A Review of Brucellosis: A Recent Major Outbreak in Lebanon
Alia Sabra1*, Bouchra el Masry2, Houssam Shaib2*
1One Health Program, Duke University, Durham, North Carolina, USA
2Department of Agriculture, Faculty of Agricultural and Food Sciences, American University of Beirut, Beirut, Lebanon
*Corresponding Author: Houssam Shaib (PhD), Faculty of Agricultural and Food Sciences, American University of Beirut, Riad El Solh 1107-2020, PO Box 11-0236, Beirut, Lebanon
Alia Sabra, (MS in Environment, MS in Energy, Certificate in One Health), One Health Program, Duke University, Durham, North Carolina
Received: 08 January 2021; Accepted: 16 January 2021; Published: 11 February 2021
Article Information
Citation:
Alia Sabra, Bouchra el Masry, Houssam Shaib. A Review of Brucellosis: A Recent Major Outbreak in Lebanon. Journal of Environmental Science and Public Health 5 (2021): 56-76.
View / Download Pdf Share at FacebookAbstract
Brucella infection remains the world’s most common bacterial zoonosis, with over half a million new cases annually, which brought renewed attention of this neglected disease. This attention is highlighted in this review manuscript, reporting worldwide outbreaks and introducing the 5th major severely prominent worldwide outbreak since 2016 which occurred in Lebanon. In this outbreak, most recent Brucella incidence was mainly observed in the Bekaa governorate with around 70% of the cases reported during the summer and spring. Furthermore, in line with the Lebanese alimentary habits, brucellosis is commonly diagnosed in adults aged between 20 and 60 years old. This paper tailors the first comprehensive One Health approach for the control of Brucellosis in Lebanon. Herein, a broad review to shed light on the complexity of Brucellosis discussing: the etiology; taxonomy; pathogenesis; epidemiology and geographic distribution of the disease; sources and transmission in both animals and humans; clinical manifestation; chemical treatment and its risk; and finally disease prevention.
Keywords
<p>Zoonosis; Brucellosis; Outbreak; One Health Approach; Control Measures; Lebanon</p>
Article Details
1. Introduction
“It has been estimated that more than 500,000 new human cases of brucellosis occur globally each year” [1]. Despite its identification in 1751 by British army surgeon Cleghorn followed by the isolation of its causative organisms in 1887 by David Bruce whose name was given to the genus “Brucella”, brucellosis is nowadays considered as a re-emerging common granulomatous zoonotic disease that is engaging health policymakers [1-3]. This highly contagious bacterium is not only negatively influencing human health via causing severely devastating and disabling sickness, but also the economy due to holding patients from their daily work and creating losses in animal production [4-5]. It is classified by the Center for Disease Control as a “Category B” bioterror agent meaning it results in moderate morbidity and low mortality rates approximately 2% as it disseminates easily, and needs specific enhancements of diagnostic capacity and surveillance [6]. In addition, the World Health Organization identified it as “one of the world’s leading neglected zoonotic diseases” due to the burden that it specifically places on low-income countries [7].
In the MENA region (Middle East and North Africa region), Brucella is an endemic zoonotic neglected tropical disease. Conflict, associated breakdowns in veterinary public health systems, and unrestricted animal transportation through open borders have promoted the re-emergence of brucellosis. Among cattle and sheep, in 2010, the highest prevalence of brucellosis occurs in Jordan, while among goats the highest rates of infection are in Iraq and Jordan, and among camels in Egypt, Iran, and Saudi Arabia. Brucellosis is also an important problem in Libya, and it is prevalent among the Bedouin community in Oman [8]. Preventive measures require surveillance, animal control, and increased use of the brucellosis vaccine for animals at risk [8].
Here comes the necessity of this review to shed light on the complexity of neglected brucellosis. It discusses the etiology, taxonomy, pathogenesis, epidemiology, sources, transmission, clinical manifestations, diagnosis, treatment and prevention of this disease. As a final point, this review has a special focus on the disease dissemination in the MENA region, and particularly introduces the 5th largest worldwide outbreak presently occurring in the country of Lebanon while suggesting a comprehensive One Health approach.
2. Etiology
Brucellosis, also known by a total of nine other names most prominently the Mediterranean and Malta fevers, is caused by infection with small (0.5–0.7 μm diameter, 0.6–1.5 μm length) [9], aerobic, gram-negative, non-spore-forming, non-motile, short rod-shaped coccobacilli bacteria of eleven Brucella species classified based on the differences in pathogenicity and host preference (Table 1) [10, 11]. They cause lifelong chronic disease and function as facultative intracellular pathogens.
|
Organism |
Animal Reservoir |
Geographic Distribution |
|
|
Classical Species |
|||
|
B. melitensis |
goat, sheep, camel (biotype 3) |
Mediterranean, Asia, Latin America, parts of Africa and some southern European countries |
|
|
B. abortus |
cow, buffalo, camel, yak, coyote (biotype 7) |
Worldwide |
|
|
B. suis |
pig (biotype 5), caribou |
South America, Southeast Asia, United States |
|
|
B. canis |
canine |
Cosmopolitan |
|
|
B. ovis |
sheep |
worldwide (No known human cases) |
|
|
B. neotomae |
rodent |
Not known to cause human disease (isolated from wood rats in North America) |
|
|
Novel Species |
|||
|
B. maris
|
B. pinnipediae |
strains from pinnipeds (seal, sea lion, walrus) |
Case reports describing some human cases (sporadic human pathogens - mainly neurobrucellosis) |
|
B. cetaceae |
isolates from cetaceans (whale, porpoise, dolphin) |
||
|
B. inopinata |
Not reported |
Case reported in Human breast abscess (implant wound) |
|
|
B. microti |
Vole |
Case during an epizootic in the Czech Republic (2001) |
|
|
B. papionis |
baboon |
Case of 13-year-old baboon captured in Tanzania |
|
|
B. vulpis |
red fox |
Case of 2 red foxes in Austria |
|
Table 1: Brucella species [11,12,13,14,15,16,17,18].
The genus Brucella consists of different serotypes also called species. The most commonly known to cause disease in humans, have a pattern of severity starting with Brucella melitensis (B. melitensis), isolated in 1887 [19], being the most virulent, invasive, and universally prevalent. It is due to the fact that B. melitensis contains genes for flagellum- specific type III and IV secretion systems, these genes are involved in different process ranging from the delivery of virulence factors into the eukaryotic cell to conjugation, transfer of genetic material, and uptake or release of DNA [11, 19]. B. melitensis infects mostly goats and form the most important source of human disease.
Brucella suis (B. suis) of intermediate virulence ranks second and mainly infects swine. In Poland, it was also isolated from wild hares [19]. Brucella suis in humans can be as severe as B. melitensis; however, in a small series, patients infected with B. suis did not have a more severe clinical course compared with those infected with Brucella abortus (B. abortus) [19].
- abortus causes abortions in cattle, being of mild-to moderate virulence that rarely causes complications is highly widespread worldwide. B. abortus was for many years the main etiologic factor of brucellosis in animals and humans (Bang’s disease) in Poland [19]. Brucella canis (B. canis), infecting dogs and rarely transmitted to humans, causes frequent relapses [7]. It was first described by Carmichael in 1966 who isolated the bacillus from the placenta, foetuses and vaginal discharge of female dog that aborted their litters. The disease was earlier diagnosed in the United States in Beagle dogs [19]. It is infrequently associated with human disease, and reported cases have usually been mild [7].
Other pathogenic species of Brucella exist, such as Brucella neotomae isolated in the United States from rats, Brucella ovis that infects sheep and rams, Brucella marina, Brucella ceti, and Brucella pinnipedialis found in sea mammals (whales, seals) in the Atlantic Ocean. Brucella microti was isolated from the common vole (Microtus arvalis) in the Czech Republic, from soil in the same area years later, and from mandibular lymph nodes of wild red foxes (Vulpes vulpes) in Austria. Brucella inopinata was isolated from a breast implant wound of a woman with clinical signs of brucellosis [19].
3. Taxonomy
The genus Brucella belongs to the family of Brucellaceae (family III) with Mycoplana and Ochrobactrum, of the order Rhizobiales in the class Alphaproteobacteria of the phylum Proteobacteria [12]. The class Alphaproteobacteria include families of organisms that are either mammalian or plant pathogens or symbionts. The genera Bartonella, Rickettsia and Ehrlichia, are examples of the organisms affecting mammals. These genera are spread by vector-based transmission. Brucella is distinguished from most genera due to the ability to infect mammalian cells, this feature is only shared with Bartonella.
Brucella genomes have preserved more of the metabolic functions shared by the plant pathogens, which gave it the potential to persist in the soil for up to 10 weeks. Due to the genome large size, brucella have the ability to survive in different environments and furthermore adapt to a number of different hosts [12]. The ability to invade mammalian hosts is a feature acquired by both Bartonella and Brucella and expected to exhibit nucleotide composition (i.e., G + C %) that is distinct from genes conserved from progenitor organisms [20].
Several gene candidates exist to fulfill this role, including those encoding biosynthesis of polysaccharides, secretion systems, adhesins and invasins [21]. However, it is possible that genes involved in uptake or invasion of mammalian cells were present in progenitor organisms, and lost from the plant pathogens [12]. In this case, the genes would not exhibit distinctive nucleotide compositions, and would require more direct approaches for identification. Evaluation of the genomes of several Brucella species indicates the loss of gene function via pseudogene formation during adaptation to the intracellular lifestyle [22]. Additionally, horizontal gene transfers unique to the Brucella species, associated with important virulence determinants, appears to be associated with adaptation to the intracellular lifestyle [23].
Species’ genome consists of two circular chromosomes as they are identified based on phenotypic characteristics, antigenic variation, and prevalence of infection in hosts and their preference [13, 24, 25]. Most open reading frames (ORF) of species share greater than 99% sequence identity [20].
In 2003, the International Committee on Systematics of Prokaryotes (ICSP) Subcommittee on the Taxonomy of Brucella agreed on the taxonomy of the classical six species, with recognized biovars of B. suis, abortus and melitensis [11]. As for the five novel species, Bruceela cetaceae, pinnipedialis and microti conform to the high genetic homogeneity by sharing identical 16S rRNA gene sequences except for Brucella inopinata which extends significantly the described genetic diversity within the atypical Brucella group. B. pinipedialis and cetaceae have a different pattern of metabolic activity by comparison to the other species [26]. The ten species, excluding Brucella inopinata, are within the core Brucella group [11]. Brucella species have the unique ability to involve almost every organ system by invading both phagocytic and non-phagocytic cells and to survive within the bloodstream [27]. To be a successful infectious agent, Brucella requires four main steps: adherence, invasion, establishment, and dissemination within the host [28].
4. Pathogenesis
Several studies point at the outer membrane being the main component for virulence factor of Brucella, this membrane contains Lipopolysaccharides (LPS) [28]. It possesses a peculiar non-classical LPS as compared to the classical LPS from Enterobacteria, such as Escherichia coli (Table 2). Generally, smooth LPS has a role in cell entry and immune evasion of the infected cell. It also alters the capacity of the infected cell to present foreign antigens, hence, prevents the immune system attack for the infected cell.
|
Classical LPS |
Non classical LPS |
|
Exhibit high toxicity |
Exhibit low toxicity for endotoxin sensitive mice and rabbit |
|
High pyrogenicity |
Low pyrogenicity |
|
Inducers of interferons and tumor necrosis factor |
Weak inducers of interferons and tumour necrosis factor |
|
Examples: E. coli |
Example: Brucella. abortus |
Table 2: Difference between classical and non-classical LPS [2, 28].
LPS has three domains: lipid A, the core oligosaccharide, and the O-antigen or O-side chain. The O-polysaccharide of smooth-type Brucella LPS (S-LPS) is an unbranched homopolymer of 1,2-linked 4,6-dideoxy-4-formamido-α-D-mannopyranosyl usually with an average chain length of 96 to 100 glycosyl subunits. The O-polysaccharide is linked to a core oligosaccharide composed of mannose, glucose, 2-amino-2,6-dideoxy-D-glucose (quinovosamine), 2-amino-2-deoxy-D-glucose (glucosamine), 3-deoxy-D-manno-2-octulosonic acid (KDO) and unidentified sugars [28]. The lipid A, linked to the core oligosaccharide, contains 2,3-diamino-2,3-dideoxy-D-glucose (diaminoglucose) as backbone, amide and ester-linked long chain saturated (C16:0 to C18:0) and hydroxylated (3-OH-C12:0 to 29-OH-C30:0) fatty acids. The hydrophobic lipid A region constitutes mostly the outer coating of the outer membrane and is responsible for many of the endotoxic properties attributed to LPS. Thermotropic phase behaviour and immunochemical analysis of B. abortus and B. melitensis lipid A suggest a disaccharide backbone molecule linked in a β1–6 configurations. Ethanolamine, neutral sugars and ester-linked acyl-oxyacyl fatty acids are not found, and phosphate is absent or present in reduced quantities. Brucella lipid A contains strongly bound outer membrane protein fragments that are not removed by conventional procedures used to release the lipid-A-associated protein of enterobacterial LPS [28].
The lipopolysaccharide coat being smooth in B. melitensis, abortus, suis and rough in B. canis can inhibit phagosomal fusion and oxidative burst activity. Phagocytes can readily kill B. abortus resulting in development of tissue granulomas and rarely ingest B. melitensis resulting in visceral micro-abscesses; thus explaining the differences in pathogenicity and clinical manifestations in human cases of brucellosis [27]. This leaves about 15 to 30% of Brucella alive which is transported into the lymphatic system and may cause systemic infection [29]. After replication in the endoplasmic reticulum, the Brucella are released with the help of hemolysins and induced cell necrosis. Development of cell-mediated immunity controls Brucella infection and helps in the recovery. Some immunity to reinfection is provided by serum immunoglobulin (Ig): IgM antibodies may remain in the serum in low levels for several months, IgG declines but persistent elevation indicate chronic or relapsed infection, and IgA may persist for very long intervals [27].
5. Epidemiology
In human-to-human transmission, six cases including trans-placental infection of the fetus were documented between 1966 and 2005. In laboratory-acquired brucellosis, 257 cases were identified during 1982 to 2007 accounting for 8% of infections. Approximately 30 cases of human B. canis infection have been reported as of 2009. Three cases of human infection by marine species were reported till 2003 [14].
Brucellosis in humans was first reported in the Middle Bronze Age in Europe [28]. Today, geographic distribution is limited by effective public and animal health programs, however its occurrence is higher in rural areas. It is mainly increasing and endemic or potentially endemic to 180 countries as some do not have effective public health and domestic animal health programs (figure 1) [14].
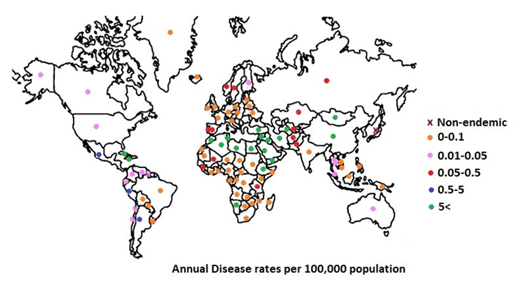
figure 1: Brucellosis Geographic Distribution in 2020 [14, 30].
The European Union states that no country with a gross domestic product above 90% of the mean had an annual rate of brucellosis exceeding ten cases per million people. Real incidence has been estimated to be 10 to 25 times higher than reported cases as often the infection is unrecognized or underreported to public health authorities; for the exception of countries where B. abortus has been eradicated i.e. no cases reported for at least five years (Cyprus, Denmark, finland, the Netherlands, New Zealand, Norway, Sweden and the UK). Incidence of infection rates are reported up to 77 cases per 100,000 people in the South of Europe [14].
|
Year |
Acquired |
Origin |
Setting |
Cases |
Death |
Outbreak Cause |
|
1965 |
UK |
Italy |
Imported Goods |
7 |
B. melitensis associated with imported pecorino cheese |
|
|
1983 |
US |
Mexico |
Imported Goods |
31 |
B. melitensis associated with imported goat cheese |
|
|
1984† |
US |
Spain |
Travel |
7 |
Tourists in Spain |
|
|
1998† |
Japan |
Iraq |
Travel |
2 |
Sexual Transmission |
|
|
2005 |
Bulgaria |
Greece |
Foreign workers |
40 |
2 |
Bulgarian Cattle workers in Greece |
|
2008† |
Morocco |
Spain |
Immigrant/Expatriate |
9 |
B. melitensis caused by ingestion of Spanish local unpasteurized milk |
|
|
2011† |
Kenya |
Somalia |
Migrants |
Outbreak among Somali nomads |
||
|
2016 |
UAE |
Mexico |
Imported Goods |
13 |
Mexican Cheese |
† Indicates publication year and not necessarily year of event
Table 3: Transboundary Brucellosis Outbreak [14].
In the US, veterinary control measures reduced cases to less than 100 per year. Human cases mostly originated from California, Florida, Texas, and Virginia as a result of relaxation of surveillance standards or because of the increasing international exchange of foodstuffs and animals and are due to B. melitensis (Table 3) [14].
In Latin American countries, Brucella recorded 0.5-10% prevalence in cattle; in Central America, 4-8% in cattle B. abortus and suis have been identified in every country but mostly localized in North America and B. melitensis in Guatemala. In Costa Rica, two cases infected with B. neotomae were reported in 2008 and 2011. In Argentina in 2008, B. abortus S19 infection was common among persons involved in the manufacturing of brucellosis vaccines [6, 14].
In Africa and Asia, the average prevalence in animals was as follows: in sheep and goats 0–88.8%, cattle 0–68.8%, camels 0.4–20%, pigs and dogs 0–12.9%. High risk human populations exist due to occupational exposure (11%), and hospital patients (7%) [6, 14].
In the Eastern Mediterranean region, brucellosis is a significant problem as seroprevalence results from small ruminant populations are high (1 to 70 cases per 100,000) [14]. B. melitensis is registered in young adult males as reported in Jordan and Saudi Arabia. Furthermore, the highest age and gender related incidence due to B. melitensis is registered in young adult males. For example, in Egypt, in 2008, there was true prevalence in sheep (41.3%) and goats (32.2%). In Saudi Arabia, there is 3 to 3.8 greater probability that an individual would be seropositive for Brucella antibodies on serology.
A study in the North of Saudi Arabia found that 60% of cases of brucellosis occurred in individuals aged 13-40 years with a 1.7:1 male-to-female ratio. Possible reasons include occupational exposure, immunologic factors, and less assiduous personal hygiene. Similarly, in Jordan, sheep seroprevalence was estimated at 2.2% in individual animal level and 45% at the herd level. As for individuals younger than 24 years old, cases were as high as 60%. In food-borne brucellosis, age and gender are not factors and thus infection is found equally in women and men [6, 14].
Lebanon has a history of brucellosis. During the 1994-1998 period, 1,137 cases with a 1.1 to 1 male to female ratio and 40% being above 60 years old were infected due to B. abortus and melitensis transmitted via animal contact (12 to 34%) and blood donors (15%) [14]. The trend continued in the 2000s with a range of 157-333 cases as well as in the 2010s with a range of 134-460 cases (figure 2) [31, 32, 33, 34].
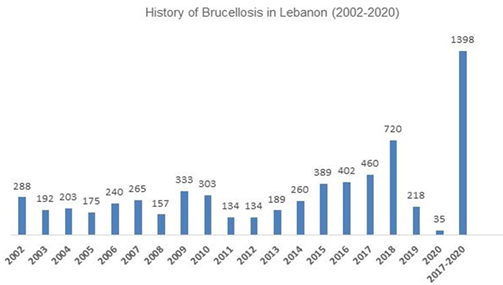
figure 2: History of Brucellosis in Lebanon [31, 32, 33, 34].
This data highlights the beginning of a major outbreak occurring in 2017 with the number of cases hitting 460 and increasing to 720 in 2018. With a total of 1,180 cases, this can be considered as the 5th major most severely prominent outbreak worldwide occurring since the last one of 2016 in Algeria (Table 4) [14, 31].
|
Year |
Region |
Cases |
|
1984-1986 |
Italy |
762 |
|
2008 |
Bosnia and Herzegovina |
757 |
|
2013 |
Syria |
336 |
|
2016 |
Algeria |
819 |
|
2017-2018 |
Lebanon |
1,180 |
Table 4: Major Brucellosis outbreaks [14, 31].
During this period, most cases were mainly observed in the Bekaa governorate followed by the Nabatiyeh and Mount Lebanon governorates (figure 3) [31, 35] and rates were reported as high as 69% during the warm and hot seasons of Spring and Summer (figure 4). The Bekaa governorate is known for the high production of dairy products, mostly cow milk [36]. In addition, the Spring and Summer seasons witness an increase in population due to expats returning to their homeland, city locals escape to the countryside and especially the Bekaa being the hub for breakfast stops on the way to adventure activities and traveling to the neighboring country by land mode. Literature consistently reported the same increase in brucellosis rate in summer time in Lebanon suggesting a relation with the lambing season [33, 37].
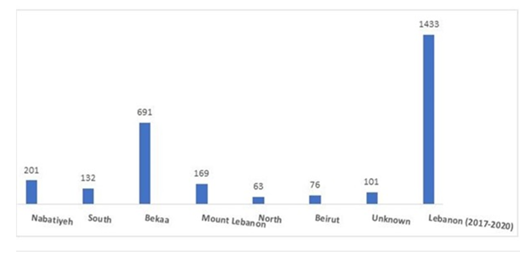
figure 3: Brucella incidence across Governorates of Lebanon for the years 2017-2020 [31, 35].
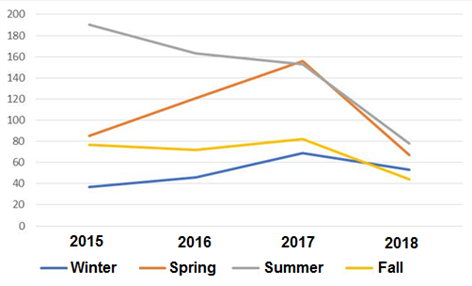
figure 4: Brucellosis rate across seasons in Lebanon (2015-2018) [31].
Furthermore, in line with the alimentary habits and the consumption of raw meat, brucellosis is more frequently reported in adults aged between 20 and 60 years old in comparison to infants and children up to 19 years of age during the 2015-2019 period (figure 5) [31].
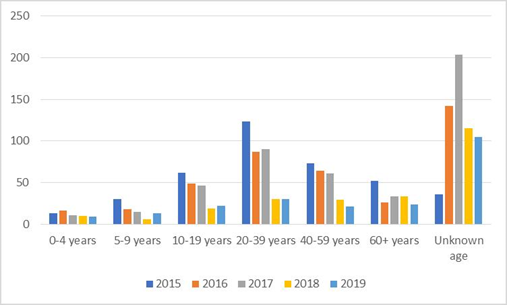
figure 5: Brucellosis for different age groups in Lebanon (2015-2018) [31].
The highest rate of infection was observed in the age range of 20-39 years (22%) followed by the 40-59 years (15%). Intriguingly, the rate of Brucella infected people with unknown age, possibly due to data and records keeping mismanagement within the healthcare system, was the highest, at 33%, prohibiting a sound comparison of the age range effect between the two periods, namely; 1994-1998 and 2015-2019.
It is worth noting that the male to female ratio during the 2015-2019 period differed from that of the 1994-1998 period by a decrease to 0.94 to 1. The rationale behind this could be due to the phenomenon of urban-rural drift, whether semi-permanently or permanently, as the country has been undergoing rapid urbanization during that period, resulting in mass migration of male manpower from rural to urban areas. Furthermore, a movement emerged across the country during the 2000s and 2010s pushing towards women empowerment that went hand in hand with the birth of Agri- and Eco-tourisms. This has led to women involvement in artisanal conserves production and consequently into more exposure to animals and their by-products.
Berger’s review of brucellosis for the year 2020 included 4 major outbreaks of human brucellosis worldwide that have been reported to date [14]. However, due to untimely publication of data by the Lebanese Ministry of Public Health, Burger’s review of the year did not include the 5th major outbreak that took place in the country of Lebanon starting from the year 2017 where the Ministry of Public Health (MoPH) reported on its epidemiological surveillance site the number of registered cases infected with Brucellosis by governorate up till December 2018 (Table 4).
Literature also reports a Brucella-induced endocarditis in a Lebanese 80-year-old patient in 2018 [38], and characterizes various B. melitensis isolates recovered from 33 patients in Lebanon [39]. In the latter study, genome comparisons revealed high levels of similarity between the strains.
In 2019, the number of reported cases dropped to 224 and appear much lower in 2020 (a total of 35 cases until May). This decrease might not be reflecting the success in implementing proper biosecurity measures but rather due to the crippling economic crisis that hit Lebanon in 2019 and continues to date intensified with COVID-19 pandemic resulting in depletion of medical supply reserves, in addition to a nationwide revolution, followed by an increase in unemployment rates and salary cuts. For the aforementioned reasons, data of the year 2020 was not included in the analysis of the age, gender and season parameters.
6. Source and Transmission
In Animals, Brucellosis can affect sheep, goats, cattle, pigs, horses, and dogs. Brucellosis can also affect rats and wild animals including deer, bison, elk, moose, camels, water buffalo, and marine mammals [40]. B. suis, abortus and melitensis are not host-specific and can transmit across species under appropriate conditions as brucellosis is highly contagious [41]. In livestock, Brucella are usually spread through contact with infected birthing tissues and fluids (e.g., placenta, aborted fetuses, fetal fluids, vaginal discharges) [7]. The bacteria can also be found in the milk, blood, urine and semen of infected animals. Animals can get the bacteria directly by ingestion (oral), contact with mucous membranes (eyes, nose, mouth), or breaks in the skin. Brucella can also be transmitted indirectly by contaminated objects (fomites) such as equipment, clothing, shoes, hay, feed or water. Some animals are carriers; they will have the bacteria but show no signs of illness. These animals can shed the bacteria into the environment for long periods of time, infecting other animals in the herd [40].
In Humans, Animal-to-Human transition may occur via Ingestion of unpasteurized milk and related dairy products or undercooked meat originating from infected animals, primarily goats, infected with B. melitensis. Occupational exposure for different occupations at small scale outbreak like slaughterhouse workers become infected with brucella through aerosolization of fluids (10-100 bacteria is sufficient to cause disease), wounds and penetration, and splashing of mucous membrane. In addition, farmers and shepherds are additionally exposed to aborted animals, and of course, veterinarians and technicians are usually infected by accidental inoculation of animal vaccines against B. abortus strain 19 and B. melitensis or by infected fluid while diagnosing animals. Laboratory workers are exposed to aerosols when processing specimens without special precautions [42].
Human-to-Human is another route of transmission from mother to child, transplacentally, breastfeeding, and rarely through sexual intercourse, organ transplantation, blood transfusions and physicians caring for infected patients [30]. Another route of transmission can be Environment-to-Human, such as outbreak of water-borne B. melitensis that is occasionally reported; furthermore, large aerosolized inocula might curtail the incubation period and increase the rate of overt and severe disease [43, 44].
7. Clinical Manifestation
Brucellosis is a systemic infection with a broad clinical spectrum, ranging from asymptomatic disease to severe and/or fatal illness [45]. In Animals, Infected livestock exhibit clinical signs of great economic significance to small and large scales livestock farmers and industries. Characteristic but not specific signs of brucellosis in most animal hosts are abortion or premature births and retained placenta. Interference with fertility is usually temporary, most infected animals will abort only once and some are unaffected [42]. In sexually mature animals the infection localizes in the reproductive system and typically produces placentitis followed by abortion in the pregnant female, usually during the last third of pregnancy [42]. Other signs can include arthritis in cows and pigs [40], Splenic abscesses and small intestinal adhesions on post-mortem examination in sows [46], orchitis or epididymitis in the case of B. melitensis and B. ovis in sheep [42], mastitis and lameness in goats, and oozing skin lesions in horses (fistulous withers) [40].
Additionally, it can induce a substantial decline in milk production over an animal’s lifespan [47], often udder is permanently infected, especially in cows and goats, with continuous shedding of the organism in milk [42]. Clinical signs of brucellosis in camels appear to be very rare [42]. In addition, clinical signs are not pathognomonic and diagnosis is dependent upon demonstration of the presence of Brucella spp. either by isolation of the bacteria or detection of their antigens or genetic material, or by demonstration of specific antibody or cell-mediated immune responses [46].
In Humans, the main presentations are acute febrile illness, with or without signs of localization, and chronic infection. Range of non-specific clinical signs may be observed including malaise, fatigue, sweats, anorexia, headache, depression, abdominal or back pain, arthritis, inconstant and prolonged fever, miscarriage. The fever of brucellosis may mimic that of enteric fever [48], and an undulant fever pattern is seen in chronic infections. Fever may be absent among patients with end-stage renal disease who acquire brucellosis. Mild lymphadenopathy is seen in 10 to 20% of patients; and splenomegaly or hepatomegaly in 20 to 30%. Hepatosplenic abscesses are visualized through imaging in 1.2% of cases and rare instances of splenic rupture have been reported. Bone and joint infections are common, including a high rate of vertebral osteomyelitis. Rare instances of acute or sternotomy infection, granulomatous myositis, bursitis, and soft tissue or muscular abscesses. Most cases of Brucella monoarthritis represent reactive rather than septic disease Infection of natural or prosthetic joints (24 cases reported to 2016) and soft tissue. Subclinical sacroilitis is common [14]. Asymptomatic infection has also been reported [49]. Clinical and laboratory features vary widely. Endocarditis is well documented including isolated case reports of Brucella infection of prosthetic valves and devices such as implantable defibrillators and pacemaker leads. Rare instances of aortitis venous or arterial thrombosis, myocarditis and pericarditis have been reported [14].
The WHO proposed a 0.150 disability weight for chronic localized brucellosis and 0.190 for acute one [48]. Individuals who are elderly are likely to manifest destructive acute localized brucellosis of the spine. Pre-pubertal children account for less than 2% of neuro-brucellosis cases. Infection among children is generally more benign than in adults with respect to likelihood and severity of complications and response to treatment [50]. Naturally-occurring infections are often asymptomatic and thus patients are seropositive. For females, Brucellosis in pregnancy is associated with risk of spontaneous abortion, premature delivery, miscarriage, and intrauterine infection with fetal death. For males, epididymoorchitis is found in 7.6% to 12.7% of male patients with brucellosis, Brucella orchitis may be mistaken for testicular tumor while Prostatitis and prostatic abscess have been reported [14].
8. Diagnosis, Treatment and Prevention
The incubation period in humans’ ranges from 1 to 4 weeks and occasionally lasts as long as several months [50]. Its length varies according to the virulence of the infecting strain, size of the inoculum, route of infection and the host resistance. As for animals, the incubation period is quite variable, it ranges from about 2 weeks to 1 year and even longer in certain instances. When abortion is the first sign observed, the minimum incubation period is usually about 30 days. Generally, infected animals that do not abort develop a positive reaction to the diagnostic test within 30 to 60 days after infection, although some may not develop a positive reaction for several months to over a year [6].
Symptoms and clinical manifestation are essential for a presumptive Brucella diagnosis. Suspected infected materials are then subjected to lab tests such as serology, culture of blood or bone marrow and molecular diagnosis. Serology diagnosis is made easier with the LPS smooth chains producing the greatest immunological responses in various hosts, however the similarity of the O-antigenic side chain of LPS of Brucella with other organisms has created a challenge for diagnosis. Also there have been an unsuccessful trial on alternative antigens being evaluated for their diagnostic potential, for a possible improvement in its specificity. Some serological tests detect antibodies against S-LPS, such as Rose Bengal plate test (RBT) that is a rapid screening test, but the results should always be confirmed. The sensitivity of RBT is over 99%, but it can give false positive reactions with cross-reactive organisms and from healthy individuals that have had contact with S- Brucella without developing disease. The second test is Serum Agglutination test (SAT), it detects antibodies to the S-LPS using heat/phenol-killed whole S-cells [42].
Although Brucella can be isolated from bone marrow, cerebrospinal fluid, wounds, pus, etc., blood is the material most frequently used for bacteriological culture [42]. Blood culture remains the first diagnosis tool of many bacterial infections including brucellosis, however it success rate is only in 40 – 70% of the cases.The oldest isolation technic of Brucella species is the Biphasic Ruiz-Castaneda system, it was than replaced by the lysis centrifugation technique, where a higher rate of positive blood culture has been reported. An automated culture system has been developed afterwards to improve the speed of detection [28]. Additionally, Bone marrow cultures provide a higher sensitivity, yield faster culture, and in case of subjects under antibiotic treatment, it may be more accurate than blood culture [28].
Since 2003, molecular diagnosis tools were introduced to test for brucellosis. ELISA offers a significant addition over the serological methods due to its ability to measure tow specific antibodies IgM & IgG. This specificity makes Elisa test a good tool to confirm the clinical stage of the disease [49]. On the other hand, the PCR test e.g. based on the bcsp31 gene, allows a rapid confirmation of the brucellosis (presenting results in less than six hours), safer for the laboratory team, and has a high sensitivity reaching 100% and an interesting specificity of 98.3% [47].
Treatment for humans, the World Health Organization (WHO) issued recommendations for the treatment of human brucellosis in 1986, suggesting the use of doxycycline, 100 mg twice daily for six weeks combined with either rifampicin, 600–900 mg daily for six weeks, or streptomycin, 1 g daily for two to three weeks [48]. In addition, Co-trimoxazole and rifampin are considered safe for pregnant women. Due to the emergence of resistant strains, more recent reviews focused on fluoroquinolone-containing regimens for the treatment of brucellosis. Also Trimethoprim / Sulfamethoxazole has been a popular choice, and was included in various combination regimens around the world especially for children younger than 8 years old [15], due to its significantly lower cost. A number of Italian studies have investigated the use of minocycline instead of doxycycline in the treatment of brucellosis. Briefly, the essential element in the treatment of all forms of human brucellosis is the administration of effective antibiotics for an adequate length of time.
In animals, a combination of antibiotic is strongly recommended, long-acting Oxytetracycline, streptomycin and Oxytetracycline intramammary infusion. Other combinations of antibiotics might be able to clear B. abortus, B. melitensis or B. suis from livestock, these treatments are currently considered to be unproven and risky, and treatment is generally discouraged. Even when Brucella seem to have disappeared, they may persist in lymph nodes or other tissues, and later reappear. Treatment is also unlikely to be cost-effective in many herds [51].
However, in an era of rapid emergence of antimicrobial resistance, controversies regarding the prolonged use of antibiotics with established activity against Brucella pose special problems [48]. Antimicrobial resistance (AMR) threatens the effective prevention and treatment of an ever-increasing range of infections caused by bacteria, parasites, viruses and fungi. It also threatens the global public health that requires action across all government sectors and society. Without effective antibiotics, the success of major surgery and cancer chemotherapy would be compromised. The cost of health care for patients with resistant infections is higher than care for patients with non-resistant infections due to longer duration of illness, additional tests and use of more expensive drugs. In 2016, 490 000 people developed multi-drug resistant TB globally, and drug resistance is starting to complicate the fight against HIV and malaria, as well [52].
In November 2006, the first International Meeting on the Treatment of Human Brucellosis was held in Ioannina, Greece. The current recommendations are summarized in Table 5 [48].
|
Treatment Regimen |
DOSE |
Comments |
|
DOX1-STR2
|
DOX1: 100 mg twice daily orally for 6 weeks; STR2: 15 mg/kg daily intramuscularly for 2- 3 weeks |
Considered the “gold standard” |
|
DOX1-RIF3
|
DOX1: as above; RIF3: 600-900 mg daily for 6 weeks, one morning dose |
Convenience of the regimen overcomes slight drawbacks concerning the pharmacokinetics of the combination and the overall outcome |
|
DOX1-GENT4
|
DOX1: as above, GENT4: 5 mg/kg daily parenterally in 1 dose for 7 days |
May be considered the preferred alternative regimen. Duration of GENT4 administration may need modification for optimal results |
|
TMP5-SMX6- containing regimens |
TMP5-SMX6: 800 + 160 mg twice daily for 6 weeks |
Recommendation referring to three drug regimens containing DOX1 |
|
Quinolone containing regimens |
Ofloxacin: 400 mg twice daily for 6 weeks; ciprofloxacin: 500 mg twice daily for 6 weeks |
Ofloxacin or ciprofloxacin may be used alternatively as second or third agent in combination regimens containing DOX1 |
1Doxycycline, 2Streptomycin, 3Rifampicin, 4Gentamicin, 5Trimethoprim, 6Sulfamethoxazole
Table 5: The recommendations of Ioannina on the Optimal Treatment of Brucellosis Without Serious Complications in Adults [48].
In order to prevent and control human brucellosis, an understanding of the immune response to Brucella is required. The relative significance of CD4 and CD8 T cells in controlling Brucella infection is unclear. However, several studies have concluded that overall, three main mechanisms of the adaptive immune response seem to be important in brucellosis. first, IFN- γ produced by CD4, CD8 and γδ T cells activates the bactericidal action of macrophages to hamper the intracellular survival of Brucella. In addition, the cytotoxic action of CD8 and γδ T cells kills infected macrophages and, finally; Th1-type antibody isotypes opsonize the bacteria to facilitate phagocytosis [9].
In the industrial world, it exists a large variety of vaccines for veterinary use. first is the B. abortus S19 and and B. melitensis Rev.1 vaccine, a high protective live vaccine that has a residual virulence. This type of live vaccine was widely used as a human vaccine in the former Soviet Union in 1954 [9], afterwards it was banned due to its high potential to introduce the infection. Another live vaccine is the RB51, very stable with a high protective efficacy and immunogenicity in companion to the previous ones. Different inactivated vaccines also exist like cell fractions and lysate, Subunit and DNA vaccine, Synthetic peptide vaccine and Vector-delivered Brucella vaccines. Like all inactivated vaccines, they are very safe, have no residual virulence, low level of protection and requires multiple boosters. Furthermore, subunit, synthetic peptide and live vectored vaccines were reported to be suitable for human use [45], however their efficiency remains uncertain.
Some trials for creating an anti-Brucella vaccine for humans were conducted. They first included the phenol-insoluble sodium dodecyl sulphate fraction of B. abortus or B. melitensis (also known as fraction PI). This combination was immunogenic and showed protection for mouse; however, it was highly reactogenic in humans and caused severe local pain at the site of injection and postvaccination fever. The vaccine is no longer produced. Another study in 1991 included a polysaccharide fraction produced by mild acid hydrolysis developed in the former Soviet Union. This combination seems to be protective with minimal reactogenicity in clinical trials; however, its availability is uncertain. Encouragingly, some antigens are able to protect (mostly against B. abortus) as effectively as a live control vaccine challenge. These include the 22.9-kDa protein, SOD (delivered as a DNA vaccine, an SFV replicon or a peptide), L7/L121–Omp16 (DNA vaccine) or Omp31 (protein or DNA vaccine against B. ovis) [9]. Disinfection is another way of prevention. Brucella spp. are killed by commonly available disinfectants including hypochlorite solutions, 70% ethanol, isopropanol, iodophors, phenolic disinfectants, formaldehyde, glutaraldehyde and xylene. Most brucella spp. are inactivated by acid pH < 3.5; however, B. microti seems to be more resistant to acidic conditions. Brucella can also be destroyed by moist heat of 121°C (250°F) for at least 15 minutes, dry heat of 160-170°C (320-338°F) for at least 1 hour, gamma irradiation and pasteurization. Boiling for 10 minutes is usually effective for liquids [51].
Biosecurity measurements includes: Appropriate program of disease diagnosis and proper vaccination schedule. Farm workers should wear adequate protective clothing when contact with infected animals after abortion or during calving, or if the environment is likely to have been contaminated by excreta, abortions or parturition products from animals with brucellosis. Aborted fetuses, placentae and contaminated litter should be collected in leak-proof containers and disposed of preferably by incineration. Any area in which an abortion or infected parturition has occurred should be washed down with an approved disinfectant (hypochlorite, iodophor or phenolic disinfectant at recommended working strength). Farm implements used for handling contaminated material should be disinfected after use by immersion in a suitable disinfectant (iodophor, phenolic soap or dilute caustic soda). Liquid manure can remain infected for long periods, especially at low temperatures. Rodent control measures should be enforced and insect infestation kept to a minimum by the use of fly screens, light traps and insecticides. Keep visitors to minimum and Maintain record for visitors and their purpose. Few vehicles or equipment should be allowed within the farm area [42].
9. One Health Approach
Prevalence of Brucella in the rural areas at the extremities (Bekaa and Nabatiyeh) is understandable. Returning of expats from the capital and cities to the rural areas, specifically during the summer, can explain the spread of the disease among adults, which are heavy consumers of animal products, whether cooked or raw, as compared to children and infants. The growing Agri- and Eco-tourism sector in the rural areas can add an additional cause for disease spread there. Again, the decreasing numbers of Brucella-reported cases in 2019-2020 can be misleading, as the country has been overwhelmed with the health, financial and security crises. Reporting the 5th largest Brucella outbreak in Lebanon, following that of Algeria in 2016, is alarming. A National Program for the Control and Prevention of Brucellosis needs to be established in the MENA region and more specifically by the local Government in Lebanon in response to the history of registered brucellosis in the country and more particularly to the recent 5th largest and most severely prominent worldwide outbreak. This program should be set by a team that follows a One Health approach for brucellosis management thus including a human, animal, and environmental health interface to prevent, prepare, detect, respond, and recover from brucellosis through the following outlined activities and interventions, 1) starting with education and raising awareness on topics of hygiene and food safety; 2) outbreak preparedness response infrastructure (a) sampling both human and animal populations, (b) carrying laboratory diagnosis and improving laboratory capacity, (c) sharing results with animal and public health authorities and all potential parties, (d) coordinating and creating partnerships at local and national levels, (e) highlighting institutional including scientific research and technical methods for implementation conducting efficient routine surveillance at the human/animal interface, (f) providing biological risk management training to veterinary and public health professionals, (g) developing the veterinary infrastructure to execute control at animal level, (h) taking action in the event of any mass gathering via establishing a biosafety and biosecurity systems for Brucellosis identification, (i) security and monitoring according to best practices); and finally 3) legal instruments and coordination mechanisms development and implementation [53]. The end-result of such national program would be an excellent platform to use for the management of future outbreaks of similar zoonotic diseases of importance in Lebanon and longstanding improvement of people livelihoods and wellbeing and thus falls back with positive repercussion on human health, animal production, and environmental management.
10. Conclusion
There is therefore the need to enforce fundamental steps in order to solve the environmental crisis in which we find ourselves by minimizing both waste and pollution in a nation with abundant reservoir of resources, whose proper utilization will result to the positive development of such a nation.
Acknowledgment
The authors thank the Lebanese Ministry of Public Health in general, and specifically Ms Hilda Harb Head of Department of Statistics. Gratitude is also extended to the Department of Agriculture at the American University of Beirut for covering the publication fees of this manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- Bakri FG, Al-Qadiri HM, Adwan MH. The highest cited papers in brucellosis: Identification using two databases and review of the papers’ major findings. BioMed Res Int (2018).
- Shaffer C. Brucellosis: Background, Types, Diagnosis, Treatment. New Medical Life Sciences (2016).
- Alavi SM, Alavi, L. Treatment of brucellosis: a systematic review of studies in recent twenty years. Caspian J Intern Med 4 (2013): 636-641.
- Dean AS, Crump L, Greter H, et al. Clinical Manifestations of Human Brucellosis: A Systematic Review and Meta-Analysis. PLOS Neglect Trop D 6 (2012): e1929.
- El-Diasty M, Wareth G, Melzer F, et al. Isolation of Brucella abortus and Brucella melitensis from Seronegative Cows is a Serious Impediment in Brucellosis Control. Vet Sci 5 (2018): 28.
- CDC- Center for Disease Control and Prevention. Bioterrorism Agents/Diseases. Emergency Preparedness and Response (2018).
- Franc KA, Krecek RC, Hasler BN, et al. Brucellosis remains a neglected disease in the developing world: A call for interdisciplinary action. BMC Public Health 18 (2018): 125.
- Hotez JP, Savioli L, Fenwick A. Neglected Tropical Diseases of the Middle East and North Africa: Review of Their Prevalence, Distribution, and Opportunities for Control. PLoS Neglect Trop D 6 (2012): 1475.
- Perkins SD, Smither SJ, Atkins HS. Towards a Brucella vaccine for humans. FEMS Microbiol Rev 34 (2010): 379-394.
- Franco MP, Mulder M, Gilman RH, et al. Human brucellosis. The Lancet Infect Dis 7 (2007): 775-786.
- Whatmore AM, Davison N, Cloeckaert A, et al. Brucella papionis sp. nov., isolated from baboons (Papio spp.). Int J Syst Evol Microbiol 64 (2014): 4120-4128.
- ficht T. Brucella taxonomy and evolution. Future Microbiol 5 (2010): 859-866.
- Lindquist D, Chu MC, Probert WWS. Francisella and Brucella. In: Manual of Clinical Microbiology, 9th ed, Murray PR, Baron EJO, Jorgensen JH, Landry ML, Pfaller MA (Eds), ASM Press, Washington, DC (2007): 824.
- Berger S. Brucellosis Global Status. Gideon ebook series, Los Angeles, CA (2020): 1-198.
- CDC- Center for Disease Control and Prevention. Human exposures to marine Brucella isolated from a harbor porpoise - Maine. MMWR Morb Mortal Wkly Rep 61 (2012): 461-463.
- Scholz HC, Hubalek Z, Sedlácek I, et al. Brucella microti sp. nov. isolated from the common vole Microtus arvalis. Int J Syst Evol Microbiol 58 (2008): 375-382.
- Scholz HC, Nöckler K, Göllner C, et al. Brucella inopinata sp. nov. isolated from a breast implant infection. Int J Syst Evol Microbiol 60 (2010): 801-808.
- Scholz C, Revilla-Fernández S, Dahouk S, et al. Brucella vulpis sp. Nov. isolated from mandibular lymph nodes of red foxes (Vulpes vulpes). Int J Syst Evol Microbiol 66 (2016): 2090-2098.
- Galinska EM, Zagórski J. Brucellosis in humans – etiology, diagnostics, clinical forms. Ann Agric Environ Med 20 (2013): 233-238.
- Paulsen IT, Seshadri R, Nelson KE, et al. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc Natl Acad Sci USA 99 (2002): 13148-13153.
- Delvecchio VG, Kapatral V, Redkar RJ, et al. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc Natl Acad Sci USA 99 (2002): 443-448.
- Chain PSG, Comerci DJ, Tolmasky ME, et al. Whole-genome analyses of speciation events in pathogenic Brucellae. Infect Immun 73 (2005): 8353-8361.
- Wattam AR, Williams KP, Snyder EE, et al. Analysis of ten Brucella genomes reveals evidence for horizontal gene transfer despite a preferred intracellular lifestyle. J Bacteriol 191 (2009): 3569-3579.
- Alton GG, Jones LM, Angus RD, et al. Techniques for the brucellosis laboratory, Institut National de la Recherche Agronomique, Paris (1988): 190 pages.
- Wright SG. Brucellosis. In: Hunter’s Tropical Medicine and Emerging Infectious Diseases, 8th ed, Strickland GT (Ed), W.B. Saunders Company, Philadelphia (2000): 416.
- Jahans KL, Foster G, Broughton ES. The characterization of Brucella strains isolated from marine mammals. Vet Microbiol 57 (1997): 373.
- Al-Nassir W, Lisgaris MV, Salata AR, et al. Brucellosis. In: Medscape from WebMD: Drugs and Diseases. New York (2017).
- Christopher S, Umapathy BL, Ravikumar KL. Brucellosis: Review on the recent trends in pathogenicity and laboratory diagnosis. J Lab Physicians 2 (2010): 55-60.
- Lecaroz C, Blanco-Prieto MJ, Burrell MA, et al. Intracellular killing of Brucella melitensis in human macrophages with microsphere-encapsulated gentamicin. J Antimicrob Chemother 58 (2006): 549-556.
- Hull NC, Schumaker BA. Comparisons of brucellosis between human and veterinary medicine. Infect Ecol Epidemiol 8 (2018): 1500846.
- MoPH- Ministry of Public Health Lebanon. General Surveillance Data (2020).
- Pappas G, Papadimitriou P, Akritidis N, et al. The new global map of human brucellosis. The Lancet Infect Dis 6 (2006): 91-99.
- Al Shaar L, Chaaya M, Ghosn N, et al. Brucellosis outbreak in Chouf district of Lebanon in 2009: a case-control study. EMHJ-East Med Health J 20 (2014): 250-256. 2014.
- Zmeter C, Tabaja H, Sharara AI, et al. Non-O1, non-O139 Vibrio cholerae septicemia at a tertiary care center in Beirut, Lebanon; a case report and review. J Infect Pub Health 11 (2018): 601-604.
- Hassan H, Salami A, Ghssein G, et al. Seroprevalence of Brucella abortus in cattle in Southern Lebanon using different diagnostic tests. Vet World 13 (2020): 2234-2242.
- United Nations Food and Agriculture Organization UNFAO. Milk for health and wealth - supporting small dairy producer communities in Lebanon (2020).
- Kalaajieh W. Epidemiology of human brucellosis in Lebanon in 1997. Méd Mal Infect 30 (2000): 43-46.
- Ephrem CG, Matar MJ, Chalhoub GC, et al. Brucella endocarditis: diagnostic challenges. J Infect Dev Ctries 12 (2018): 25S.
- Abou Zaki N, Salloum T, Osman M, et al. Typing and comparative genome analysis of Brucella melitensis isolated from Lebanon. FEMS Microbiol Lett 364 (2017).
- CSFPH - The Center for Food Security and Public Health Iowa State University. Fast facts: Brucella- Udulant fever (2008).
- Robinson A. Guidelines for coordinated human and animal brucellosis surveillance ISSN: 0254-6019. FAO Animal Prod Health (2003): 156.
- Corbel MJ. Brucellosis in humans and animals. Produced by the World Health Organization in collaboration with the Food and Agriculture Organization of the United Nations and World Organization for Animal Health (2006).
- Pappas G, Panagopoulou P, Christou L, et al. Brucella as a biological weapon. Cell Mol Life Sci 63 (2006): 2229-2236.
- Doganay GD, Doganay M. Brucella as a potential agent of bioterrorism. Recent Pat Antiinfect Drug Discov 8 (2013): 27-33.
- Lalsiamthara J, Lee JH. Development and trial of vaccines against Brucella. J Vet Sci 18 (2017): 281-290.
- Poester FP, Samartino LE, Santos RL. Pathogenesis and pathobiology of brucellosis in livestock. Rev Sci Tech Off Int Epiz 32 (2013): 105-115.
- Queipo-Ortuño MI, Morata P, Ocón P, et al. Rapid diagnosis of human brucellosis by peripheral-blood PCR assay. J Clin Microbiol 35 (1997): 2927-2930.
- Ariza J, Bosilkovski M, Cascio A, et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLOS Medicine (2007).
- Asaad AM, Alqahtani JM. Serological and molecular diagnosis of human brucellosis in Najran, Southwestern Saudi Arabia. J Infect Public Heal 5 (2012): 189-194.
- Khan MZ, Zahoor M. An overview of Brucellosis in cattle and humans, and its serological and molecular diagnosis in montrol strategies. Multidisciplinary Digital Publishing Institute (MDPI). Trop Med Infect Dis 3 (2012): 65.
- OIE- World Organisation for Animal Health. Report of the meeting of the OIE ad hoc group on prioritisation of diseases for which vaccines could reduce antimicrobial use in cattle, sheep, and goats (2018).
- WHO- World Health Organization. Antimicrobial resistance (2018).
- Godfroid J. Brucellosis in livestock and wildlife: zoonotic diseases without pandemic potential in need of innovative one health approaches. Arch Public Health 11 (2017): 75-34.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 