Effects of Turbidity and Acidity on Predator-Prey Interactions
Shixu Zhu1 and Yixin Zhang1,2,3*
1Department of Health and Environmental Sciences, Xi’an Jiaotong-Liverpool University, Suzhou, China
2Huai’an Research Institute of New-Type Urbanization of Xi’an Jiaotong-Liverpool University, Huai’an, China
3Suzhou Urban and Environmental Research Institute of Xi’an Jiaotong-Liverpool University, Suzhou, China
*Corresponding Author: Yixin Zhang, Department of Health and Environmental Sciences, Xi’an Jiaotong-Liverpool University, Suzhou, China
Received: 28 March 2019; Accepted: 12 April 2019; Published: 06 May 2019
Article Information
Citation:
Shixu Zhu and Yixin Zhang. Effects of Turbidity and Acidity on Predator-Prey Interactions. Journal of Environmental Science and Public Health 3 (2019): 246-256.
View / Download Pdf Share at FacebookAbstract
Predator-prey interactions are influenced by environmental condition changes, such as increased turbidity and acidification caused by human disturbance. These anthropogenic factors can affect trophic interactions from pisciovorous fish, invertivorous fish, and shredder invertebrates to leaf litter leaching. In this study, we investigated effects of increased turbidity and acidification on predator-prey interaction through a four-level detritus-based food chain, which are top predator (pisciovorous fish), intermediate predator (invertivorous fish), shredder invertebrates, and allochthonous leaf litter. The experiment had a top predator-snakehead (Channa argus), an intermediate predator-black carp (Mylopharyngodon piceus), shredder prey-a freshwater crustacean isopods (Asellus sp.), and allochthonous leaf litter sakura (Cerasus sp.). The pisciovorous snakehead was caged, providing non-lethal predation effect on black carp. The effects of turbidity condition changes with different Nephelometric Turbidity Unit (high level: 60 NTU, and low: 10 NTU) and acidic condition changes (weakly acidic: pH 6.0 and normal: pH 7.5) on leaf litter weight loss. The experiment measured shredder density change and leaf litter weight change that was caused by both leaf litter leaching and shredder’s foraging processes. Results indicated that: the high turbid treatment (60 NTU) reduced the black carp’s antipredator defense to snakehead, so that the black carp’s foraging caused the high mortality of isopod shredders, which reduced leaf litter weight loss. By contrast, the weakly acidic treatment (pH=6.0) did not influence black carp’s top-predator avoidance, but induced the loss of predator avoidance of isopod Asellus sp. shredders that caused isopod high mortality. The acidic treatment did not influence litter weight change. Our study highlights that increased tur
Keywords
<p>Predator-prey interactions, Trophic cascading effect, Visual cue, Chemical stimuli, Antipredator, Ecosystem functioning</p>
Article Details
1. Introduction
Interactions between predators and prey rely on their sensory pathways to detect each other [1], and such ability varies in different surrounding environments [2]. Those sensory pathways of predators and prey, including the chemical stimulus and visual cues, can be interfered by anthropogenic disturbance, such as increased turbidity and acidification [2-4]. Turbidity is usually related to increased sediment inputs through surface runoff caused by anthropogenic disturbance, such as deforestation and urbanization [5-7]. High water turbidity can influence the interaction between predators and preys through deterring their visual cues [8, 9]. For example, high turbidity in water can provide a refuge for prey, because prey visual cues to predators are limited [10-12]. However, little is known about how the impact of turbidity will induce the trophic cascading effect on leaf litter weight loss, which can be that the top-predator’s non-lethal predation effect constrains intermediate predator foraging, and releases its predation risk on shredders, and thus speeds up the leaf litter weight loss.
On the other hand, acidification resulting from atmospheric pollution with high rainfall events [13] can influence on leaf litter leaching, such as leaf litter decomposition [14], and can cause impairment of the chemosensory system of aquatic organisms, consequently, affecting their ability to obtain chemical cues [15-17]. Our understanding on how increased acidification and turbidity influence predator-prey interaction to trigger trophic cascade effect on leaf litter leaching is still limited. In the present study, through a four-trophic level experiment: piscivorous fish, invertivorous fish, shredders, and basal resource (leaf litter) (Figure 1A), an experiment was conducted by manipulating treatments with increased levels of turbidity and acidification in mesocosms.
The high turbid treatment and acidic treatment can constrain the visual sensory and chemosensory of foragers, in terms of predators and prey, respectively. We hypothesized that macroinvertebrate shredders are top-down controlled by invertivorous fish, and a top predator (piscivorous fish) can trigger a non-lethal trophic-cascade on leaf litter weight loss. We compared these treatment effects on changes of shredder invertebrate abundance and leaf litter biomass.
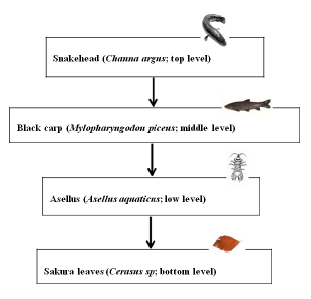
Figure 1: (A) Diagram of the relationships within four levels of tropic interactions. It was tested that the black carp did not eat leaf litter and the snakehead did not eat Asellus sp. shredders.
The objective of this study was to examine how increased turbidity and acidity affect predator-prey interactions, and the consequence on leaf litter weight loss. With different treatment conditions, we further hypothesized:
- Under natural conditions, the cage-constrained top predator (snakehead) will pose non-lethal predation effect on intermediate predator (black carp) through visual and chemical cues, causing black carp’s antipredator defense by reducing foraging. Such non-lethal effect of the top predator will release the predation pressure of intermediate predator on Asellus sp. shredders and promote a trophic cascade effect to cause ‘prey release’ and to enhance leaf litter leaching, i.e., ‘prey release’ hypothesis [18]. Thus, shredders will increase leaf litter weight-loss process (Figure 1B-a)
- Under the turbid environment, visual cue and chemical cue of the top predator are at different levels to the intermediate predator, in which the visual cue is restricted and the chemical cue remains. Stephenson [19] found that ambiguous visual cues of guppies are more reliable spatially and temporally than unambiguous chemical cues, but visual risky detection must be in close proximity for the prey and predator. Though fish can firstly detect unambiguous chemical cues over longer distances than ambiguous visual cues, guppies showed more attentive to visual cues than those exposed to chemical cues. Thus, we assume that visual cue from the top predator in this study should be more influential than its chemical cue for the intermediate predator. The restriction on visual cue access can reduce non-lethal predation effect of the top predator on the intermediate predator to cause “intermediate predator release”, which may impact shredders to influence leaf litter leaching. Such turbid condition will provide visual refuge for the intermediate predator to enhance its foraging activity. Thus, the mortality of shredder will increase, so that in consequence it will reduce leaf litter weight loss (Figure 1B-b)
- Under weakly acidic environment (pH 6.0), due to more sensitive to increased acidic condition, shredders’ ability to detect chemical stimuli of predators will be constrained, i.e., acid-mediated impairment of predator avoidance, so that shredders will be impacted with a high mortality. Thus, leaf litter weight loss should slow down. Yet this weakly acidic level will not impact the foraging of intermediate predator as it should be more tolerated to this acidic condition, but more influenced by non-lethal visual cue from the top predator (Figure 1B-c)
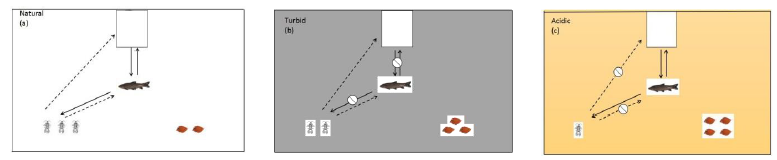
Figure 1: (B) Schematic of predicted abundance change of Asellus sp. shredders and dry biomass change of leaf litter under three simulated experimental conditions: (a) natural condition, with ‘prey release’ hypothesis; (b) increased turbid condition, with “intermediate predator release” effect; (c) increased acidic condition, with acid-mediated impairment of predator recognition for prey. Solid lines represent access of visual cue by an intermediate predator on detecting both prey (Asellus sp.) and a top predator (snakehead). Dashed lines represent the detection of chemical stimuli by Asellus sp.from the intermediate predator (black carp) and a top predator (snakehead).
2. Methods
This experiment was conducted in the aquatic field lab at Xi’an Jiaotong-Liverpool University (XJTLU), Suzhou, China (31°16′30″ N, 120°43′59″ E). Snakehead (Channa argus ) was chosen as the top predator (standard length: 20-25 cm). Snakehead is a widely distributed piscivorous fish in China [20] and recently became an invasive species in North America and Europe [21]. Juvenile black carp, Mylopharyngodon piceus (Cypriniformes: Cyprinidae), is a native fish to lakes and rivers in East Asia, which was chosen as the invertivorous predator (body standard length: 7-10 cm). Black carp are widely cultured distributed in China and mainly feed on benthic invertebrates such as snails, clams, shrimps, and aquatic insect larvae [22] and Asellus sp. (body length: 0.5-1 cm) as the low-level consumer-a shredder which mainly feed on organic matters [23]. For the primary producer, the sakura (Cerasus sp.) leaf litter was used.
The sakura leaves were collected on ground along the Renai Road nearby XJTLU. Sakura leaves were washed carefully in lab and dried at 60°C for 24 h. Both snakehead (piscivorous predator) and juvenile black carp (invertivorous predator) were obtained from the local fishermen in Suzhou. The isopods (Asellus sp.) were collected in the stream nearby XJTLU. In lab condition, Asellus sp. individuals were observed to feed on Cerasus sp. leaf litter. For all fishes and shredder Asellus sp. were all isolated respectively in the water at the field lab for 3 days without providing food, as this standardized hungered level should reduce the variation of previous foraging efforts. Animal individuals in this experiment only were used in a single mesocosm with different treatments. The indication of hypothetic trophic relationship in the experiment is shown as Figure 2. The experiment was conducted in the aquatic station at XJTLU along a river at field condition with natural light.
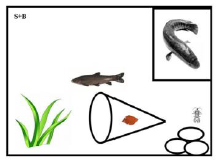
Figure 2: The indication of the experimental mesocosm structure. We put the gravels/cobbles right under the niche of snakehead and the leaf litter bag in the middle of watergrass and cobbles. To make sure the black carp can eat the prey Asellus sp., we kept the mouth of the leaf litter pack opened.
Three experimental treatments were: (1) natural condition (NTU of 10 and pH of 7.5); (2) high turbid level (NTU of 60 and pH of 7.5) (3) acidic conditions (NTU of 10 and pH of 6.0). Each treatment had 8 replicates (Figure 1). Each replicate was set in a mesocosm (60 cm × 40 cm × 34 cm, 30 L). For the top predator, one snakehead was put in a transparent plastic niche (20 cm × 20 cm × 16 cm) covered net screen with 0.55-mm mesh size (Figure 2). Three-gram sakura leaf litter (dry weight) was put in the small yellow nylon pack (8 mm mesh size). We used the stones (diameter of 5-10 cm) as the refuge for 10 individuals of freshwater isopod Asellus sp. in each mesocosm. We used artificial aquatic grasses (one individual ranges from height of 10-15 cm) as the refuge for the two black carps in the mesocosm. During the experimental period, the institutional animal use and care guideline was followed.
2.1 Effect of turbid and acid environment
The pH of water in mesocosms was measured by a portable electric pH meter and the turbidity by the automatic nephelometric analyzer. To simulate the natural condition, we used the river water from the river channel beside the aquatic lab with pH of 7.5 and Nephelometric Turbidity Unit (NTU) of 10. To simulate the high turbidity treatment, 10 g fine particulate Kaolin clay powder (diameter <0.05 mm) was added into the mesocosms based on treatment of natural to the turbidity about 60NTU (about 3.33 g/L [12]). To simulate the acidic environment with four-level food chain, the pH of the water was buffered to 6.0. The pH of 6.0 was regarded as a major freshwater acidification threshold for many biota species [24]. We acidized the river water by dropping in minute amounts of dilute H2SO4 under the monitoring of portable water pH meter.
The experiment was ran for 48 hours. The reason for having 48-h experimental time was that the prey could have a chance of survival under predator treatment through this experimental period, which allowed to obtain the survival data with variation that is necessary for statistical analysis. If the experimental time is too long, there would be not be any prey individual left at the end of the experiment, which would cause no data suitable for statistical analysis. For each treatment, after 48 hours, the leaf litter packs were taken out and sent to the laboratory, gently washed and dried for 24 h at 60°C, and weighed. The dry weight change of the leaf litter was caused by both processes of leaf litter leaching and shredder’s foraging.
For Asellus sp. shredders, the remained individuals were checked in mesocosms after 48 hours and recorded. In addition, during the 48 h experimental period, careful observations on organisms’ behaviors were conducted about every 12 hours. During the observation, behaviors of black carp and Asellus sp. (foraging or hiding in the refuge) were recorded and each observation lasted about 20 minutes for all the mesocosms.
All statistical analyses were performed using R 3.1.2 (R Development Core Team 2016). For the first hypothesis, one-way analyses of variance (ANOVAs) were performed with data of leaf litter dry-weight loss (%) or consumed Asellus sp. shredder (%) to test the effects of turbid treatment. For the second hypothesis, we performed one-way analyses of variance (ANOVAs) with leaf litter dry-weight loss (%) or percentage of consumed Asellus sp. shredder (%) to test the effects of increased acidity treatment. All data were log-transformed to stabilize variance.
3. Results
In natural condition, treatment the caged top predator (snakehead) caused the antipredator response of the intermediate predator (black carp) through visual cue and chemical stimuli, which reduced black carp’s movement so that triggered the situation of releasing its predation pressure on Asellus sp. Shredders. Thus, the Asellus sp. individuals moved out of their refuge (cobbles) for foraging on leaf litter. Under turbid environment (NTU of 60), the weight loss of leaf litter (%) caused by Asellus sp. shredders was decreased (F1,15=10.12, p<0.01; Figure 3a) and there was a stronger predation effect of black carp on Asellus sp. shredders, which enhanced loss of Asellus sp. (%) by 25% under turbid condition (F1,15=9.615, P < 0.05; Figure 3b). observed that the black carps were moving around in the mesocosm but not hiding among the water grass or beneath the leaf litter packs, and the Asellus sp. shredders were also not hiding among the cobbles but creeping in the mesocosm bottom or on the leaf litter packs in all replicate mesocosms. Under acidic (pH of 6) treatment, results indicated no significant difference of leaf litter dry weight change between the acidic group and the natural group (F1,15=1.289, P = 0.283; Figure 3a), whereas the Asellus sp. shredders (%) decreased significantly by 25% under acidic condition comparing with the natural group (F1,15=10.71, P < 0.01; Figure 4b). For behavior observation, it was found that the black carps hid under the leaf litter packs, but not the artificial refuge we provided in all replicates, whereas the Asellus sp. shredders still were creeping in the bottom of the mesocosm and on the leaf litter packs.
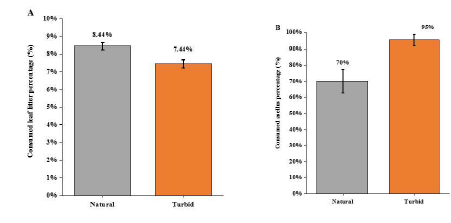
Figure 3: (A) Comparison of consumed leaf litter weight percentage under natural and turbid environment (± 1SE); (B) Comparison of consumed number of Asellus sp. percentage under natural and turbid environment (± 1SE).
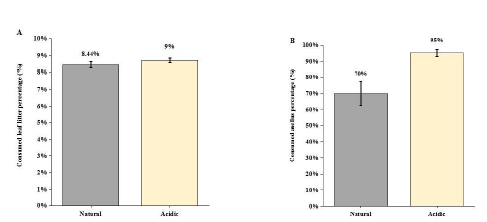
Figure 4: (A) Comparison of leaf litter dry-weight loss (%) under the natural and acidic environment (± 1SE); (B) Comparison of consumed Asellus sp. (%) under the natural and acidic environment (± 1SE).
4. Discussion
4.2 Turbid condition
Through manipulating the high turbidity condition, the increased mortality of Asellus sp. shredders was found due to black carp’s predation, which triggered trophic cascading effect on leaf litter weight loss. This is in accordance with our hypothesis (Figure 1B-b). High water turbidity can affect interaction between predators and preys through deterring their visual cues [8]. Although some other studies suggested that high turbidity can amplify the non-lethal effect of predators on prey which reduced the foraging activities of prey [12, 27], yet in our study, we observed that the black carp swam around and did not hide in the refuge or under the leaf litter pack under high turbid environment. The high turbid condition can provide the ‘visual refuge’ for invertivorous black carp by limiting visual cues to the top predator-snakehead [10, 28, 29]. However, high turbid environment may have no effect on predators that rely on chemical cues for detection [27]. for fish species which rely on visual cues would be more sensitive to detect those mobile prey [32-34]. Our experiment was conducted under natural light condition, so that visual cues should function well at all day time.
4.3 Acidic condition
Our results suggest that the Asellus sp. shredders in weakly acidic condition were impaired in their ability to detect potential predation risk. The high mortality of Asellus sp. shredders under acidic condition was consistent with our third hypothesis (Figure 1B-c). During the experiment, we observed that, under the weakly acidic condition, no Asellus sp. shredders hid under cobbles, but the black carp hid under the leaf litter pack. When Asellus sp. shredders went to leaf litter pack for feeding, the black carp can capture them easily. The acidic environment could interfere the predator detection of Asellus sp. shredders that rely on chemical stimuli, but no influence the black carp because it is more influenced by visual cue of the top predator [19]. Though the situation of pH of 6 in freshwater ecosystems is regarded as a threshold for many species due to sensory system impacted [24, 35], the acidification condition with pH <6 in freshwater bodies can be found worldwide [36-38]. Further studies should explore how predator-prey interactions will be changed under the stronger acidic condition [39-40].
5. Conclusion
This mesocosom experiment revealed that high turbidity and acidic condition can restrict target detection by the access of visual cue and chemical stimuli for both predators and preys, which can cause the loss of antipredator response of intermediate predator (more rely on visual cue) and basal prey (more rely on chemical stimuli). In this study, both cases finally led to the high mortality of isopod Asellus sp. shredders. Under high turbidity condition, a trophic cascading effect caused by changed predator-prey interaction further led to reduction of leaf litter weight loss, because the turbidity condition reduced the top-predator’s predation effect on the intermediate predator, so that the reduction of shredder density caused by black carp foraging decreased leaf litter weight loss. Our results indicated that abiotic condition changes due to disturbance can change and modify trophic interaction in aquatic ecosystems, and such anthropogenic impacts with multiple stressors on predator-prey interaction should be further investigated for understanding their ecological mechanisms.
Acknowledgments
We thank Zhaofeng Yuan and Hongyong Xiang for their help in the field to collect shredders and providing lab support. We thank several reviewers for their constructive comments and suggestions on earlier versions of the manuscript. This work was supported by XJTLU Research Development Fund (RDF-15-01-50), Huai’an Science & Technology Bureau Program (HAS201617), and Jiangsu Science and Technology Program (BK20171238).
References
- Webster DR, Weissburg MJ. The hydrodynamics of chemical cues among aquatic organisms. Annual Review of Fluid Mechanics 41 (2009): 73-90.
- Weissburg MJ, Zimmer-faust RK. Life and death in moving fluids: hydrodynamic effects on chemosensory-mediated predation. Ecology 74 (1993): 1428-1443.
- Ferner M, Smee D, Weissburg M. Habitat complexity alters lethal and non-lethal olfactory interactions between predators and prey. Marine Ecology Progress Series 374 (2009): 13-22.
- Robinson EM, Smee DL, Trussell GC. Green crab (Carcinus maenas) foraging efficiency reduced by fast flows. PLoS ONE 6 (2011):
- Ricciardi A, Rassmusen JB. Extinction rates of North America freshwater fauna. Conservation Biology 13 (1999): 1220-1222.
- Dudgeon D, Arthington AH, Gessner MO, et al. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81 (2006): 163-182.
- Donohue I, Molinos JG. Impacts of increased sediment loads on the ecology of lakes. Biological Reviews 84 (2009): 517-531.
- Abrahams M, Kattenfeld M. The role of turbidity as a constraint on predator-prey interactions in aquatic environments. Behavioural Ecology and Sociobiology 40 (1997): 169-174.
- Van de Meutter F, De Meester L, Stoks R. Water turbidity affects predator–prey interactions in a fish-damselfly System. Oecologia 144 (2005a): 327-336.
- Jacobsen L, Berg S, Jepsen N. Does roach behavior differ between shallow lakes of different environmental state? Journal of Fish Biology 65 (2004): 135-147.
- Lunt J, Smee DL. Turbidity influences trophic interactions in estuaries. Limnology and Oceanography 59 (2014): 2002-2012.
- Figueiredo BRS, Mormul RP, Chapman BB, et al. Turbidity amplifies the non-lethal effects of predation and affects the foraging success of characid fish shoals, Freshwater Biology 61 (2016): 293-300.
- Likens GE, Butler TJ, Buso DC. Long- and short-term changes in sulfate deposition: Effects of the 1990 Clean Air Act Amendments. Biogeochemistry 52 (2001): 1-11.
- Wu WT, Zhang YX. Effects of particulate matter (PM2.5) and associated acidity leaf litter leaching: response of leaf litter breakdown. Environmental Science and Pollution Research 25 (2018): 30720-30727.
- Brown GE, Adrian JC, Jr. Lewis MG, et al. The effects of reduced pH on chemical alarming signaling in ostariophysan fishes. Canadian Journal of Fisheries and Aquatic Sciences 59 (2002): 1331-1338.
- Brown GE, Elvidge CK, Ferrari MCO, et al. Understanding the importance of episodic acidification on fish predator-prey interactions: Does weak acidification impair predator recognition? Science of the Total Environment 439 (2012): 62-66.
- Leduc AOHC, Ferrari MCO, Kelly JM. Learning to recognize novel predators under weakly acidic conditions: the effects of reduced pH on acquired predator recognition by juvenile rainbow trout. Chemoecology 14 (2004): 107-112.
- Rodríguez-Lozano P, Verkaik I, Rieradevall M, et al. Small but powerful: top predator local extinction affects ecosystem structure and function in an intermittent stream. PLoS ONE 10 (2015): e0117630.
- Stephenson JF. Keeping eyes peeled: guppies exposed to chemical alarm cue are more responsive to ambiguous visual cues. Behav Ecol Sociobiol 70 (2016): 575-584.
- Liu JS, Cui YB, Liu JK. Food consumption and growth of two piscivorous fishes, the mandarin fish and the Chinese snakehead. Journal of Fish Biology 53 (1998): 1071-1083.
- Lapointe NWR, Odenkirk JS, Angermeier PL. Seasonal movement, dispersal, and home range of northern snakehead Channa argus (Actinopterygii, Perciforms) in the Potomac river catchment. Hydrobiologia 709 (2013): 73-87.
- Hao SF, Lan LL, Li GF. The biological character and aquaculture technology of Black Carp (Mylopharyngodon piceus). Heilongjiang Aquaculture 1 (2007): 11.
- Graca MAS, Maltby L, Calow P. Comparative ecology of Gammarus pulex (L.) and Asellus aquaticus (L.) l: population dynamics and microdistribution. Hydrobiologia 281 (1994): 155-162.
- Holt CA, Yan ND, Somers KM. pH 6 as threshold use in critical load modeling for zooplankton community change with acidification in lakes of south-central Ontario: accounting for morphometry and geography. Canadian Journal of Fisheries and Aquatic Science 60 (2003): 151-158.
- Ryer CF, Stoner AW, Titgen RH. Behavioral mechanisms underlying the refuge value of benthic habitat structure for two flatfishes with differing anti-predator strategies, Marine Ecology Progress Series 286 (2004): 231-243.
- Donelan SC, Grabowski JH, Trussell GC. Refuge quality impacts the strength of nonconsumptive effects of prey. Ecology 98 (2017): 403-411.
- Lunt L, Smee DL. Turbidity interferes with foraging success of visual but not chemosensory predators. PeerJ 3 (2015): e1212.
- Ranaker L, Jonsson M, Nilsson PA, et al. Effects of brown and turbid water on piscivore-prey fish interactions along a visibility gradient. Freshwater Biology 57 (2012): 1761-1768.
- Carter MW, Shoup DE, Dettmers JM, et al. Effects of turbidity and cover on prey selectivity of adult smallmouth bass. Transactions of the American Fisheries Society 139 (2010): 353-361.
- Heads PA. The effect of invertebrate and vertebrate predators on the foraging movements of Ischnura elegans larvae (Odonata, Zygoptera). Freshwater Biology 15 (1985): 559-571.
- Van de Meutter F, Stoks R, De Meester L. Macroinvertebrate communities diverge in function of turbidity state and microhabitat: a pilot study in six shallow lakes. Hydrobiologia 144 (2005b): 327-336.
- Banks PB, Norrdahl K, Korpimaki E. Nonlinearity in the predation risk of prey mobility. Proceedings of the Royal Society B: Biological Sciences 267 (2000): 1621-1625.
- Gegenfurtner KR, Hawken MJ. Interaction of motion and color in the visual pathways. Trends Neurosci 19 (1996): 394-401.
- Derrington AM. Vision: can color contribute to motion? Current Biology 10 (2000): 268-270.
- Olivier A, Leduc AOHC, Roh E, et al. Impaired detection of chemical alarm cues by juvenile wild Atlantic salmon (Salmo salar) in a weakly acidic environment. Canadian Journal of Fisheries and Aquatic Sciences 63 (2006): 2356-2363.
- Mallory ML, McNicol DK, Cluis DA, et al. Chemical trends and status of small lakes near Sudbury, Ontario, 1983-1995: evidence of continued chemical recovery. Canadian Journal of Fisheries and Aquatic Science 55 (1998): 63-75.
- Jeffries DS, Lam DCL, Wong I, et al. Assessment of changes in lake pH in southeastern Canada arising from present levels and expected reductions in acidic deposition. Canadian Journal of Fisheries and Aquatic Science 57 (2000): 40-49.
- Doka SE, McNicol DK, Mallory ML, et al. Assessing potential for recovery of biotic richness and indicator species due to changes in acidic deposition and lake pH in five areas of southeastern Canada. Environmental Monitoring Assessment. 88 (2003): 53-101.
- Ajemian MJ, Sohel S, Mattila J. Effects of turbidity and habitat complexity on antipredator behavior of three- spined sticklebacks (Gasterosteus aculeatus). Environmental Biology of Fishes 98 (2015): 45-55.
- Pangle KL, Malinich TD, Bunnell DB, et al. Context-dependent planktivory: interacting effects of turbidity and predation risk on adaptive foraging. Ecosphere 3 (2012): UNSP114.
|
Citation: Shixu Zhu and Yixin Zhang. Effects of Turbidity and Acidity on Predator-Prey Interactions. Journal of Environmental Science and Public Health 3 (2019): 246-256. |


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 