Air-Mycoflora of Some Eating Places on University of Lagos Campus, Nigeria
Article Information
Adeyinka Odebode*, Ajikobi Omodolapo, Adedotun Adekunle
Department of Botany, Faculty of Science, University of Lagos, Akoka, Nigeria
*Corresponding Author: Adeyinka Odebode, Department of Botany, Faculty of Science, University of Lagos, Akoka, Nigeria
Received: 15 July 2019; Accepted: 30 July 2019; Published: 05 August 2019
Citation: Adeyinka Odebode, Ajikobi Omodolapo, Adedotun Adekunle. Air-Mycoflora of Some Eating Places on University of Lagos Campus, Nigeria. Journal of Environmental Science and Public Health 3 (2019): 407-418.
View / Download Pdf Share at FacebookAbstract
Fungi are found everywhere and can cause infections when inhaled. In view of this, fungal flora of some eating places on the University of Lagos campus was investigated within two seasons. Ten eating places were chosen at various parts of the Akoka-campus of the University. Air mycoflora of these eating places was carried out by sedimentation method with Potato Dextrose Agar and Dichloran Glycerol-18 plates exposed for ten minutes. The air sampling was carried out in Five months within the dry and wet seasons (February, March, June, July, and August). The results of the investigation reveal that a total of 814 spores were obtained from the ten locations during the five months of study. The fungi obtained are of the genera, Aspergillus, Penicillium, Fusarium, Curvularia, Rhizopus, Neurospora and Trichoderma. From the results, the Aspergillus spp were the most frequent observed fungi while Fusarium spp and Curvularia spp were the least frequently sampled. Culture Media comparison was carried out throughout the study months. Meteorological data was also obtained from The Nigerian Meteorological Agency, Lagos, Nigeria, to determine the relationship between weather parameters and the growth and distribution of fungi spores. Some of the fungi isolated are opportunistic in nature and are allergens which cause various diseases, irritations and allergic reactions to human.
Keywords
Air mycoflora, Campus, Fungi
Air mycoflora articles Air mycoflora Research articles Air mycoflora review articles Air mycoflora PubMed articles Air mycoflora PubMed Central articles Air mycoflora 2023 articles Air mycoflora 2024 articles Air mycoflora Scopus articles Air mycoflora impact factor journals Air mycoflora Scopus journals Air mycoflora PubMed journals Air mycoflora medical journals Air mycoflora free journals Air mycoflora best journals Air mycoflora top journals Air mycoflora free medical journals Air mycoflora famous journals Air mycoflora Google Scholar indexed journals allergic asthma articles allergic asthma Research articles allergic asthma review articles allergic asthma PubMed articles allergic asthma PubMed Central articles allergic asthma 2023 articles allergic asthma 2024 articles allergic asthma Scopus articles allergic asthma impact factor journals allergic asthma Scopus journals allergic asthma PubMed journals allergic asthma medical journals allergic asthma free journals allergic asthma best journals allergic asthma top journals allergic asthma free medical journals allergic asthma famous journals allergic asthma Google Scholar indexed journals microscope articles microscope Research articles microscope review articles microscope PubMed articles microscope PubMed Central articles microscope 2023 articles microscope 2024 articles microscope Scopus articles microscope impact factor journals microscope Scopus journals microscope PubMed journals microscope medical journals microscope free journals microscope best journals microscope top journals microscope free medical journals microscope famous journals microscope Google Scholar indexed journals public health articles public health Research articles public health review articles public health PubMed articles public health PubMed Central articles public health 2023 articles public health 2024 articles public health Scopus articles public health impact factor journals public health Scopus journals public health PubMed journals public health medical journals public health free journals public health best journals public health top journals public health free medical journals public health famous journals public health Google Scholar indexed journals ANOVA articles ANOVA Research articles ANOVA review articles ANOVA PubMed articles ANOVA PubMed Central articles ANOVA 2023 articles ANOVA 2024 articles ANOVA Scopus articles ANOVA impact factor journals ANOVA Scopus journals ANOVA PubMed journals ANOVA medical journals ANOVA free journals ANOVA best journals ANOVA top journals ANOVA free medical journals ANOVA famous journals ANOVA Google Scholar indexed journals public health articles public health Research articles public health review articles public health PubMed articles public health PubMed Central articles public health 2023 articles public health 2024 articles public health Scopus articles public health impact factor journals public health Scopus journals public health PubMed journals public health medical journals public health free journals public health best journals public health top journals public health free medical journals public health famous journals public health Google Scholar indexed journals animal health articles animal health Research articles animal health review articles animal health PubMed articles animal health PubMed Central articles animal health 2023 articles animal health 2024 articles animal health Scopus articles animal health impact factor journals animal health Scopus journals animal health PubMed journals animal health medical journals animal health free journals animal health best journals animal health top journals animal health free medical journals animal health famous journals animal health Google Scholar indexed journals antibiotics articles antibiotics Research articles antibiotics review articles antibiotics PubMed articles antibiotics PubMed Central articles antibiotics 2023 articles antibiotics 2024 articles antibiotics Scopus articles antibiotics impact factor journals antibiotics Scopus journals antibiotics PubMed journals antibiotics medical journals antibiotics free journals antibiotics best journals antibiotics top journals antibiotics free medical journals antibiotics famous journals antibiotics Google Scholar indexed journals toxins articles toxins Research articles toxins review articles toxins PubMed articles toxins PubMed Central articles toxins 2023 articles toxins 2024 articles toxins Scopus articles toxins impact factor journals toxins Scopus journals toxins PubMed journals toxins medical journals toxins free journals toxins best journals toxins top journals toxins free medical journals toxins famous journals toxins Google Scholar indexed journals genome articles genome Research articles genome review articles genome PubMed articles genome PubMed Central articles genome 2023 articles genome 2024 articles genome Scopus articles genome impact factor journals genome Scopus journals genome PubMed journals genome medical journals genome free journals genome best journals genome top journals genome free medical journals genome famous journals genome Google Scholar indexed journals
Article Details
1. Introduction
Often times we go to some indoor environments and we leave with itchy eyes or other allergies and reactions. To a large extent, medical mycology has been able to relate these allergies and irritations to the mycoflora often present in the air. Air mycoflora usually include spores of fungi, including Penicillium, Aspergillus etc. [1]. Each day, we are in one way or the other exposed to these spores with their beneficial and harmful effects. Over the course of time, lifestyle changes have occurred from outdoor environments to indoor environments which contribute a great deal to the human health [2, 3]. Fungal spores are liberated into the air from various sources in massive concentrations so upsetting a mould source can send the spores into the air. Generally, spores occur in both indoor and outdoor environments. Fungal spores can enter indoor environments through various ways such as: open doorways, windows and air conditioning systems and some other avenues. Spores may also be attached to people’s clothing’s and animal’s skin, making clothing, shoes, bags and pets a major carrier for transporting fungi from outdoor indoors. Furthermore, improper building maintenance, which causes leakage, poor architectural building design or occupant of building activities often result in a condition called ‘Sick Building Syndrome’ (SBS), where those living in the building experience adverse health effects which is linked with time of exposure to the spores in the building [1, 4, 5].
The occurrence and distribution of fungi is largely influenced by meteorological factors such as sunlight, wind, rainfall and relative humidity. These also affect the occurrence of spores all year round. This explains why some spores are either present or absent during the various seasons, especially dry and wet seasons. Allergic diseases caused by fungi include: allergic asthma, allergic rhinitis, allergic sinusitis, Broncho Pulmonary mycoses and hypersensitivity pneumonia, etc. Human exposure to fungal spores is mainly related to direct mucosal irritation and elicitation of an IgE mediated hypersensitivity responses that precipitate rhinitis and upper airways irritation, eye irritation and sinusitis that characterize allergic syndrome. The principal fungal allergens are either cell wall components (1-3) - β –D glucan or water soluble glycoproteins. These allergens become airborne when these materials are aerosolized. Several species of fungi come in contact with human daily, but only a few have been identified and described. Therefore, this research work was done to determine the air mycoflora of eating places on the University of Lagos Akoka-campus. The findings of this study will be helpful to inform the level of hygiene of these eating places.
2. Materials and Method
2.1 Preparation of media
Two media: Potato Dextrose Agar (PDA) and Dichloran Glycerol-18-Agar (DG-18 Agar) were used for collection of isolates during the sampling period.
2.2 Sampling sites
Ten sites all located within the campus were selected for this work. The eating places chosen cover almost all sites on the campus where students can easily go to eat. They are places of interest and have won themselves a large number of customers ranging from students to lectures, non-teaching staff and even visitors of the University. The sampling height was approximately 1 m above feet level, which is a human breathing zone. Four plates were exposed at each site. Two containing PDA, and the other two containing DG-18 agar. The surfaces of plates containing media were exposed horizontally exposed in the air for ten minutes so that fungal spores present in the air can blow and settle on the agar in the Petri dishes and were covered after each exposure. The Petri dishes were labelled according to each sampling site chosen and transported to the laboratory and incubated at room temperatures (28- 31°C) for 3 to 5 days. Colony count and growth appearance was monitored.
The selected sampling sites include;
- Glamos Rare Bits (GPS: 6.5166°N, 3.3983°E, 11 m)
- Mavise (GPS: 6.51667°N, 3.39828°E, 5 m)
- QSS foods Ltd (GPS: 6.51813°N, 3.39737°E, 30 m)
- Mz Valerie Cafeteria (GPS: 6.51618°N, 3.39704°E, 30 m)
- Cafeteria 2001 (GPS: 6.51933°N, 3.39191°E, 13 m)
- Mommy Mariam Kenny Gold (GPS: 6.5117°N, 3.39264°E, 10 m)
- Jesus Embassy Catering (GPS: 6.51553°N, 3.39232°E, 25 m)
- Baba Ding Cuisine (GPS: 6.51573°N, 3.39232°E, 20 m)
- Salado Catering service and Restaurant (GPS: 6.51783°N, 3.8964°E, 7 m)
- Olaiya Special Homely Catering (GPS: 6.51572°N, 3.38499°E, 20 m).
2.3 Morphological studies and Identification of fungi
The growth pattern, colour and cultural characteristics of each of the isolates were observed. With the use of sterile inoculating needle, small portions of mycelium from the sporulating medium of each culture were placed on microscope glass slides after which a drop of lacto phenol cotton blue was added on each slide and then covered carefully with a cover slip in such a way that air bubbles will be avoided. The slides were observed under the light microscope. The fungi were then identified with the use of cultural and microscopic characteristics according to descriptions in the texts given by Talbot [6], Deacon [7] and Bryce. The Percentage frequency of each fungus was calculated as the number of the fungus over the total number of colonies of fungi from all the sites, throughout the five study months (February, March, June, July and August).
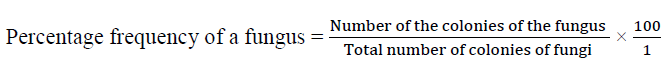
2.4 Weather data
Weather data was obtained from the Nigerian Meteorological Agency (NIMET). The data contained parameters for Dry bulb temperature, Wind speed, Relative humidity and Monthly total of rainfall for the months February, March, June, July and August 2016.
2.5 Statistical analysis
Data obtained were analysed using multiple analysis of variance (ANOVA) and means were separated using Duncan Multiple Range Test (DMTR) with the level of significance at P<0.05 (95% confidence interval).
3. Results
The result of the study carried out showed the following fungal genera as being the component of air mycoflora present in some eating places on the University of Lagos Akoka-campus. The fungi obtained include: Aspergillus niger, A. flavus, A. fumigatus, A. ochraceus, Trichoderma harzianium, T. viride, Penicillum sp, P.chrysogenum, Fusarium spp, Rhizopus sp, Curvularia spp, Yeast.
|
S/N |
LOCATION |
FEB |
MAR |
JUN |
JULY |
AUG |
TOTAL |
|
1 |
Glamos rare bits |
12 |
14 |
22 |
18 |
16 |
82 |
|
2 |
Mavise |
15 |
17 |
19 |
18 |
17 |
86 |
|
3 |
QSS |
13 |
18 |
35 |
22 |
17 |
105 |
|
4 |
Cafeteria 2001 |
16 |
16 |
24 |
19 |
13 |
88 |
|
5 |
Mz Valerie |
13 |
11 |
18 |
21 |
17 |
70 |
|
6 |
Jesus embassy |
14 |
13 |
20 |
15 |
8 |
82 |
|
7 |
Baba ding |
16 |
15 |
15 |
14 |
8 |
60 |
|
8 |
Mommy mariam |
15 |
18 |
21 |
18 |
10 |
68 |
|
9 |
Salado |
12 |
15 |
20 |
11 |
16 |
77 |
|
10 |
Olaiya |
13 |
18 |
22 |
12 |
14 |
79 |
Table 1: Number of colonies in all eating places throughout the study months.
Table 1 shows the number of colonies obtained in each location in the various study months. A total of 82 colonies were obtained in Glamos rare bits, a total of 86 from Mavise and 105 from QSS. Cafeteria 2001 had a total of 88 colonies, Mz Valerie had 70 colonies in total and Jesus embassy had a total of 82. A total of 60 colonies were obtained from Baba ding, 68 from Mommy Mariam, 74 colonies from Salado and a total of 79 colonies from Olaiya.
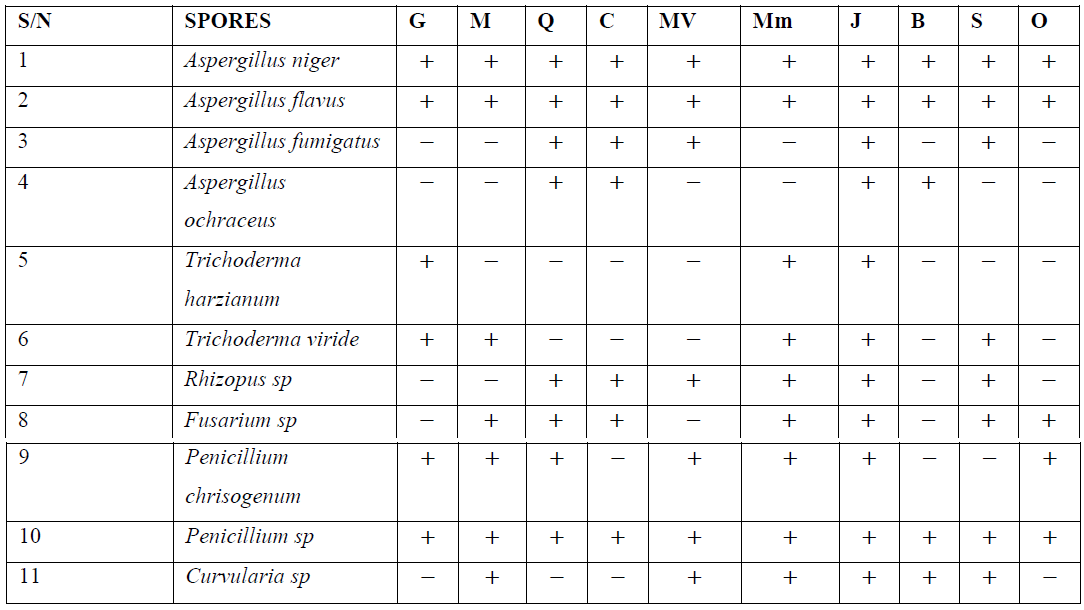
Table 2: Fungi occurrence in all eating places throughout the study months.
The eating places are represented as follows: Glamos rare bits -G, Mavise -M, QSS –Q, Cafeteria 2001 –C, Mz Valerie –V, Jesus embassy –J, Baba ding –B, Mommy mariam –Mm, Salado –S, Olaiya –O. Table 2 shows the occurrence of fungi spores in all eating places studied. Aspergillus niger and A. flavus were present in all the eating places. Aspergillus fumigatus was present in all eating places except Mavise, Baba ding and Olaiya. A. ochraceus was present only in QSS, Cafeteria 2001 and Baba ding. Trichoderma harzianum was present only in Glamos rare bits, Mavise Mommy maria and Cafeteria 2001. Trichoderma viride was absent in QSS, Cafeteria 2001, Mz Valerie, Baba ding and Olaiya. Rhizopus sp was present in all eating places except Glamos rare bits, Mavise, Baba ding and Olaiya. Fusarium sp was also present in all eating places except Glamos rare bits and Baba ding. Penicillium chrysogenum was present in all eating places except in Baba ding and Salado. The second penicillium species obtained was present in all the eating places without an exception and Curvularia sp was present in all eating places except QSS, Cafeteria 2001 and Olaiya.
|
Fungi isolated |
February |
March |
June |
July |
August |
|
Aspergillus niger |
4.33ab |
4.11ab |
4.30a |
3.44ab |
2.22c |
|
Aspergillus flavus |
4.90a |
5.40a |
4.50a |
4.70a |
2.25c |
|
Trichoderma harzianum |
1.67cd |
3.00abc |
- |
- |
- |
|
Trichoderma viride |
- |
- |
2.66a |
3.00ab |
2.50bc |
|
Aspergillus fumigatus |
- |
- |
3.75a |
3.50ab |
5.00ab |
|
Aspergillus ochraceus |
2.00bcd |
3.00abc |
3.00a |
- |
2.00c |
|
Rhizopus spp |
2.00bcd |
- |
2.16a |
2.50ab |
2.00c |
|
Penicillium spp |
3.67abc |
3.6abc |
4.22a |
3.60ab |
3.43bc |
|
Fusarium spp |
- |
1.00c |
3.00a |
- |
3.20bc |
|
Penicillium chrysogenum |
2.33bcd |
1.00c |
3.25a |
2.00b |
3.00bc |
|
Neurospora crassa |
- |
2.00bc |
- |
4.00ab |
6.00a |
|
Curvularia spp |
2.00bcd |
1.50bc |
2.33a |
2.33ab |
3.00bc |
|
Yeast |
2.50bcd |
1.00c |
1.67a |
1.67b |
2.00c |
|
Unidentified species |
1.00d |
2.17cb |
2.83a |
3.00ab |
2.87bc |
|
EMS |
0.97 |
1.08 |
2.57 |
1.39 |
1.46 |
Table 3: Occurrence of fungi spores monthly.
Mean with different superscripts are significantly different. Mean separation done with Duncan Multiple Range Test at P<0.05. Results also showed that the month of June had highest collection of fungi spores compared to other months. Also, Aspergillus fumigatus, Neurospora crassa, A. niger and A. flavus were the highest occurring fungal colonies during the period of sampling. Fusarium spp, Yeast, T. harzanium, Curvularia and P. Chrysogenum recorded lowest number of colonies throughout the months of sampling. Higher values were observed during the periods of June and July which are significantly different (P ≤ 0.05) from the values observed for Feb and March (Table 3).
|
LOCATION |
FEBRUARY |
MARCH |
JUNE |
JULY |
AUGUST |
|
QSS Foods LTD |
2.17d |
3.60bc |
4.37a |
5.50a |
2.43a |
|
Glamous Rare Bits (SHOP 10) |
2.40d |
2.80c |
3.14a |
3.60bc |
3.20a |
|
MAVISE |
3.00cd |
3.40bc |
3.80a |
4.50ab |
2.83a |
|
CAFETARIA 2001 |
5.33ab |
5.33a |
3.43a |
3.80abc |
2.60a |
|
VALERIE |
3.66bcd |
2.75c |
3.00a |
3.50bc |
2.43a |
|
Jesus Embassy Catering |
2.80cd |
2.60c |
2.50a |
2.50c |
2.67a |
|
Baba Ding Catering |
6.00a |
3.75abc |
3.33a |
2.20c |
3.60a |
|
Mommy Mariam Kenny Gold Canteen |
5.33ab |
3.75abc |
3.00a |
3.50bc |
2.00a |
|
Salado Catering Service and Restaurant |
6.00a |
3.75abc |
3.33a |
2.20c |
3.60a |
|
Olaiya Special Homely Catering |
4.33abc |
5.00ab |
4.40a |
4.00abc |
3.50a |
|
EMS |
0.97 |
1.08 |
2.57 |
1.39 |
9.25 |
Mean with different superscripts are significantly different. Mean separation done with Duncan Multiple Range Test at P<0.05.
Table 4: Abundance of fungi spores in each location.
Abundance of fungal spores with respect to different locations in the University campus showed that all sampled locations had higher proportions during the months of June and July. Mavise, Baba Ding, Cafetaria 2001, Salado, Mommy Mariam, QSS, Olaiya, locations recorded the highest abundance during the sampling months (Table 4).
|
Parameter |
Variables |
Pooled Number of Colonies |
|
Time (Months) |
February |
6.95c |
|
March |
7.70bc |
|
|
June |
10.80a |
|
|
July |
8.21b |
|
|
August |
7.65b |
|
|
EMS |
7.84 |
|
|
Location |
QSS Foods LTD |
10.50a |
|
Glamous Rare Bits (SHOP 10) |
8.30ab |
|
|
MAVISE |
8.60ab |
|
|
CAFETARIA 2001 |
9.60ab |
|
|
VALERIE |
7.56b |
|
|
Jesus Embassy Catering |
7.00b |
|
|
Baba Ding Catering |
7.50b |
|
|
Mommy Mariam Kenny Gold Canteen |
8.20ab |
|
|
Salado Catering Service and Restaurant |
7.50b |
|
|
Olaiya Special Homely Catering |
7.80ab |
|
|
EMS |
7.84 |
Mean with the different letter across the column are significantly (p<0.05) different from one another with respect to each parameter
Table 5: Abundance of fungal spores in different months and locations.
The fitted model of the analysis of variance for locations and monthly abundance of fungi spores produced a highly significant (p<0.01) effect on the abundance of fungi in the locations investigated. A significant (p<0.01) number of fungi were collected in the month of June while months of July and August were not significantly different from each other. The month of February recorded lowest number of fungi spores. For location effect, QSS foods recorded significant (p<0.01) number of fungi spores compared to other locations followed by Cafetaria 2001. Jesus embassy, Baba Ding and Salado showed no significant difference from each other (Table 5).
|
S/N |
Months |
PDA |
DG-18 |
|
1 |
February |
46 |
93 |
|
2 |
March |
59 |
95 |
|
3 |
June |
87 |
129 |
|
4 |
July |
63 |
93 |
|
5 |
August |
60 |
93 |
|
Total |
315 |
503 |
|
Table 6: Differences in the total number of colonies on culture media.
Table 6 shows the difference in the number of colonies obtained on the two different culture media used. A total of 315 colonies were obtained on the Potato Dextrose Agar (PDA) and a total of 503 on the Dichloran Glycerol-18- Agar.
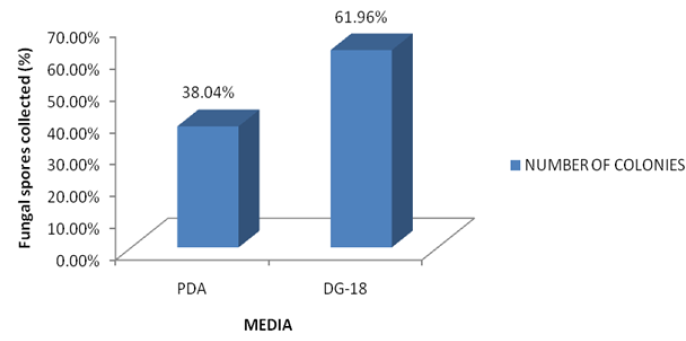
Figure 1: Comparison Percentage fungi spores collected with respect to media.
Source: Nigerian Meteorological Agency (NIMET), Lagos.
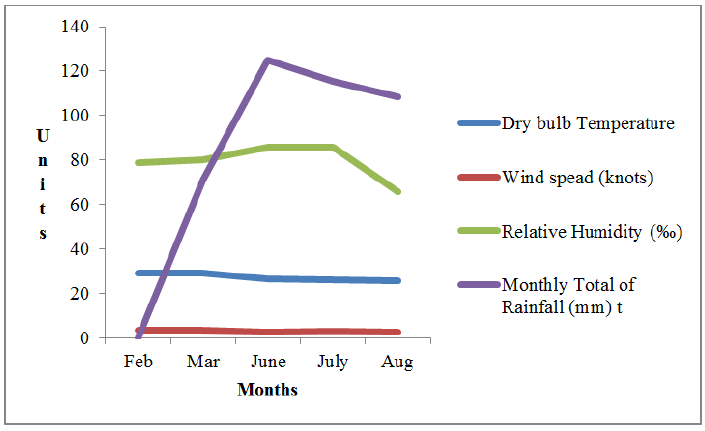
Figure 2: weather parameters for all the study months.
Figure 2 shows that Temperature decreased from February to August, wind speed increased between February and March, decreased in June, increased in July and then reduced in August. Relative humidity increased from February to July and reduced in August, while no rainfall was recorded in February with a drastic increase in rainfall between March and June and a slight decrease between July and August.
4. Discussion
The effect of fungal exposure to human health has been subject of debate in recent years because fungi exposure can cause several adverse health such as infections hypersensitivity disorders and toxic/ irritant effects from their by-products [8]. Only few people are sensitized to specific genera of fungi during exposure. This had led many researchers to ultimately provide measures or means by which indoor air quality can be improved. The results of the investigation reveal that a total of 814 spores were obtained from the ten locations during the five months of study. Aspergillus niger (21%) had the highest occurrence followed by A. Flavus (14%) with a total of 174 and 115 colonies respectively while Neurospora crassa (2.8%), Trichoderma harzianum,(1.5%) and A. Ochraceus (1.9%) had the least occurrences in all the locations in the study period. The species of Aspergillus had the most occurrences in all the sample sites and other allergenic fungi like Penicillium were also isolated during this investigation which corroborates the works of Lumpkins et al. [9], Mabadeje [10] and Boyacioglu et al. [11], Adekunle [12] and Odebode [13], who also recorded higher number of allergenic fungal spores especially during the rainy season. It is very disturbing to know that Aspergillus flavus, which is known to produce mycotoxin more often than the other Aspergillus species and has also been reported by Robbins et al. [14] and Hadayati et al. [15] to cause allergies (brewer’s lungs) was one of the most frequently isolated species. Tiwari et al. [16] also recorded that Aspergillus flavus could cause Septic arthritis and Aspergillosis in immunocompromised persons.
Most of these isolated fungi are known allergens and they cause allergies, irritations and even infectious diseases in humans with suppressed immune system. Aspergillus niger causes hearing problems that may even lead to hear loss. Another isolated fungus is Curvularia, a dangerous fungus that can be a cause of human infections, including onchomycosis, pneumonia, cerebral abscess, etc. It is also known to cause eye irritations when in excess in air spora as reported by Al-Doory. Rhizopus has also been implicated in causing deep subcutaneous mycosis to man and it is also opportunistic in nature. Some other fungi species isolated are Trichoderma species, which is not a major human pathogen, Neurospora and Fusarium species which also produce mycotoxins.
The effects of meteorological factors on the frequency and distribution of fungi during this study was also considered. Aerobiological studies enable us to ascertain the concentrations of the fungal spores present in the air and give a better understanding on the relationship between their concentrations and weather parameters [17]. Based on the data obtained from The Nigerian Meteorological Agency (NIMET), Lagos and the results obtained from the investigation, it was observed that there was a positive correlation between the month with the highest number of colonies which is June, and the month with the highest rainfall and Relative humidity. NIMET also recorded little or no rainfall in February and March which can also be related to the low amount of colonies obtained in these months. Temperature is also an important factor because airborne fungi, especially allergenic types are mainly mesophilic (optimal temperature for growth is 20-40°C) according to Gravesen [18]. The meteorological data revealed that the dry bulb temperature for all the study months was within the range of (20-40°C) meaning that the temperature for the months was favourable for the growth of fungi hence, their abundance in the air.
Two culture media were used for this study mainly to determine the most appropriate and for comparison. Potato dextrose agar is known to have constituents which favour the luxuriant growth of some fungi while suppressing others. The Dichloran glycerol-18 agar has constituents which allow the fair growth of a very wide range of fungi, both slow and fast growers. The results of this study showed a positive correlation to this as more colonies were obtained from DG-18 agar (61.96%) compared to PDA (38.04%). This was also in agreement with the work of Odebode [13] who reported that DG-18 agar recorded more fungal colonies than PDA. In most of the eating places with air conditioning units, there is a common practice of switching off the A/C to save electricity during off business hours [19]. This may lead to condensation of water and a rise in relative humidity [20, 21] and temperature favouring fungi growth. In this case it is advisable to ensure frequent cleaning of the A/C filters and the entire room, but the best approach is to keep the A/C units switched on continuously according to Yau et al. [22] stated that A/C filters also need to be replaced or cleaned periodically as they can be clogged due to dust load and fungi infestation.
The most effective way to reduce fungi growth in a building is to remove the conditions that favour the growth of fungi [8, 23]. Therefore the owners and workers of the eating places must always ensure that available moisture should be avoided to eliminate fungi growth [24]. The steps to reduce moisture include, maintenance of indoor relative humidity to less than 50 per cent, sealing leaks to prevent water intrusion, increasing ventilation and keeping all moisture sensitive materials dry [25]. There is also an increased interest in the use of germicidal treatment or irradiation to clean indoor environments for the control of certain infectious diseases [26]. Further research is needed to establish the relationship between casualties and improve our understanding of fungal and dampness related health effects.
A number of the eating places use the eating rooms as their food store. The stored seeds or grains can serve as source of fungal spores since some of the seeds or grains may be infected and it could account for the presence of plant pathogens such as Penicillium and Rhizopus during the survey which corroborates the work of Adekunle [12]. The eating places should be washed regularly with disinfectants, at least once weekly and the eating rooms should not serve as food stores. Most importantly, the eating places on the Akoka-campus should be fumigated at least once quarterly as recommended by Young and Duncan [27] to make their environments healthy for living.
5. Conclusion
Fungi are one of the major causes of microbial damage in indoor environments and are a serious threat to public health. It is therefore a must to create awareness on the importance of prevention and control of these human pathogens. Good sanitation practices and fumigation should be encouraged in eating places on school campuses and other indoor environments alike. Factors that contribute to or favour the growth of fungi should also be mitigated. Eating places should not be built near bushes, air conditioning systems should be well taken care of, leakages should also be repaired and eating places should also not be converted to stores as these are all sources of indoor fungi. Public health practitioners have a big role to play in creating awareness to the public so as to reduce the health effects of this micro organisms because they are a part of the air we breathe in and it is not a matter of choice to breathe in air.
References
- Zeliger HI. Toxic effects of chemical mixtures. Archives of Environmental Health: An International Journal 58 (2003): 23-39.
- Chao HJ, Schwartz J, Milton DK, et al. The work environment and workers health in four large office buildings. Environment Health Prospectives 111 (2003): 1242-1248.
- Molhave L. Sick building syndrome. Encyclopaedia of Environmental Health (2011): 61-67.
- Ebbehoj NE, Hansen MO, Sigsgaard T, et al. Building related symptoms and molds: a two-step intervention study. Indoor Air 12 (2002): 273-277.
- Bakke JV, Norback D, Wieslander G, et al. Symptoms, complaints, ocular and nasal physiological signs in relation to indoor environment temperature and gender interactions. Indoor Air 18 (2008): 131-143.
- Talbot PHK. An introduction to seed technology Leonard Hill, London (1971): 252.
- Deacon JW. Introduction to Mordern Mycology. Blackwell Scientific Publications. London (1980): 197.
- De Blay F, Casel F, Spirlet F, et al. Elimination of airborne allergens from the household environment. Revue Des Maladies Respiratoires17 (2000): 29-39.
- Lumpkins ED, Corbit SL, Tiedeman GM. Airborne fungi survey. Culture-plate survey of the indoor environment. Annals of allergy 31 (1973): 361-370.
- Mabadeje SA. The fungal flora of the air in Lagos, Nigeria. Nigerian Journal of Natural Sciences 3 (1981): 107-111.
- Boyancioglu H, Hahki AJ, Ates M, et al. The statistical investigation on airborne fungi and pollen grains of atmosphere in Izmir-Turkey. Environmental Monitoring and Assessment135 (2007): 327-334.
- Adekunle AA. Airborne fungi of some eating places on the University of Lagos Campus. Bioscience Research Communications 13 (2000): 81-90.
- Odebode AJ. Characterization of allergenic fungal spores from selected locations in Lagos and Ibadan, Nigeria. PhD unpublished thesis, University of Lagos, Akoka, Nigeria (2017): 241.
- Robbins SL, Cotran RS and Kumar V. Robbins Pathologic basis of disease. (5th) WB Saunders Company Philadelphia (1995): 620.
- Hadayati MT, Mayahi S and Dnning DW. A study of Aspergillus species in houses of asthmatic patients from Sari city, Iran and a brief review of the health effects of exposure to indoor Aspergillus. Environmental Monitoring and Assessment 68 (2010): 481-487.
- Tiwari V, Khatri K, Khan SA, et al. Disseminated Aspergillusflavus following Septic arthritis in an immunocompetent patient. Biomedical Central Research Notes 7 (2014): 709.
- Agnieszka G and Beata B. Effects of meteorological factors on the composition of selected fungal spores in the air. Aerobiologia 31 (2015): 63-72.
- Gravesen S, Nielson PA, Iversn R, et al. Microfungal contamination of damp buildings-examples of risks construction and risk materials. Environmental Health Perspectives 107 (1999): 505-508.
- Hsu NY, Chen PY, Chang HW, et al. Changes in profiles of airborne fungi in flooded homes in southern Taiwan after Typhoon Morakot. Science of Total Environment 409 (2011): 1677-1682.
- Cleri DJ, Ricketti AJ and Vernaleo JR. Fever of unknown origin due to Infection Prevention and Control in Healthcare 21 (2007): 963-996.
- Gaffin JM and Phipatanaku W. The role of indoor allergens in development of Current Opinion in Allergy and Clinical Immunology 9 (2009): 128-134.
- Yau YH, Ng WK. A comparison study on energy savings and fungus growth control using heat recovery device in a modern tropical operating theatre. Energy Conversion and Management 52 (2011): 1850-1860.
- Khan AA and Karuppayil SM. Potential natural disinfectants for indoor environment. Internation Journal of Chemical Aromatherapy 7 (2010): 1-5.
- Barnes CS, Dowling P, Van-Osdol T, et al. Comparison of indoor fungal spore levels before and after professional home remediation. Annals of Allergy, Asthma Immunology 98 (2007): 262-268.
- Cole EC and Cook CE. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. American Journal of Infection Control 26 (1998): 453-466.
- Cardenas MX, Cortes JA and Parra CM. Aspergillus sp in risk areas of transplant patients in a university hospital. Revista Iberoamericana de Mycologia 25 (2008): 232-236.
- Young DB and Duncan K. Prospects for new interventions in the treatment and prevention of mycobacterial disease. Annual Review of Microbiology 49 (1995): 644-674.


 Impact Factor: * 3.6
Impact Factor: * 3.6 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 