Ammonium Interference Reduced Copper uptake by Formaldehyde Crosslinked Sargassum sp. Seaweed
Wenfa Ng
Department of Chemical and Biomolecular Engineering, National University of Singapore
*Corresponding Author: Wenfa Ng, Department of Chemical and Biomolecular Engineering, National University of Singapore
Received: 01 November 2021; Accepted: 11 November 2021; Published: 16 November 2021
Article Information
Citation:
Wenfa Ng. Ammonium Interference Reduced Copper uptake by Formaldehyde Crosslinked Sargassum sp. Seaweed. Journal of Environmental Science and Public Health 5 (2021): 451-461.
View / Download Pdf Share at FacebookAbstract
Sargassum sp., a marine brown macroalgae, is an efficient sorbent for various heavy metals at high concentrations. However, the efficiency at which seaweed removes heavy metals from dilute solutions and the effect of ammonium on metal removal is not well understood; an issue of importance given the ubiquity of nitrogenous compounds in the environment arising from various surface runoffs. Herein, the effect of ammonium on copper removal (at trace to low concentration) by formaldehyde crosslinked Sargassum sp. (treated SW) was studied. Due to high copper background, equilibrium sorption experiments was inconclusive concerning treated SW’s ability in removing copper (<1000 ppb), but rapid copper sorption observed in kinetic experiments suggested potential feasibility of the process. Within initial copper concentration of 4 to 20 ppm and pH 2 to 5, experiments revealed that, above a threshold concentration of [NH4+-N] of 50 ppm, ammonium impede copper update on treated SW in a concentration dependent manner. Specifically, sorption kinetics slowed, and uptake capacity decreased with increase in [NH4+-N] from 0 to 2500 ppm. Collectively, beyond demonstrating that treated SW could remove copper from dilute solutions, revelations that ammonium reduced copper sorption highlighted the importance of accounting for the effect in data interpretation and modelling.
Keywords
<p>Metal adsorption; Trace concentration; Chemical pretreatment; Seaweed; Copper; Sargassum sp.; Ammonium interference; Formaldehyde crosslinking; Kinetic studies; Equilibrium studies</p>
Article Details
Graphical Abstract
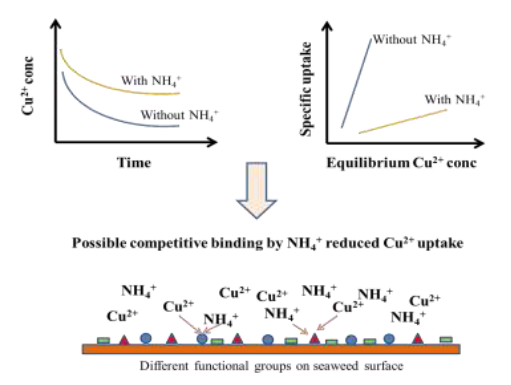
Short Description
Reduced kinetic and equilibrium uptake of copper on formaldehyde crosslinked Sargassum sp. seaweed from low concentration solutions suggested interference effect from ammonium ion co- solute.
1. Introduction
Multiple useful properties such as high conductivity and malleability facilitates the use of metals in diverse applications. Metal production from pristine ores generates fugitive emissions to the environment. Similarly, secondary processing of metals (such as in electroplating and semiconductor manufacturing) also results in metal release, for example, as soluble ions in industrial effluents. Well documented toxicity of metals in humans, in combination with increasingly stringent environmental regulations motivates the development of new technologies for remediating metal impacted waters. Biosorption, a surface mediated passive uptake of heavy metals by dead biomass, is one such example. Compared to commercial metal removal techniques such as ion exchange, biosorption offers a couple of key advantages [1, 2]. Specifically, biosorption utilizes dead biomass with minimal processing. This approach generates cost savings while simultaneously solving a waste disposal problem. Various bacteria, yeast, fungi, micro and macroalgae, and agricultural waste products are shown to be useful in removing metal ions [3-9]. However, uptake capacities differ between biosorbents, given different cell wall structure as well as availability and accessibility of functional groups for metal uptake [10].
The brown marine marcoalgae, Sargassum sp., is an efficient sorbent (up to 20% of the biomass’s dry weight) for a variety of heavy metal ions [10-12]. Commonly found in the intertidal zones of coastal areas in tropical and subtropical climes, seaweed is a plentiful and inexpensive resource. More importantly, evolutionary pressure unique to the marine environment (i.e., the relatively high Ca2+ and Mg2+ content of seawater compared to freshwater) likely endowed Sargassum sp. with high metal sorption capacity through the evolution of cell wall structures comprising specific functional groups.
Copper is intermediate in toxicity compared to more toxic metals such as cadmium, chromium and mercury. But its utility in a variety of applications, such as wires in homes and commercial buildings or as interconnects in silicon chips meant that it is in high demand; thus, its inevitable release during production makes it an important element from the environmental remediation perspective. Demonstrated to bioaccumulate in kidney, liver and other organs, chronic exposure to copper by ingestion is known to cause Wilson’s disease, even though it is an essential micronutrient with diverse physiological functions, chief amongst which is its role as cofactors in enzymes [1, 13]. Industry is the major source of metal release into the environment; thus, studies in the literature have predominantly focused on remediating high copper concentration wastewater [14]. Metals at concentration of less than 20 ppm (defined as trace to low concentration) have been detected in environmental water samples [15]. Although significantly lower than industrial wastewater, metal concentration described above still represents a health threat, one given less attention than it deserves from a water treatment and environmental protection perspective. Thus, the focus of this study was to understand the efficacy of formaldehyde crosslinked seaweed (previously demonstrated to be very effective in removing copper from high concentration synthetic wastewater) in removing copper at trace and low concentration. Given that many water-scarce regions rely on untreated surface waters for drinking and domestic uses, a need also exist for understanding the efficacy of biosorption technology as a potential low cost and point-of-use treatment technique for removing low concentration metal contaminants in source water.
Increasing population and demand for higher food production necessitates increased application of fertilizers on fields. This, together with suboptimal application techniques lead to substantial run-offs of nitrogen rich fertilizers into the natural environment. Hence, many surface water bodies would likely contain elevated concentrations of ammonium and other nitrogenous compounds. At the circumneutral pH typical of most environmental waters, ammonia exists in the soluble, positively charged, ammonium ion form (i.e., NH4+), which, in addition to competing with other cations for binding sites in environmental matrices such as river sediments and clays, it also substitutes for water molecules in the hydration shells of cations when present at high concentration (i.e., ammoniacal solutions) [16]. Thus, co-occurrence of metals in waters at trace concentration with elevated concentrations of ammonium compounds is possible, and poses a relevant research question concerning ammonium’s effect on trace metal biosorption.
Overall, a knowledge gap exists in our understanding of both the utility of seaweed in removing copper at trace and low concentrations, and the roles of ammonium ion in affecting metal removal from dilute solutions. More specifically, removing metal at the trace/low concentration range requires strong affinity between seaweed functional groups and metal, which could not be directly assessed in sorption experiments conducted at high metal concentrations typical of industrial effluents. Thus, using batch kinetic and equilibrium experiments, the present study aimed to (i) assess the feasibility of adsorbing copper from water at trace and low concentrations and quantitating the speed and extent of such an uptake, and (ii) investigating possible interference effect of NH4+ ion on Cu2+ biosorption by formaldehyde crosslinked Sargassum sp. (treated SW). Formaldehyde crosslinking was chosen for chemically modifying Sargassum sp. since pristine seaweed is known to leach significant amounts of organics during biosorption, which translates into progressive reduction of sorption capacity and deterioration of mechanical properties in cycles of sorption/desorption [17]. More intriguingly, the crosslinking technique has also been reported to increase sorption capacity [18].
Experiment results were inconclusive concerning the feasibility of removing copper at trace concentrations (<1000 ppb) in batch equilibrium studies. However, kinetic experiments revealed a consistent trend of exceptionally rapid copper uptake. The preliminary data thus highlighted the need for extra precautions in accounting for copper bound to seaweed and sorption apparatus in trace concentration studies. The focus of the study subsequently shifted to higher [Cu2+] of 4 to 20 ppm, where experimental data revealed rapid uptake of Cu2+, even under NH4+ interference. Speed of Cu2+ sorption on treated SW, however, decreased in a concentration dependent manner with increase in [NH4+-N], where a threshold concentration of 50 ppm (or 3.57 mM) exist, below which interference effect was not observed. Batch equilibrium experiments revealed similar trends, which together with kinetic sorption data, suggested that within the pH range investigated (i.e., pH 2 to 5), NH4+ reduced Cu2+ sorption. Collectively, experimental data revealed that ammonium ion impeded copper sorption on treated SW in a concentration dependent manner; thereby, highlighting the importance of factoring this effect in modelling metal biosorption processes and interpreting experimental results.
2. Materials and Methods
2.1 Chemicals
Copper and ammonium stock solutions were prepared by dissolving crystalline Cu(NO3)2.3H2O salts and amorphous NH4Cl (Merck, min purity 99.5%) in deionized water. Formaldehyde (37 wt%) for crosslinking seaweed was from Riedel-deHaen.
2.2 Collection and pre-treatment of seaweed
Sargassum sp. was harvested at Labrador Nature Reserve, Singapore. The seaweed was washed with copious quantities of deionized water, sun dried for a couple of days, and further dried at 60 oC for 8 to 10 hours in an oven. The seaweed was then grounded into a heterogeneous mixture and sieved by a granulated separator into different size fractions. Only one particle size fraction (500850 μm) was used to prepare formaldehyde crosslinked seaweed since uptake capacity of seaweed is independent of particle size [19].
2.3 Preparation of formaldehyde crosslinked seaweed
10 g of seaweed prepared above was added to 1 L of 0.2 wt% formaldehyde solution in a glass beaker and continuously stirred for 24 hours at 350 rpm [17]. The reaction mixture was allowed to settle to facilitate the collection of formaldehyde crosslinked seaweed (treated SW). The supernatant was decanted and the treated SW washed with copious quantities of ultrapure water and dried at 60 oC for 24 hours. The treated SW was subsequently cooled and kept in a plastic container before use.
2.4 Kinetic biosorption experiments
Solution chemistry modelling, via the chemical equilibrium software MINEQL+ 4.5, revealed that at pH 5.0, about 99% of the total copper exists as Cu2+ and ammonia as NH4+. Copper nitrate and ammoniacal copper solution (2L volume) was prepared in 2L borosilicate glass beaker. Solution pH was measured with an Orion 525A pH/ISE meter and maintained at 5.0±0.1 with 0.1M HNO3 or 0.1M NaOH throughout the experiment to prevent copper precipitation. Biosorbent concentration was 1 g/L. In ammonium effect experiments, solutions were constituted by adding an aliquot (volume depending on target concentration) of NH4Cl stock solution to copper nitrate solution. The solution was stirred at 400 rpm on a stirring hotplate to ensure solution homogeneity (Fisher Scientific, USA). 10 mL of solution was withdrawn at stipulated time points, filtered through a 0.45 μm PTFE membrane filter into 20 mL plastic sample bottles. All samples were acidified with 2% HNO3 and stored at 4 oC prior to analysis of metal concentrations by ICP-OES (Perkin Elmer Optima 3000DV) when [Cu2+] > 1 ppm, and ICP-MS (Perkin Elmer Elan 6100, upper detection limit, 200 ppb) when [Cu2+] < 1 ppm.
2.5 Equilibrium biosorption experiments
Copper nitrate or ammoniacal copper solutions (100 mL) were prepared in 125 mL polyethylene Azlon bottles. Ammoniacal copper solutions were constituted by adding an aliquot from a stock solution of NH4Cl into copper nitrate solutions of differing concentrations within the experimental range. 10 mL of solution was withdrawn before and after equilibration for determining the copper concentration either via ICP-MS or ICP-OES. The biosorbent concentration used was 1 g/L. The sorption mixtures were equilibrated on an orbital shaker for 24 hours at 200 rpm. All aliquots were filtered through 0.45 μm PTFE membrane filter into 20 mL plastic sample bottles, followed by acidification with 2 % HNO3, and stored at 4 oC before metal analysis. The initial and final solution pH was measured with an Orion 525A pH/ISE meter but not controlled during the experiment. Duplicate experiments were conducted at 22 oC.
2.6 Data analysis
The copper uptake per gram of dry treated SW was calculated by Eq. 1.
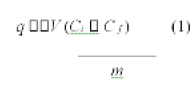
where q is specific uptake (mg/g), V is volume of solution (mL), Ci and Cf are the initial and final concentrations, respectively, and m is the mass of dry treated SW added (mg).
3. Results and Discussion
3.1 Biosorption of copper at trace concentration
Kinetic experiments with initial [Cu2+] of 1000 ppb were performed, and revealed rapid uptake of Cu2+, with ~90% of uptake occurring within the first 10 seconds (Figure1a). This fine-grained snapshot of copper sorption was enabled by high temporal resolution sampling within the first 2 mins of the kinetic experiment. In contrast, typical kinetic experiments tend to have lower sampling resolution within the first few minutes because, depending on the metal concentration, tens of minutes to hours would be needed for the system to reach equilibrium. Over the entire kinetic run, there was a transient increase in [Cu2+] after initial rapid sorption, which was followed by a gradual decrease in [Cu2+] till the end of experiment at 120 mins (Figure 1b). Focusing on Figure 1a, which depicted rapid decrease in [Cu2+] within the first 2 mins of sorption, it can be seen that almost all copper uptake occurred within 10 seconds, with the [Cu2+] essentially constant thereafter – albeit with a slight upward trend. A more holistic view of the phenomenon in Figure 1b, however, showed that there was slow decline in [Cu2+] up to 120 mins. Besides metal analysis, the TOC of samples were also analyzed in experiments designed to investigate possible correlation between organic release and copper sorption. However, the trace concentration used (1000 ppb Cu2+) was not sufficient to afford the discrimination of the relative contribution of metal uptake mediated organic leaching and that without metal influence, given that amount of organics leached was similar to background levels observed when ultrapure water was the contact solution.
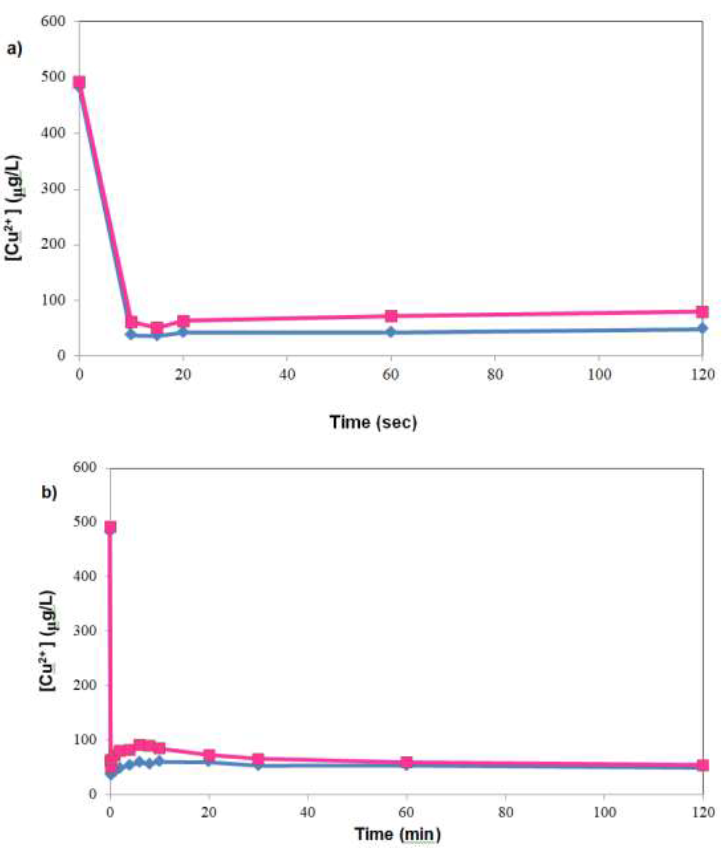
Figure 1: Rapid decrease in copper concentration upon contact with treated SW, a) evolution of [Cu2+] in first 2 minutes of sorption; b) over the entire sorption run. Biomass concentration = 1 g/L, pH not controlled after initial adjustment, data points were of individual runs of the same experiment.
Batch equilibrium experiments (initial [Cu2+] between 1 and 1000 ppb, data not shown), however, were inconclusive. Specifically, large data scatter with no discernible trends were observed. The effect was especially pronounced at the lower end of [Cu2+] (1 to 100 ppb), possibly due to the high background residual on polypropylene bottles, and the trace amount of metal ions present on treated SW. Preliminary results suggested net desorption of copper ions from the treated SW into the bulk solution. Further work in the trace concentration region would likely require use of Teflon bottles, and ultrapure acids for preserving the samples prior to ICP-MS analysis. In view of the inconclusive results obtained, the experimental focus shifted to higher initial [Cu2+] of 4 to 20 ppm.
3.2 Low concentration kinetic biosorption experiment
Uptake of Cu2+ from low concentration solutions onto treated seaweed was biphasic, with a fast initial phase and a slower secondary phase, similar to sorption of other metals to seaweed (Figure 2). The fast initial phase could be attributed to Cu2+ sorption onto treated SW’s exterior surfaces, while diffusion of Cu2+ into the seaweed’s interior accounted for the observed slower secondary phase [10]. From the relative amount of copper sorption that occurred in each phase, it could be inferred qualitatively, that surface sorption played a more important role in Cu2+ uptake relative to sorption on intra-particle binding sites, since the gelatinous nature of alginate severely retarded the microporous diffusion of metal ions into the seaweed interior [17]. From another perspective, the extracellular alginate matrix is akin to a thin polyelectrolyte film through which the metal ions diffuse into the seaweed’s interior during the slower secondary sorption phase [11]. Finally, independent of ammonium interference, a higher initial [Cu2+] used would result in corresponding higher equilibrium [Cu2+], consistent with trends reported in the literature [20].
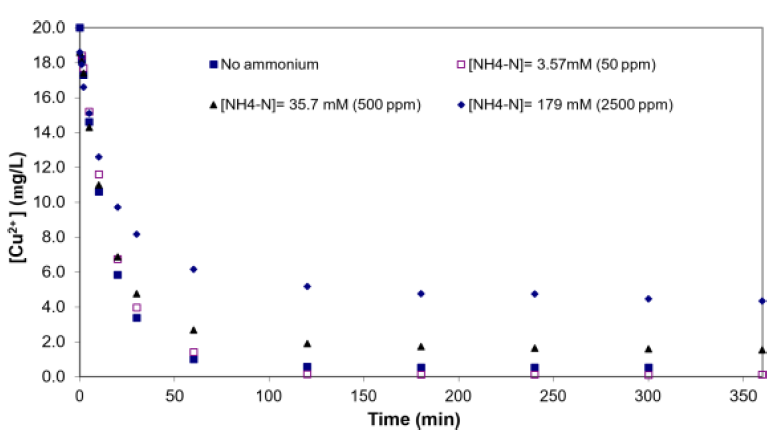
Figure 2: Kinetics of copper biosorption by treated SW with and without added ammonium (0-179 mM), biomass concentration = 1 g/L, pH = 5.0 (controlled) and initial copper concentration of 20 ppm.
The data for NH4+ effect experiments revealed higher residual [Cu2+] (i.e., poorer uptake) with increase in [NH4+-N]. Specifically, NH4+ impeded Cu2+ uptake in a concentration-dependent manner, with [NH4+-N] of 179 mM severely affecting Cu2+ uptake (equilibrium [Cu2+] ~5 ppm relative to ~0.5 ppm in experiments without added NH4+). However, there was no appreciable effect on Cu2+ sorption at [NH4+-N] = 3.57 mM, which suggested a threshold concentration existed beyond which NH+ 4+ would impede Cu2+ sorption on treated SW. Besides higher [Cu2+] residuals, longer equilibration times were also observed with increasing [NH4+-N]. Further evidence of the threshold effect could be seen in the similar equilibration time observed in systems with [NH4+-N] of 0 and 3.57 mM (50 ppm). Specifically, 120 mins was required for the system to reach equilibrium at [NH4+-N] of 0 mM and 3.57 mM, while longer equilibration times (~240 mins and > 360 mins) were needed at [NH4+-N] of 35.7 mM and 179 mM, respectively. One possible explanation for the decreased uptake capacity might be direct binding of NH4+ on treated SW; but questions on the type of functional groups mediating the binding and whether they were the same ones that bind Cu2+ remain to be elucidated. Alternatively, ammonium-copper complexation could also hinder copper binding to seaweed.
3.3 Equilibrium biosorption experiments
The equilibrium specific uptake of copper increased with pH, independent of ammonium interference (Figure 3). Specifically, the gradient of the linearized lines at pH 3 and 4.5 were larger than that at pH 2, indicating that biosorption was more favourable at higher pH, which broadly agreed with other studies in the literature [10, 17, 21]. At initial pH = 2, there was almost no uptake of Cu2+, most likely due to the competitive binding effect from the high concentration of protons [10]. Possible explanations of the enhanced copper uptake at higher pH include: (i) deprotonation of the carboxyl sites (of alginate) and sulfated polysaccharides sites (of fucoidan) in the treated SW cell wall matrix at high pH, which increased the number of negatively charged sites available for binding Cu2+ ions, and (ii) reduced competition from solution H+ ions for the same binding sites at high pH [17, 22].
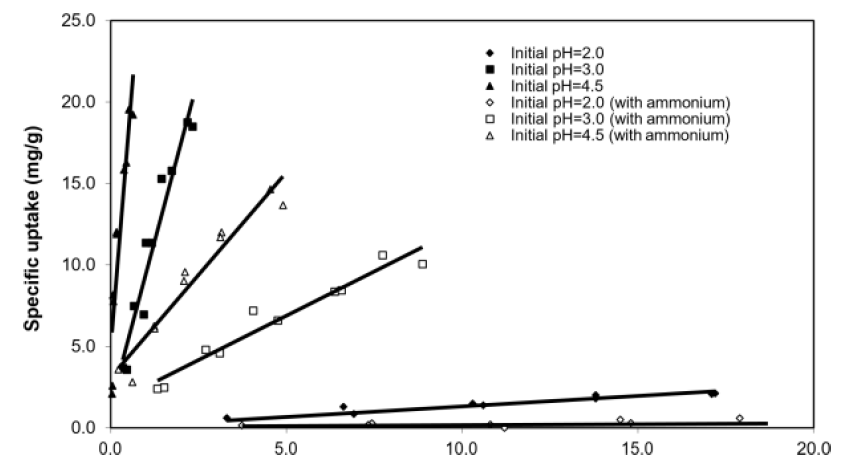
Figure 3: Batch equilibrium studies with and without ammonium interference. Biosorbent concentration = 1 g/L, no pH control, [NH4+-N] = 179 mM. Data points were of individual experiment runs.
The specific uptake and equilibrium [Cu2+] showed a positive linear relationship for [Cu2+] of 0.1 to 20 ppm, which indicated that initial [Cu2+] was positively correlated with equilibrium specific uptake. Such a linear relationship concurred with predictions from various isotherm models such as Langmuir and Freundlich, and is typical of metal sorption from low concentration solutions. At the same initial pH, the presence of NH4+ reduced the equilibrium specific uptake for [Cu2+] within 0.1 to 20 ppm.
4. Conclusions
Taken together, the feasibility of removing copper (at trace concentration) by formaldehyde crosslinked Sargassum sp., was equivocal, even though rapid sorption was observed in kinetic experiments. Future research in this concentration region should adopt better contaminant control practices. Kinetic and equilibrium experiments in the low copper concentration region (i.e., 4 to 20 ppm), however, provided strong evidence of Cu2+ uptake by treated SW even under NH4+ interference4 . Specifically, NH4+ reduced Cu2+ sorption in a concentration-dependent manner beyond a threshold concentration of 50 ppm NH4+-N.
Conflicts of Interest
The author declares no conflict of interest.
Funding
The author thank the National University of Singapore for financial support.
References
- Volesky B. Biosorption of Heavy Metals, CRC Press (1990).
- Raize O, Argaman Y, Yannai S. Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol. Bioeng 87 (2004): 451-458.
- Vijayaraghavan K, Yun YS. Bacterial biosorbents and biosorption. Biotechnol. Adv 26 (2008): 266-291.
- Dhankhar R, Hooda A. Fungal biosorption – an alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol 32 (2011): 467-491.
- Kordialik-Bogacka E. Surface properties of yeast cells during heavy metal biosorption. Cent. Eur. J. Chem 9 (2011): 348-351.
- Kuroda K, Ueda M, in Kotrba P, et al. Yeast Biosorption and Recycling of Metal Ions by Cell Surface Engineering Cell Surface Engineering, Springer Netherlands (2011): 235-247.
- Hanbali M, Holail H, Hammud H. Remediation of lead by pretreated red algae: adsorption isotherm, kinetic, column modeling and simulation studies. Green Chemistry Letters and Reviews 7 (2014): 342-358.
- Rodrigues MS, Ferreira LS, Carvalho JCMd, et al. Metal biosorption onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris: Multi-metal systems. J. Hazard. Mater 217-218 (2012): 246-255.
- Hossain MA, Ngo HH, Guo WS, et al. Biosorption of Cu (II) From Water by Banana Peel Based Biosorbent: Experiments and Models of Adsorption and Desorption. Journal of Water Sustainability 2 (2012): 87-104.
- Volesky B. Sorption and Biosorption, BV Sorbex, Inc. (2003).
- Fourest E, Volesky B. Contribution of Sulfonate Groups and Alginate to Heavy Metal Biosorption by the Dry Biomass of Sargassum Environ. Sci. Technol 30 (1995): 277-282.
- Davis TA, Volesky B, Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37 (2003): 4311-4330.
- S. National Research Council. Physiological Role of Copper, National Academies Press (2000).
- Niad M, Rasoolzadeh L, Zarei F. Biosorption of copper (II) on Sargassum angostifolium C.Agardh phaeophyceae biomass. Chem. Spec. Bioavailab 26 (2014): 176-183.
- Ada FB, Ayotunde EO, Offem BO. Surface and Ground Waters.Concentrations of Metal Elements in Central Cross River State, Nigeria, and their Suitability for Fish Culture. International Journal of Environment and Sustainability 1 (2012): 9-20.
- Copcia V, Hristodor C, Luchian C, et al. Ammonium nitrogen removal from aqueous solution by natural clay. Revista de Chimie (Bucharest) 61 (2010): 1192-1196.
- Chen JP, Yang L. Chemical Modification of Sargassum for Prevention of Organic Leaching and Enhancement of Uptake during Metal Biosorption. Ind. Chem. Eng 44 (2005): 9931-9942.
- Veit MT, Gonçalves GdC, Fagundes-Klen, et al. Organic leaching and metal removal with Sargassum Acta Scientiarum: Technology 36 (2014): 429-435.
- Davis TA, Ali FEC, Giannitti E, et al. Cadmium Biosorption by S. fluitans: Treatment, Resilience and Uptake Relative to Other Sargassum and Brown Algae. Water Quality Research Journal of Canada 39 (2004): 183-189.
- Tsui MTK, Cheung KC, Tam NFY, et al. A comparative study on metal sorption by brown seaweed. Chemosphere 65 (2006): 51-57.
- Sheng PX, Ting YP, Chen JP, et al. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interf. Sci 275 (2004): 131-141.
- Carsky M, Mbhele FN. Adsorption of heavy metals using marine algae. South African Journal of Chemical Engineering 18 (2013): 40-51.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 