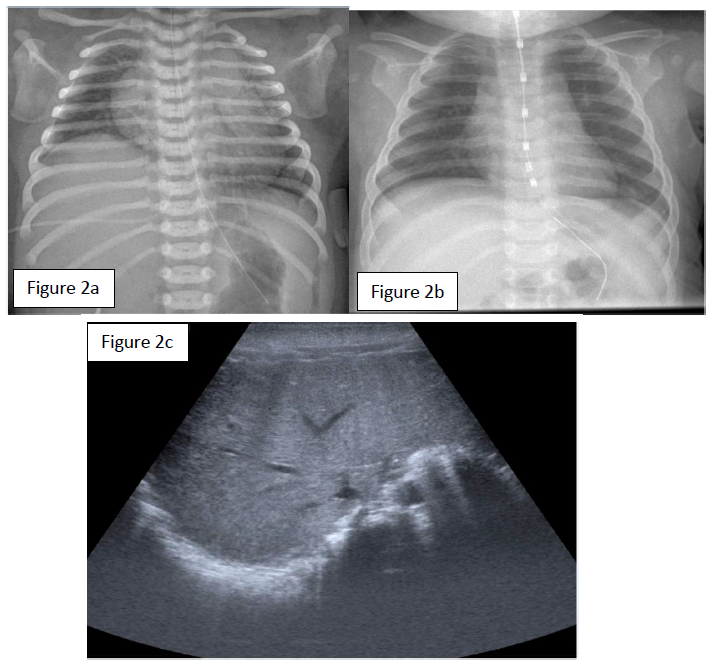
A female neonate was born to a 24-year-old G5P2 at 38 and 1/7 weeks of gestation. The pregnancy was marked by a poorly controlled type 1 maternal diabetes and a significant weight gain of 24 kg. To note, an antecedent of macrosomia with shoulder dystocia at a prior delivery. In consideration of risk factors, including an estimated fetal weight on the 98th centile, an induced delivery was planned at 38 2/7 weeks of gestation. A baby girl was born 1 day ahead of the planned date. Birth weight was 4745g (>P95). The vaginal delivery was complicated by shoulder dystocia, requiring obstetrical maneuvers (two Mac Roberts maneuvers and one Jacquemier maneuver), but no instrumentation. Variable decelerations were noted during delivery and amniotic fluid was meconium stained. The APGAR score was 3, 3, and 7, at 1, 5 and 10 minutes respectively, and the arterial and venous umbilical cord pH values were 7.21 and 7.26. Bag and mask ventilation was necessary for 10 minutes due to absent breathing and subsequently transitioned to nasal CPAP for respiratory distress. Due to respiratory distress with high oxygen dependency the girl was then nasotracheally intubated. The first chest x-ray at 20 minutes of life showed a right hemidiaphragm elevation (figure 2a). Diaphragmatic ultrasound was performed and confirmed absent right hemi-diaphragm and normal left hemi-diaphragm movements (figure 2c). The right upper extremity remained adducted with internal rotation and minimal movements. On examination a significant weakness of the wrist and fingers extensions as well as shoulder abduction was consistent with Erb’s palsy. We concluded to an ipsilateral phrenic nerve injury with hemi-diaphragmatic paralysis secondary to right BPI. During the first day of life, we also observed spells of persistent fetal circulation with documented pulmonary hypertension.
The respiratory outcome was rapidly favorable, allowing extubation at 24 hours of life and weaning from CPAP at 4 DOL. The chest x-ray and focus ultrasonography confirmed resolution of the hemi diaphragmatic paralysis within one week (figure 2b). At 3 months the girl had complete resolution of the Erb’s palsy but had required a gastrostomy for motoric oral feeding difficulties of unknown origin. At 8 months, she had a normal psychomotor development except residual axial hypotonia and she still has failure to thrive of unknown origin.
3. Discussion
3.1 Epidemiology
With only few cases reported in the literature, the exact incidence of DP in neonates is not known. Iatrogenic DP after pleural drain is by far less common than after cardiac surgery, particularly PDA ligation, with an estimated incidence of 5% [1, 7] , and obstetrical trauma with an incidence between 1/15’000 and 1/30’000 live births [8], but affecting 2-6% of newborns with BPI. Very few other etiologies were reported in the literature; neck surgery, congenital varicella syndrome [9], lower brainstem hemorrhage, and associations with congenital hypomyelination neuropathy and congenital myotonic dystrophy [10]. Subclavian or jugular catheter insertion or also peripherally inserted central catheter lines with diffusion of parenteral nutrition around the phrenic nerve have also sporadically been reported [2].
3.2 Physiopathology and presentation of DP
Whatever the etiology, the younger the patient, the less DP is tolerated. The higher diaphragmatic dependence for adequate gas exchanges is explained by the high chest wall compliance and mediastinal mobility, hindering contralateral lung function as a compensatory mechanism [4]. DP typically presents as respiratory distress with an asymmetrical breathing pattern and paradoxical breathing. Gastrointestinal manifestations such as gastroesophageal reflux or vomiting [2], or digestive intolerance are common, as reported in our first case, probably secondary to ascension and reduced capacitance of the stomach. Unexplained weaning difficulties from mechanical ventilation, increased oxygen requirement and tachypnea are other clinical features that may reveal DP [11].
DP spectrum extends from minor traction palsy with rapid recovery to more severe injury leading to chronic respiratory failure, with an average spontaneous recovery delay from 7 days to 6 months [9]. Atelectasis and recurrent pneumonia are the more frequent midterm complications [2]. Increased work of breathing and chronic hypoxia may cause failure to thrive [3]. In the most severe cases, such as in our second case, persistent pulmonary hypertension and neurodevelopmental delays have been reported [2]. In summary, DP may lead to prolonged hospital stay, infections, failure to thrive, developmental delays, and even death [1].
3.3 Pleural drain
Pleural drain associated DP is very rare. Pigtail catheters have an excellent safety profile, and are today considered more secure than straight tubes, though occasional complication may occurs [12]. Reed and al. underlined that contextual emergency during the pleural drain procedure and the catheter length were both associated with higher phrenic nerve injury rate [13]. Mechanism of phrenic nerve injury reported are axonotmesis caused by pleural drain friction or compression, or necrosis and edema due to hemorrhage, but also direct stretching of the phrenic nerve due to tension pneumothorax [14,15].
From a procedural point of view, a safety margin between the pleural drain tip and the mediastinum or vertebrae of 1cm for newborn [14] and 2cm for children [12] is recommended on frontal chest x-ray. Nakagama and al. review 13 cases of DP due to drain malposition and report 6 spontaneous recoveries, suggesting favorable outcome after prompt drain withdrawal (less than 4 days). The maximal observed delay to full spontaneous recovery was 3 months. They proposed phrenic nerve compression as the causal mechanism, whose duration is crucial regarding the chances of recovery [6]. Our first case had removal of the drain at 4 days, however without rapid recovery, suggesting a traumatic injury rather than compression or irritation secondary to hemothorax.
3.5 Shoulder dystocia
Nerve traction injury is a known complication of shoulder dystocia during vaginal delivery, most often resulting in a BPI. However, 2-6% of infants with BPI also develop DP [3-5, 8]. Such patients may have associated fractures and poor adaptation, such as in our second case [2]. Most phrenic nerve injuries are ipsilateral to brachial plexus palsy (4,5); the right hemi-diaphragm is affected more often in 70-80% of cases [8, 11]. Risk factors of simultaneous phrenic nerve and BPI include breech presentation, maternal diabetes, instrumental extraction, shoulder dystocia, macrosomia and uterine malformations [3,4, 11].
The mortality associated with birth trauma DP is estimated 10-15% (4,8). BPI severity does not correlate with the severity of the respiratory disease (4), but concurrent DP and BPI predict often poor motor BPI recovery (5). The literature reports large differences in spontaneous recovery of DP associated with birth injury. Bowerson (4) and Stramrood (16) report that 70-75% of newborn needed diaphragmatic plication, while in the study of Rizeq (3) and Yoshida (5) only 23% and 5% underwent surgical repair, respectively. Our case showed a spontaneous recovery of the DP within one week and of Erb’s palsy after 3 months.
3.6 Diagnosis and follow-up of DP
Despite its low sensitivity, with false negative imaging due to respiratory support, chest x-rays represent a simple first investigation to detect an abnormally elevated hemidiaphragm. An elevation of more than one intercostal space (ICS) on the left side, and more than two ICS on the right side are considered relevant. It is advised though to realize the chest x-ray on spontaneous ventilation. Ultrasound is advantageous in allowing a dynamic diaphragmatic assessment, is feasible at bedside, and valuable for regular follow up. A transverse B-mode ultrasound in the subxiphoid plane may detect hemi-diaphragmatic dysfunction compared to the healthy hemi-diaphragmatic movement and precise local anatomical relations [17].
In a series of 278 pediatric patients, Epelman and al showed that the frontal chest radiograph had a low sensitivity(Sn) of 34% only to detect abnormal diaphragmatic motion, a specificity (Sp) of 86%, a positive predictive value (VPP) of 85% and negative predictive value (NPV) of 37% comparing to M-mode US [18]. Although fluoroscopy has historically been considered the gold standard for the diagnosis of DP, ionizing radiation and risks of patients transportation needs, made it obsolete [2]. A prospective study on 25 patients with post-cardiac surgery DP showed that US was perfect in predicting fluoroscopy result (100% Sn, Sp, PPV, NPV). Moreover, it significatively reduced the mean delay to diagnosis, from 17 hours for fluoroscopy to 15 minutes for US [9].
The use of electrophysiology studies with compound muscle action potential (CMAP) and conduction analysis after magnetic or electrical phrenic nerve stimulation are promising [2, 9, 20]. The prognostic contribution of this technique resides in categorizing the injury, for example distinguishing between contusion (prolonged latency) and laceration (absent signal), thus guiding clinicians in the choice between conservative or surgical therapy (16); a poor re-innervation pattern on the EMG is predictive of an inadequate recovery [2, 21]. Unfortunately, this practice remains today accessible in only few specific centers and its application may not be handy in the NICU context.
3.7 Management of DP
3.7.1 Conservative management
The first goal of neonatal DP management is the respiratory stabilization to restore adequate gas exchanges. CPAP alone is recognized to alleviate tachypnea and improve respiratory distress in such patients [5] and neurally adjusted ventilatory assist (NAVA) appears to be associated with less complications [2]. Roosens et al describe that the electric signal of one single hemi-diaphragm is sufficient to trigger non-invasive ventilation (NIV-NAVA): an optimal synchronization of the ventilation with the healthy hemi-diaphragm appears to compensate the dysfunctional hemidiaphragm [22] and may avoid (re-)intubation.
In our single case experience (first case), NIV-NAVA was ineffective to clear CO2, as the high driving pressures required resulted in abdominal distention and discomfort. However, nasal high-frequency oscillation (nHFOv) was effective for CO2 clearance at lower mean airway pressures (max 15mmHg). Generally, patients require mechanical ventilation for stabilization, with risk of weaning failure. Unfortunately, long term mechanical ventilation exposes to volo-/barotrauma, pneumonia and might induce diaphragm atrophy even of the healthy side [23].
Likewise, long term oxygen therapy favors chronic lung disease and development of its free radial complications such as retinopathy in preterm neonates and chronic lung disease. For most patients, weaning from the ventilator still requires several weeks [4] and alternate non-invasive ventilation strategies appear promising.
3.7.2 Surgical management with plication
Surgical plication of the diaphragm by thoraco- or laparoscopic approach is the most common therapeutic intervention and presents less complication than classical open thoracotomy. Diaphragmatic plication decreases lung compression, stabilizes the thoracic base, mediastinum, thus supporting respiratory and abdominal muscles [24]. The security profile of the procedure, which doesn’t prevent subsequent recovery of the diaphragm motility, is now well accepted [2]. There is no clear consensus though, on the best timing of surgical repair that remains controversial. As learned mainly from studies following cardiac surgery or post-obstetrical trauma, early plication for DP seems rapidly beneficial for the respiratory improvement compared to the relatively slow spontaneous recovery. This was particularly true in children younger than 1 year old with increased mortality risk due to mechanical ventilation exceeding 10 days [1, 11].
The literature review reports the earliest interventions at 10 days of life extending up to 15 months, with a median age at surgery of 35 days [3]. More recent studies suggest surgical repair should be rapidly considered despite a possibility of spontaneous recovery, particularly for infants with respiratory insufficiency, where more aggressive intervention results in a shorter total hospital stay, lower costs, and lower readmission rates. The intervention is considered simple, safe and durable, even in extremely low birth weight neonates [3, 21, 24, 25]. The suggested best age for surgery in obstetrical trauma DP varies from 19 days (16) to 45 days (2), with an apparent 30 days threshold below which a better ventilatory outcome and lower morbidity can be expected [8]. Indications mainly rely on patient’s age, dependence on respiratory support, weaning failure and failure to thrive.
Our first patient recovered before the surgical management time-threshold, but illustrates the direct and indirect morbidities of DP, with prolonged mechanical ventilation, oxygen exposition and hospital stay, as well as infectious risk associated with the development of chronic lung disease. Our second patient on the other hand required prolonged recovery time and we can wonder whether a diaphragmatic plication would really have improved our first patient’s outcome. Different factors, the etiology, the patient’s mild clinical condition, and notably the response to novel ventilation strategies guided our management. Regardless of the short and long-term benefits of a conservative vs surgical approach, this case highlights novel strategies to support DP, particularly the respiratory and nutritional modalities that significantly impact the disease outcome.
Rare follow up studies of neonatal DP suggest some loss of respiratory function and exercise capacity, whether surgically managed or not (10,23). There is however, a critical lack of clinical trials evaluating efficacity of early diaphragmatic plication in neonates and children. Prospective studies, including possibly electro-physiologic evaluations, may help categorize severity of phrenic nerve injury and spontaneous recovery potential and thus improving future DP management.
4. Conclusion
DP in neonates is a rare but serious cause of respiratory compromise. Cardiac surgery and birth trauma are the main causes of phrenic nerve injury; DP should therefore be part of the differential diagnostic in neonatal respiratory compromise in patients with complicated delivery or after cardiac surgery. Iatrogenic DP after pleural drain insertion is a very rare and potentially life-threatening condition. Ultrasound, accurate and easy to perform, is the modality of choice to visualize diaphragmatic motion in children. Supportive care, with complex respiratory and feeding management is crucial for a favorable conservative outcome. We believe the clinical condition and progress, as well as the complications and duration of conservative management should be balanced against a surgical intervention as reinnervation may occur even months after the primary insult. Neonatal DP management needs to be addressed in a multidisciplinary team of neonatologists, surgeons and radiologists.
Nomenclature:
DP: diaphragmatic paralysis
BPI: brachial plexus injury
CPAP: continuous positive airway pressure
ICS: intercostal space
DOL: day of life
NAVA: Neurally adjusted ventilatory assist
HFO: high frequency oscillatory
nHFOv: nasal high frequency oscillatory ventilation
PDA: patent ductus arteriosus
US: ultrasonography
CMAP: compound muscle action potential
EMG: electromyography
NIV-NAVA: non-invasive NAVA
MRI: magnetic resonance imaging
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication:
Written informed consent was obtained from the parents of both patients for publication.
Availability of data and materials:
All data generated or analysed during this study are included in this published article.
Competiting interest:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding:
No funding for the article.
Author’s Contributions:
AC and OO contributed to performing the database searching and evaluation of the included articles. All authors contributed to the drafting of the manuscript, acquisition of the data and analyzing of the data, to designing of the study, interpretation the data and final approval of the version to be published. All authors have read and approved the final manuscript.
Acknowledgements:
Not applicable.
References
- Akay TH, Ozkan S, Gultekin B, Uguz E, Varan B, Sezgin A, et al. Diaphragmatic paralysis after cardiac surgery in children: incidence, prognosis and surgical management. Pediatric Surgery International 22 (2006): 341-6.
- Gerard-Castaing N, Perrin T, Ohlmann C, Mainguy C, Coutier L, Buchs C, et al. Diaphragmatic paralysis in youg children: A literature review. Pediatric Pneumology 54 (2019): 1367-73.
- Rizeq YK, Many BT, Vacek JC, Reiter AJ, Raval MV. Diaphragmatic paralysis after phrenic nerve injury in newborns. Journal of Pediatric Surgery 55 (2020): 240-4.
- Bowerson M, Nelson VS, Yang LJ-S. Diaphragmatic Paralysis Associated With Neonatal Brachial Plexus Palsy. Pediatric Neurology 42 (2010): 234-6.
- Yoshida K, Kawabata H. The Prognostic Value of Concurrent Phrenic Nerve Palsy in Newborn Babied With Neonatal Brachial Plexus Palsy. Journal of Hand Surgery 40 (2015): 1166-9.
- Nakagama Y, Kaneko Y, Ono H. Reversible diaphragmatic paralysis caused by malpositioned chest tube. Cardiology in the Young (2014): 1-3.
- Joho-Arreola AL, Bauersfeld U, Stauffer UG, Baenziger O, Bernet V. Incidence and treatment of diaphragmatic paralysis after cardiac surgery in children. European Journal of Cardio-thoracic Surgery (2005): 53-7.
- Reiter AJ, Rizeq YK, Many BT, Vacek JC, Abdullah F, Goldstein SD. A Rare Case of Controlateral Diaphragm Paralysis following Birth Injury with Brachial Plexus Palsy: A Case Report and Review of the Literature. Case Reports in Pediatrics 2020 (2020): 1-6.
- de Toledo JS, Munoz R, Landsittel D, Shiderly D, Yoshida M, Komarlu R, et al. Diagnosis of Abnormal Diaphragm Motion after Cardiothoracic Surgery: Ultrasound Performed by Cardiac Intensivist vs. Fluoroscopy. Congenital Heart Disease 5 (2010): 565-72.
- Lemmer J, Stiller B, Heise G, Alexi-Meskishvili V, Hübler M, Weng Y, et al. Mid-term follow-up in patients with diaphragmatic plication after surgery for congenital heart disease. Intensive Care Medicine (2007).
- France NE. Unilateral diaphragmatic paralysis and Erb’s palsy in the newborn (1954): 357-9.
- Nahum E, Ben-Ari J, Schonfeld T, Horev G. Acute diaphragmatic paralysis caused by chest-tube trauma to phrenic nerve. Pediatric Radiology 31 (2001): 444-6.
- Reed RC, Siebert JR. Complications of percutaneous thoracostomy in neonates and infants. Journal of Perinatology 36 (2016): 296-9.
- Odita JC, Khan ASSI, Dincsoy M, Kayyali M, Masoud A, Ammari A. Neonatal phrenic nerve paralysis resulting from intercostal drainage pneumothorax. Pediatric Radiology 22 (1992): 379-81.
- Gupta V, Pandita A, Panghal A, Hassan N. Unusual cause of brachial palsy with diaphragmatic palsy. BMJ Case Reports (2018): 1-3.
- Stramrood CAI, Blok CA, van der Zee DC, Gerards LJ. Neonatal phrenic nerve injury due to traumatic delivery. Journal of Perinatal Medicine 37 (2009): 293-6.
- Chavhan GB, Babyn PS, Cohen RA, Langer JC. Multimodality Imaging of the Pediatric Diaphragm: Anatomy and Pathologic Conditions. RadioGraphics 30 (2010): 1797-817.
- Epelman M, Navarro OM, Daneman A, Miller SF. M-mode sonography of diaphragmatic motion: description of techniques and experiences in 278 pediatric patients. Pediatric Radiology 35 (2005): 661-7.
- Ross Russell RI, Helms PJ, Elliott MJ. A prospective study of phrenic nerve damage after cardiac surgery in children. Intensive Care Medicine 34 (2008): 728-34.
- Arya R, Jain P, Kumar A, Gulati S. Spontaneous Spinal Epidural Hematoma in an Infant. Journal of Child Neurology 27 (2012): 1577-9.
- Ahmadpour-Kacho M, Zahedpasha Y, Hadipoor A, Akbarian-Rad Z. Early Surgical Intervention for Diaphragmatic Paralysis in a Neonate; Report of a Case and Literature Review. Iran Journal Pediatric 21 (2010): 116-20.
- Roosens S, Derriks F, Cools F. Case Report: Non-Invasive Neurally Adjusted Ventilatory Assist in a Newborn With Unilateral Diaphragmatic Paralysis. Pediatric Pulmonology 51 (2016): E37-9.
- Similowski T, Duguet A, Prodanovic H, Straus C. Studying diaphragm function in the intensive care unit. Réanimation 12 (2003): 6-18.
- Jog SM, Patole SK. Diaphragmatic paralysis in extremely low birthweight neonates: Is waiting for spontaneous recovery justified. Journal of Pediatric Child Health 38 (2002): 101-3.
- Georgiev S, Konstantinov G, Latcheva A, Mitev P, Mitev I, Lazarov S. Phrenic nerve injury after paediatric heart surgery: is aggressive plication of the diaphragm beneficial? European Journal of Cardio-Thoracic Surgery. 2013;44:808-12.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 