Utility of Estimated Glomerular Filtration Rate in Hypertensive and Type 2 Diabetic Patients: Results from a Community-Based Study
Eduardo Gutiérrez-León1,2, Ricardo Escamilla-Santiago1, Reyna Pacheco-Domínguez3, Pablo Martínez-Amezcua4, Ricardo Correa-Rotter5, Malaquías López-Cervantes1*
1Department of Public Health; School of Medicine, Universidad Nacional Autónoma de México, Mexico City, Mexico
2PECEM (MD/PhD); School of Medicine, Universidad Nacional Autónoma de México, Mexico City, Mexico
3Center for Research in Policies, Population and Health; School of Medicine, Universidad Nacional Autónoma de México, Mexico City, Mexico
4Columbia University Irving Medical Center, Department of Medicine, Division of General Medicine, New York, EE.UU
5Department of Nephrology and Mineral Metabolism, National Institute of Medical Science and Nutrition Salvador Zubirán, Mexico City, Mexico
*Corresponding author: Malaquías López-Cervantes, Sexto piso, Edificio B, Departamento de Salud Pública, Facultad de Medicina, Circuito Escolar 411A, Copilco Universidad, Coyoacán, 04360, Ciudad de México, Mexico.
Received: 11 February 2022; Accepted: 22 February 2022; Published: 09 March 2023
Article Information
Citation: Eduardo Gutiérrez-León, Ricardo Escamilla- Santiago, Reyna Pacheco-Domínguez, Pablo Martínez- Amezcua, Ricardo Correa-Rotter, Malaquías López- Cervantes. Utility of Estimated Glomerular Filtration Rate in Hypertensive and Type 2 Diabetic Patients: Results from a Community-Based Study. Archives of Clinical and Biomedical Research. 7 (2023): 315-324.
View / Download Pdf Share at FacebookAbstract
Background: The usefulness of eGFR for screening chronic kidney disease (CKD) in early stages among people with type 2 diabetes (T2D) and hypertension is still under investigation and has not been definitively established.
Methods: We used a cross-sectional and longitudinal design and recruited adults over 19 years of age from Hidalgo, Mexico between 2012 and 2016. The study aimed to determine the use of eGFR as a screening method for comorbidity among people with type 2 diabetes and hypertension. The mean difference was compared between periods and longitudinal linear regression models were used to analyze the relationship between eGFR and blood pressure and HbA1c levels, which were divided into quartiles. The results of this study may provide information on the effectiveness of using eGFR for screening chronic kidney disease in this population.
Results: They recruited 1721 participants, of whom 102 had T2D, 319 had hypertension, 142 had both, and 208 had neither. The results showed that an increase in blood pressure was associated with a decrease in eGFR across all groups, with the greatest decrease observed in the last quartile compared to the first quartile in the second evaluation, and among those with hypertension only (-14.20, 95% confidence interval [95%CI] -24.33 to -4.08, P 0.001) and those with both T2D and hypertension (-16.06, 95% CI -31.94 to -0.17, P 0.04). The trend in eGFR was more stable in relation to an increase in HbA1c, which was more marked in the second measurement among those with T2D only (2.32, 95%CI -15.11 to 19.74, P≥0.05). The longitudinal analysis of the absolute change in eGFR showed similar trends across all groups. These findings suggest that blood pressure may have a greater impact on eGFR compared to HbA1c levels in this population.
Conclusions: The increase in blood pressure is associated with a decrease in eGFR regardless of the presence of T2D or hypertension. This suggests that measuring eGFR over a short period of time (2 years) can be useful in detecting early-stage kidney damage.
Keywords
<p>Blood Pressure; Estimated Glomerular Filtration Rate; Hypertension; Type 2 Diabetes</p>
Article Details
1. Introduction
According to worldwide projections, the number of people with hypertension is expected to reach 1.56 billion by 2030, and 439 million people are expected to have type 2 diabetes T2D by the same year [1-2]. Hypertension is a common problem among T2D patients, with a prevalence ranging from 39% to 85.8%, depending on the diagnosis [3-4]. Both hypertension and T2D are known to be major contributors to chronic kidney disease, which is a major health concern for many countries. These projections highlight the importance of addressing hypertension and T2D as major risk factors for chronic kidney disease [5]. In the context of the growing number of people with hypertension and T2D, early detection of chronic kidney disease (CKD) is of crucial importance. The early stages of CKD are the most prevalent and offer the greatest opportunity for intervention to prevent progression to end-stage renal disease (ESRD) [6-7]. To this end, organizations recommend that people with T2D or hypertension undergo testing for albuminuria and estimated glomerular filtration rate (eGFR) to detect kidney disease even in the absence of symptoms [8-9]. Screening for kidney disease using these methods has been shown to be a cost-effective screening method for T2D, but its effectiveness for hypertension is less clear [10-13]. These findings underscore the importance of continued research to improve screening methods for CKD in at-risk populations. While these conclusions have been drawn from cross-sectional and cost-effectiveness studies, they do not take into account the temporal aspect of CKD definition [10,14]. Additionally, short-term variations in eGFR in individuals with T2D and hypertension, especially in those with poor control of blood pressure and HbA1c, are not well understood. Hence, it is important to determine if short-term eGFR measurement screening is useful in diagnosing kidney disease, especially in light of the lack of therapeutic control of these diseases. In this study, data from a community-based cohort in Mexico was used to examine the usefulness of eGFR in its variability as a screening method for declining kidney function in individuals diagnosed with T2D, hypertension, or both diseases. The investigation looked at the relationship between eGFR and blood pressure and HbA1c levels in these individuals at two different points in time.
2. Methods
2.1 Study Design
The Emiliano Zapata cohort is a prospective study in Hidalgo, Mexico that began in 2012, with the aim of studying chronic non-communicable diseases. The study population was selected using a geostatistical framework provided by the Instituto Nacional de Estadística y Geografía [15]. Using the ArcGIS 9.3, ESRI software [16], we obtained a probabilistic sample of homes in each area based on their size. A total of 1721 participants who were over 18 years old and met the inclusion criteria participated in the study. The study excluded pregnant women. The study followed ethical guidelines and each participant provided informed consent prior to participation. The protocol was approved by the Ethics and Research Committees of the Faculty of Medicine at the National Autonomous University of Mexico (UNAM). The data collection process was carried out by trained personnel and followed established protocols for collecting sociodemographic, lifestyle, medical, anthropometric, and biochemical information, from November 2012 to August 2016. No financial compensation was provided to participants in accordance with Mexican legislation.
2.2 Study Population and Follow-up
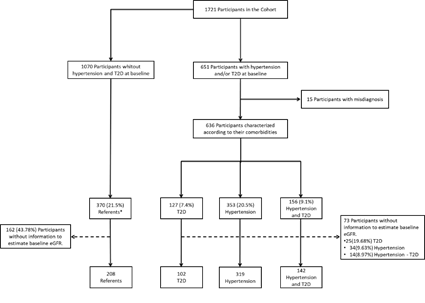
From 1721 participants, only 563 (32.7%) of the 636 participants with T2D and/or hypertension had complete data to calculate their basal eGFR and were used in the analysis. There were 102 participants (5.9%) with T2D, 319 (18.5%) with hypertension, 142 (8.3%) with both T2D and hypertension, and 208 (12.1%) without either condition (fasting glucose serum values <100mg/dL, HbA1c <5.7%, and blood pressure <120/80mmHg). The 208 participants without T2D or hypertension were used as referents during the follow-up.
2.3 Blood Pressure Measurement
Blood pressure was measured twice by trained nurses and medical students using the Riester© Sphygmomanometer with a precision of ±3 mmHg [17]. The average of the two measures was used to define systolic and diastolic blood pressure.
2.4 Glycemia and Blood Pressure Assessment
The subjects were divided into three groups based on the presence of T2D, hypertension, or both according to the diagnostic criteria set by the American Diabetes Association 2020 (fasting glycemia ≥126mg/dL [7.0 mmol/L] or HbA1c ≥6.5%) and the Eighth Joint National Committee - JNC8 (blood pressure ≥ 140 systolic / 90 diastolic mmHg) [18-19]. Individuals with prehypertension or prediabetes were excluded to avoid variability in the analysis.
2.5 Assessment of Glomerular Filtration Rate
The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation based on creatinine serum levels, according to the 2012 KDIGO guide [20-21]. The eGFR was entered into the model as a continuous variable to identify variations in kidney function within each group.
2.6 Statistical Analysis
In summary, we used chi2 tests and ANOVA tests to contrast the baseline demographic, anthropometric, and medical characteristics across the different study groups. If the assumptions for ANOVA were not met, a median extension test was used.
2.7 Quartile Effect
Quartiles were estimated for HbA1c, and systolic blood pressure based on the distribution of the variables in the groups. The effect of glycemia and blood pressure on eGFR was assessed by looking at HbA1c and systolic blood pressure individually and combined, forming four categories based on the baseline status. HbA1c and systolic pressure combination was assessed for each group over eGFR, forming four categories according to the baseline status recorded when they did not remain within the two lowest quartiles for HbA1c and the systolic pressure in both assessments. The remaining three categories are: systolic pressure in the highest quartiles and HbA1c in the lowest two quartiles, systolic pressure in the lowest quartiles and HbA1c in the two highest quartiles, and subjects with HbA1c and systolic pressure in the highest two quartiles. The effect of HbA1c and systolic pressure on eGFR was studied using ANOVA tests by stratified baseline status, and the absolute difference and its confidence intervals were measured using a Bonferroni post-estimation test on the baseline measurement and at 2-year follow-up. The temporal variability of eGFR between the two measurements was measured with a longitudinal mixed-effects model by stratified condition, which allows everyone to be his own referent over time. The results are presented as the eGFR means with their 95% confidence intervals per quartile, as well as the values of the contrasted absolute differences and their P-values per quartile. The temporal analysis is shown in beta values with their 95% confidence intervals. The statistical analysis was carried out using the Stata© 15.1 program.
3. Results
The participants were divided into 4 groups based on their chronic conditions and baseline eGFR: 1) type 2 diabetes only (n=102, 5.9%), 2) hypertension only (n=319, 18.5%), 3) both, type 2 diabetes and hypertension (n=142, 8.3%), and 4) a reference group without these conditions (n=208, 12.1%).
* Participants without prehypertension, hypertension, prediabetes, and type 2 diabetes at baseline and at the end of follow-up.
Abbreviations: T2D- Type 2 Diabetes; eGFR- estimated Glomerular Filtration Rate.
Table 1 shows the baseline characteristics of the 4 groups of participants in the study. The reference group had a younger mean age compared to the other groups (32 years [SD±9] vs 51 [12], 55 [15], and 60 [12] for T2D only, hypertension only, and both diseases, respectively). Participants with both diseases were more likely to have prior knowledge of their conditions and to receive treatment with insulin. They also had lower fasting glucose and HbA1C levels compared to those with T2D only (mean=162.97 SD±82.53 vs 164.45 ±83.32 mg/dL, and HbA1C 8.67 ±2.49 vs 9.0 ±2.44 %, respectively), but higher systolic blood pressure compared to those with hypertension only (142.10 mmHg ±17.61 vs 135.88 ±18.35, p<0.001). The participants with both diseases were also more likely to take antihypertensive medication compared to those with hypertension only (33.10% vs. 22.26%, p=0.01).
|
Variable |
Referent (N=208)¶ |
T2D (N=102) |
Hypertension (N=319) |
Both Diseases (N=142) |
P |
|
Age - yr |
32±9 |
51±12 |
55±15 |
60±12 |
<0.001 |
|
Female sex - no (%) |
173 (83.17) |
55 (53.92) |
212 (66.46) |
98 (69.01) |
<0.001 |
|
Type of community - no (%) |
0.011 |
||||
|
Urban |
151 (72.60) |
58 (56.86) |
234 (73.35) |
96 (67.61) |
|
|
Rural |
57 (27.40) |
44 (43.14) |
85 (26.65) |
46 (32.39) |
|
|
Duration of disease - yr |
|||||
|
T2D † |
- |
7±3 |
- |
8±3 |
0.522 |
|
Hypertension ‡ |
- |
- |
7±3 |
8±3 |
0.201 |
|
Previous diagnosis - no (%) |
|||||
|
T2D † |
- |
55 (53.92) |
- |
110 (77.46) |
<0.001 |
|
Hypertension ‡ |
- |
- |
161 (50.47) |
84 (59.15) |
0.104 |
|
Fasting serum glucose - mg/dL |
<0.001 |
||||
|
Mean |
72.57±8.62 |
164.45±83.32 |
78.31±12.45 |
162.97±82.53 |
|
|
Median |
72 |
137.5 |
78 |
136 |
|
|
Interquartile range |
67-79 |
93-218 |
71-85 |
98-221 |
|
|
Glycated hemoglobin - % |
<0.001 |
||||
|
Mean |
4.95±0.44 |
9.00±2.44 |
5.51±0.49 |
8.67±2.49 |
|
|
Median |
5 |
8.65 |
5.5 |
8.1 |
|
|
Interquartile range |
4.7-5.3 |
6.8-10.5 |
5.2-5.9 |
6.7-10.2 |
|
|
Blood pressure - mmHg Systolic |
<0.001 |
||||
|
Mean |
103.75±7.70 |
116.71±9.07 |
135.88±18.35 |
142.10±17.61 |
|
|
Median |
105 |
120 |
130 |
140 |
|
|
Interquartile range |
100-110 |
110-120 |
125-145 |
130-150 |
|
|
Dyastolic |
<0.001 |
||||
|
Mean |
66.42±5.56 |
74.52±6.98 |
88.71±11.42 |
90.63±8.90 |
|
|
Median |
70 |
75 |
90 |
90 |
|
|
Interquartile range |
60-70 |
70-80 |
80-95 |
90-100 |
|
|
Serum creatinene - mg/dL |
0.013 |
||||
|
Mean |
0.76±0.21 |
0.80±0.22 |
0.84±0.61 |
0.92±0.82 |
|
|
Median |
0.71 |
0.76 |
0.75 |
0.76 |
|
|
Interquartile range |
0.65-0.82 |
0.65-0.90 |
0.65-0.90 |
0.65-0.92 |
|
|
Estimated GFR - mL/min/1.73m2 |
<0.001 |
||||
|
Mean |
109.45±16.26 |
95.41±18.22 |
92.13±21.47 |
85.50±22.38 |
|
|
Median |
112.16 |
99.82 |
93.44 |
88.36 |
|
|
Interquartile range |
100.50-120.53 |
87.83-105.74 |
83.62-104.38 |
72.87-101.21 |
|
|
Estimated GFR <60 mL/min/1.73m2 - no (%) Other risk factors |
01 (00.48) |
06 (05.88) |
20 (06.27) |
16 (11.27) |
<0.001 |
|
Cholesterol - mg/dL |
|||||
|
LDL |
93.11±29.79 |
105.24±31.70 |
108.17±31.47 |
109.66±34.08 |
<0.001 |
|
HDL |
47.33±11.60 |
43.26±10.47 |
44.64±12.57 |
42.66±9.95 |
0.003 |
|
Triglyceride - mg/dL |
145.80±98.18 |
244.75±268.11 |
178.98±118.22 |
265.44±237.33 |
<0.001 |
|
Body-mass index |
25.35±4.04 |
28.96±4.89 |
29.37±5.33 |
30.09±5.2 |
<0.001 |
|
Current and former smoker - no (%) |
99 (47.60) |
48 (47.06) |
131 (41.07) |
56 (39.44) |
0.27 |
|
Hypoglycemic medications |
|||||
|
Insulin - no (%) † |
0 (0.00) |
10 (9.80) |
0 (0.00) |
23 (16.20) |
0.312 |
|
None - no (%) † |
208 (100.00) |
68 (66.67) |
319 (100.00) |
79 (55.63) |
0.082 |
|
Antihypertensive medications |
|||||
|
None - no (%) ‡ |
208 (100.00) |
96 (94.12) |
248 (77.74) |
95 (66.90) |
0.014 |
|
Other medications |
|||||
|
NSAIDs - no (%) |
14 (6.73) |
8 (7.84) |
52 (16.30) |
22 (15.49) |
0.003 |
|
Cholesterol lowering - no (%) |
2 (00.96) |
5 (4.90) |
9 (2.82) |
6 (4.23) |
0.158 |
|
Exercise as a treatment - no (%) |
- |
14 (13.73) |
28 (8.78) |
29 (20.42) |
0.728 |
|
Diet as a treatment - no (%) |
- |
1 (0.98) |
23 (7.21) |
17 (11.97) |
0.552 |
|
Medical check-up for the disease in the last year - no (%) |
- |
35 (34.31) |
62 (19.44) |
66 (46.48) |
0.072 |
|
Hospitalizations associated with the disease in the last year - no (%) |
- |
1 (0.98) |
5 (01.57) |
10 (7.09) |
0.066 |
Table 1: Characteristics of the participants at baseline, by groups. *
* Plus-minus values are means ±SD. The body-mass index is the weight in kilograms divided by the square of the height in meters.
¶ Group with fast glucose serum values of <100mg/dL, HbA1c <5.7%, and systolic/diastolic blood pressure of <120/80mmHg during the follow-up.
† P value considering only the group with T2D without hypertension and the group with both diseases.
‡ P value considering only the group with hypertension without T2D and the group with both diseases.
Abbreviations: T2D, type 2 diabetes; GFR, glomerular filtration rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein; NSAIDs, nonsteroidal anti-inflammatory drugs.
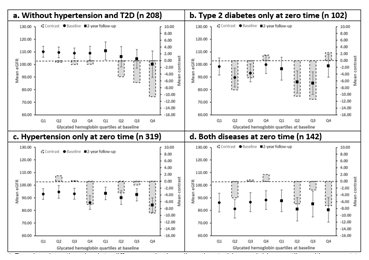
The mean basal eGFR for the reference group was 109.45 mL/min/1.73m2 (SD ±16.26). Participants with type 2 diabetes only had a mean basal of 95.41 mL/min/1.73m2 (±18.22), while those with hypertension only had a mean basal of 92.13 mL/min/1.73m2 (±21.47). Participants with both type 2 diabetes and hypertension had the lowest mean basal eGFR, which was 85.50 mL/min/1.73m2 (±22.38). They found that the trend in mean eGFR among the four groups of participants showed a stable or even increasing trend in the first assessment when HbA1c increased. However, this trend was not statistically significant (P≥0.05). In the second evaluation, the groups showed a downward trend in eGFR with increasing HbA1c, except for the group with type 2 diabetes only. The absolute difference in mean eGFR of the last quartile compared to the reference quartile showed no significant contrast for the group with type 2 diabetes only in both first 1.61 (95%CI -11.76 to 14.98, P≥0.05) and second 2.32 (-15.11 to 19.74, P≥0.05) measurements, and for individuals with both diseases in the first 2.04 (-12.24 to 16.32, P≥0.05) and second -7.20 (-24.80 to 10.40, P≥0.05) measurements (Figure 2).
*Panel a show the absolute differences by baseline glycated hemoglobin quartiles with respect to the first baseline glycated hemoglobin quartile at baseline and at 2-year follow-up for the mean eGFR in the participants without hypertension and T2D. While panel b, c and d show the stratified by baseline comorbidity condition, being T2D without hypertension, hypertension without T2D and both conditions, respectively. The bars indicate the absolute difference contrasted with the first quartile. The vertical lines above the dots indicate the confidence interval for the mean eGFR.
Abbreviations: eGFR- estimated Glomerular Filtration Rate; T2D- Type 2 Diabetes.
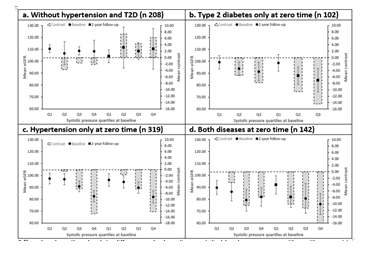
The increase in systolic blood pressure had a tendency to decrease the mean eGFR in the groups with chronic conditions, with a greater decrease in those with baseline hypertension, in both first and second evaluations. This decrease was more significant in the second evaluation, with significant differences in the last quartile compared to the first quartile for the groups with hypertension only -14.20 (-24.33 to -4.08, P 0.001) and both diseases -16.06 (-31.94 to -0.17, P 0.04). The trend was not observed in the reference group, which showed a stable and upward trend in both evaluations (first -2.13 [-15.47 to 11.21] and second 6.26 [-18.21 to 30.73] evaluations, P≥0.05). Figure 3.
* Panel a show the absolute differences by baseline systolic blood pressure quartiles with respect to the first baseline systolic blood pressure quartile at baseline and at 2-year follow-up for the mean eGFR in the participants without hypertension and T2D. While panel b, c and d show the stratified by baseline comorbidity condition, being T2D without hypertension, hypertension without T2D and both conditions, respectively. The bars indicate the absolute difference contrasted with the first quartile. The vertical lines above the dots indicate the confidence interval for the mean eGFR.
Abbreviations: eGFR- estimated Glomerular Filtration Rate; T2D- Type 2 Diabetes.
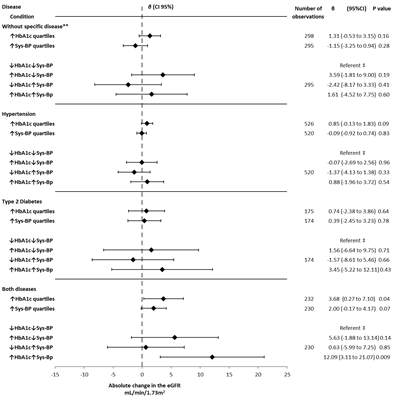
The longitudinal analysis integrating changes in eGFR, HbA1c, and systolic pressure over time, showed a trend similar to the cross-sectional analysis. As HbA1c increased, eGFR increased (e.g., both diseases β 5.63 [-1.88 to 13.14, P 0.14]), while as systolic pressure increased, eGFR decreased or had a lower increase (e.g., both diseases β 0.63 [-5.99 to 7.25, P 0.85]). However, the increase in both HbA1c and systolic pressure resulted in a greater increase in eGFR compared to when either HbA1c or systolic pressure were elevated individually (e.g., both diseases β 12.09 [3.11 to 21.07, P 0.009]). This was seen in all groups analyzed (Figure 4).
**Participants without hypertension and type 2 diabetes.
‡ Comparison group within groups (consisting of individuals with HbA1c and systolic blood pressure within the two lowest quartiles over time).
Abbreviations: eGFR- estimated Glomerular Filtration Rate; T2D- Type to Diabetes; HbA1c- Glycated Hemoglobin; Sys-BP- Systolic Blood Pressure.
4. Discussion
The results showed that there was a downward trend in eGFR in individuals with T2D and/or hypertension in both cross-sectional and longitudinal analyses. However, the relationship between HbA1c and eGFR was not significant, with stable or even increasing trends in eGFR as HbA1c increased, particularly in the T2D-only group. On the other hand, systolic pressure was found to be a significant factor associated with decreased eGFR in individuals with disease. We concluded that eGFR could be a useful screening method for short-term deterioration of renal function, especially with increasing blood pressure, but not for serum glucose elevation. However, hypertension and decreased eGFR may occur in future years for the diabetic group without hypertension, given the high prevalence of hypertension with increasing age and in people with diabetes [22-23]. There is evidence supporting the use of eGFR for screening worsening renal function in people with T2D and/or hypertension, and it is more effective in people over 50 years old or those with albuminuria higher than 50 mg/dL [24-26]. The evidence is more conclusive in the T2D population. Our data do not support the implementation of eGFR as a screening method for the deterioration of renal function in people with uncontrolled T2D, due to the observed increase or stability of eGFR. This increase or stability can be partially explained by the phenomenon of glomerular hyperfiltration that occurs in the natural history of a diabetic kidney. Additionally, the values of eGFR obtained during the study match with those obtained during the natural history of a diabetic kidney in the T2D population. However, we demonstrated the usefulness of eGFR as a screening method for the early stages of deterioration of renal function in people with hypertension and lack of therapeutic control, even though the evidence for this outcome is not as conclusive. An increase in eGFR was observed in participants from all groups with increased HbA1c over the 2-year follow-up period. These results could be due to a phenomenon of glomerular hyperfiltration and maintenance of regular ratios by adaptive mechanisms of the kidneys, which corresponds to the early stages of the natural history of diabetic kidney disease [27-28]. It is important to note that this increase in eGFR with elevated HbA1c also occurred in the group of participants with hypertension only. On the other hand, regarding the effect of changes in eGFR caused by the combination of HbA1c and systolic pressure, we observed a different pattern in the trends of evaluation of HbA1c and systolic pressure separately and together on eGFR in the group with both diseases compared to the group with T2D or hypertension alone, suggesting an interaction effect (effect modification) when both factors were combined in the group with both diseases. It must be highlighted that these results could partially explain the findings related to the lowest probability of an individual with chronic kidney disease to have a linear trajectory or progression to ESRD [29]. According to what we observed, HbA1c is accompanied by a minimum elevation in eGFR. It is possible to think that this lack of progression in kidney disease is explained by this phenomenon. However, this does not imply the absence of kidney damage, but rather an adaptive phenomenon. Finally, more than 50% of the participants were without pharmacological treatment for their chronic disease. Moreover, a scarce use of hygienic-dietetic measures was observed, partially explained by the number of individuals unaware of their disease at the beginning of the study (more than 30% in any of the three comorbidity groups). Without adequate diagnosis and treatment, diabetes and hypertension would follow their natural course, leading, in many cases, to advanced stages of chronic kidney disease. The main strength of this study was being an open population cohort, which allowed us to understand the natural history of eGFR in this type of population without the characteristics observed in participants of clinical trials such as having a better prognosis and a lower estimate of GFR compared to the general population. Additionally, dividing subjects with T2D and hypertension from the group with both comorbidities represented an advantage in identifying interactive effects between both factors on renal function. Some of the limitations of the study were the categorization of the participants into groups with both comorbidities and one with only one, as well as the sub-categorization of the groups based on the levels of HbA1c and blood pressure, which resulted in a significant decrease of the population in the analysis and unstable results. Secondly, we believe that the follow-up time of the cohort should have been longer since the worsening of eGFR by T2D and/or hypertension is a chronic process that takes years to manifest. Finally, there was a significant age discrepancy between the groups at baseline. With aging, the prevalence of certain comorbidities (such as hypercholesterolemia) that can affect renal function increases, and it is possible that the presence or absence of these conditions may have implications for our results, which were not considered in our analysis.
5. Conclusion
Variations related to the increase in HbA1c levels showed a trend of elevating eGFR in the short term for subjects with T2D, probably due to an adaptive phenomenon of the kidney to impairment; however, it does not suggest that renal function is in a state of homeostasis. Conversely, the increase in systolic pressure levels showed a trend towards a decrease in eGFR for participants with T2D or hypertension. Finally, the short-term measurement of eGFR in open population with some of these comorbidities represents a benefit for early detection of impaired renal function, given the decrease observed with increasing blood pressure and the high prevalence of coexisting T2D and hypertension at the same time, while continuing to use other studies, such as albuminuria, for detecting kidney damage in the early stages.
Ethics Approval and Consent to Participate
This study protocol was reviewed and approved by the Ethics and Research Committees of the Faculty of Medicine at Universidad Nacional Autónoma de México (UNAM), approval number FM/DI/077/2019; and each participant signed an informed consent agreement under the Mexican Legislation, moreover, no economic support was offered to the participants.
Relevant Guidelines
The present study followed the items proposed in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement, guideline for reporting observational studies.
Consent for Publication
Not applicable.
Availability of Data and Materials
The data that supports the findings of this study are not publicly available since the containing information can compromise the privacy of research participants, however, they are available from the corresponding author MLC upon reasonable request.
Competing Interests
The authors have no conflict of interest to declare.
Funding
This Project was supported by UNAM-PAPIIT (grant IA200912) and National Council of Science and Technology (grant S0008-1-202253).
Authors' Contributions
EGL carried out the analysis and prepared the appendix. EGL, RAES, RPD, PMA, RCR and MLC drafted the initial manuscript. EGL and RAES oversaw database construction, assessed, compiled, and updated the database. All authors interpreted and reviewed model results, edited the manuscript and agreed on the final version of the manuscript.
Acknowledgements
The authors wish to thank Mrs. María Josefina Bolado Garza, Head of the Scientific Paper Translation Department, from Division of Investigation at Faculty of Medicine, UNAM, for translating this manuscript into English.
References
- Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 365 (2005): 217-223.
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87 (2010): 4-14.
- Hypertension in Diabetes Study (HDS): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens 11 (1993): 309-317.
- Kabakov E, Norymberg C, Osher E, et al. Prevalence of hypertension in type 2 diabetes mellitus: impact of the tightening definition of high blood pressure and association with confounding risk factors. J Cardiometab Syndr 1 (2006): 95-101.
- Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 382 (2013): 260-272.
- Levey AS, Coresh J. Chronic kidney disease. Lancet 379 (2012): 165-180.
- Levey AS, Schoolwerth AC, Burrows NR, et al. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis 53 (2009): 522-535.
- Moyer VA; U.S. Preventive Services Task Force. Screening for chronic kidney disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157 (2012): 567-570.
- Qaseem A, Hopkins RH Jr, Sweet DE, et al. Clinical Guidelines Committee of the American College of Physicians. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: A clinical practice guideline from the American College of Physicians. Ann Intern Med 159 (2013): 835-847.
- Romagnani P, Remuzzi G, Glassock R, et al. Chronic kidney disease. Nat Rev Dis Primers 3 (2017): 17088.
- Hoerger TJ, Wittenborn JS, Segel JE, et al. A health policy model of CKD: 2. The cost-effectiveness of microalbuminuria screening. Am J Kidney Dis 55 (2010): 463-473.
- Manns B, Hemmelgarn B, Tonelli M, et al. Population based screening for chronic kidney disease: cost effectiveness study. BMJ 341 (2010): c5869.
- Komenda P, Ferguson TW, Macdonald K, et al. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis 63 (2014): 789-797.
- Fink HA, Ishani A, Taylor BC, et al. Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: a systematic review for the U.S. Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 156 (2012): 570-581.
- Instituto Nacional de Estadística y Geografía. Marco Geoestadístico Municipal (2010), versión 4.2.
- Software: ArcGIS [software GIS]. Version 9.3. Redlands, CA: Environmental Systems Research Institute, Inc., (2009).
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289 (2003): 2560-2572.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 43 (2020): S14-S31.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). 311 (2014): 507-520.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 150 (2009): 604-612.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3 (2013): 1-150.
- Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int 82 (2012): 270-277.
- Pottel H, Delanaye P, Weekers L, et al. Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J 10 (2017): 545-551.
- Plantinga LC, Boulware LE, Coresh J, et al. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med 168 (2008): 2268-2275.
- Komenda P, Ferguson TW, Macdonald K, et al. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis 63 (2014): 789-797.
- Moyer VA; U.S. Preventive Services Task Force. Screening for chronic kidney disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157 (2012): 567-570.
- Vora JP, Dolben J, Dean JD, et al. Renal hemodynamics in newly presenting non-insulin dependent diabetes mellitus. Kidney Int 41 (1992): 829-835.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 12 (2017): 2032-2045.
- Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 59 (2012): 504-512.


 Impact Factor: * 5.8
Impact Factor: * 5.8 Acceptance Rate: 71.20%
Acceptance Rate: 71.20%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 