Sodium Arsenite in Drinking Water is a Continuous Threat to Maturing Reproductive System: a Study in Prepubertal Male Mice
Irfan Zia Qureshi*1, Naureen Anwar1, Sumaira Hassan2
1Laboratory of Animal and Human Physiology, Department of Animal Sciences (Zoology), Quaid-i-Azam University, 45320 Islamabad, Pakistan. 2 Department of Veterinary Science, PMAS Arid Agriculture University, 44000 Rawalpindi
*Corresponding Author: Irfan Zia Qureshi. Laboratory of Animal and Human Physiology, Department of Zoology, Quaid-i-Azam University, 45320 Islamabad, Pakistan.
Received: 20 April 2023; Accepted: 27 April 2023; Published: xxxxx
Article Information
Citation: Irfan Zia Qureshi, Naureen Anwar, Sumaira Hassan. Sodium Arsenite in Drinking Water is a Continuous Threat to Maturing Reproductive System: a Study in Prepubertal Male Mice. Journal of Environmental Science and Public Health. 7 (2023): 66-78.
View / Download Pdf Share at FacebookAbstract
Human exposure to arsenic in countries known for heavy arsenic load, where ground water arsenic level exceeds the WHO limit, can be detrimental for developing gonads. Developmental and reproductive toxicity of arsenic in rodents after in utero exposure is although known but information on the toxic effects of arsenic on postnatal gonadal development is scant. This aspect was the focus of the present investigation. Postnatal day 25 prepubertal male mice were challenged with sub-chronic and chronic (up to PND 53 & PND 114 respectively) oral exposure (drinking water) to low and high doses (0.01, 5, and 10 mg.L-1) of sodium arsenite. Data were compared statistically at P<0.05. Sub-chronic exposure to high arsenic doses led to significant increase in the production of reactive oxygen species and lipid peroxidation, while simultaneous significant reduction occurred in the activity of antioxidant enzymes catalase, superoxide dismutase and peroxidase, hormone concentrations (folliclestimulating hormone, luteinizing hormone, testosterone) and sperm parameters. Testicular cell damage and sperm DNA damage, as revealed by comet assay, were evident at 10 mg.L-1arsenic dose. Chronic exposure further aggravated the adverse effects. Severe testicular oxidative stress, reduction in hormones and pronounced histological alterations in testis and epididymis, and sperm parameters together with excessive sperm DNA damage were noticeable even at the lowest test dose of 0.01 mg.L-1. The study concludes that arsenic exposure considerably affects the prepubertal gonad, may cause irreversible damage to the developing reproductive system of males and that minute quantity of arsenic in drinking water can be a serious health hazard.
Keywords
<p>Sodium arsenite, Prepubertal mice, Reproductive toxicity, Reproductive hormone, Oxidative stress, Sperm parameters, Genotoxicity</p>
Article Details
1. Introduction
The heavy metal arsenic, which has a ubiquitous occurrence in soil, water, and atmosphere, is considered a major toxic metal element due to its carcinogenic, mutagenic, and teratogenic properties [1, 2]. Although 90% of arsenic poisoning is believed to be geogenic, anthropogenic sources like use of pesticides and dumping of tons of quantities of industrial waste have considerably increased its occurrence in the environment [3]. Contamination of natural water reservoirs with the arsenic in West Bengal, China, India, Bangladesh, Pakistan, Afghanistan, Mongolia, Myanmar, Cambodia, DPR Korea, and Nepal is of special concern [1,4,5]. In these countries, arsenic concentration in the drinking water has been found to be well above the permissible limit of 10 ppb or 10mg.L-1 as outlined by the WHO [6,7]. It has been estimated that more than 230 million people worldwide, which include 180 million from Asia, are at risk of arsenic poisoning [8].
In humans, chronic arsenic exposure is known to cause numerous health related problems. For instance, lung cancer, skin cancer, bladder cancer, coronary artery disease, hyperkeratosis, peripheral vascular disease, melanosis, gangrene, and lung diseases are associated with arsenic exposure [9, 10]. Arsenic poisoning has also been linked with infant mortality, impaired intellect and motor dysfunction in children [10, 11]. Depending on the route [12], arsenic adversely affects the male reproduction [13,14]. Its exposure leads to a decrease in the testes and accessory sex organs weight and reduction in testosterone concentrations. This eventually causes spermatotoxicity and general reproductive toxicity in experimental animals [15-17]. Studies on animal models have suggested that arsenic induces necrosis, apoptosis and, through diminished testosterone production, may cause permanent gonadal dysfunction [18-20].
At tissue and cell level, arsenic induces malformations in the male reproductive system through increased production of reactive oxygen species (ROS) [21]. Developmental toxicity caused upon exposure to arsenic includes retardation of fetal growth, gross tissue abnormality and mortality [22]. Numerous studies have reported in vivo toxic outcomes of arsenic on the adult male rat reproductive system [19, 23-25].
However, pre- and post-natal developmental time periods are even more critical, since exposure to a toxicant metal element, chemical agent or a drug in the early days of gonadal maturation can lead to adverse reproductive health effects immediately or in later life. In this context, prenatal exposure of mice to high arsenic concentrations is known to produce cell damaging effects [16, 26,27]. For instance, a low dose of 50 ppb arsenic was shown to induce learning impairments [28] and liver dysfunctions in mice [29]. Adverse outcomes of low doses of arsenic on pre-and post-natal development of mice have been reported [30]. Although it is known that sexual maturation in prepubertal female rats is delayed upon exposure to arsenic [31], similar data on prepubertal male mice are lacking.
The present study therefore aimed to investigate the impact of arsenic exposure on postnatal male mouse reproductive system following exposure to sub-chronic and chronic oral doses of NaAsO2 beginning from PND 25 to PND 53 and PND 25 to PND 114 respectively.
2. Material and Methods
2.1 Animals and maintenance
Eighty Swiss albino male mice (age 21-25 days old) were obtained from the National Institute of Health Islamabad and maintained in the Animal House Facility of Quaid-i-Azam University, Islamabad. Before experiments, mice were kept in rodent cages under standard laboratory conditions: room temperature (24 ± 1 °C), photoperiod (14:10 light:dark hours) and 40% relative humidity. Mice were maintained on standard rodent feed and had free access to drinking water ad libitum. Animal handling and the experimental design were approved by the “Bioethical committee of the Faculty of Biological Sciences”, Quaid-i-Azam University, Islamabad. Animal handling also strictly complied with the European Union Guidelines on Animals and in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and further guidelines, the European Communities Council Directive of 24 November 1986 (86/609/EEC).
2.2 Experimental design
The mice were divided into four groups, each containing ten mice. NaAsO2 was administered orally in drinking water at 0.01, 5.0, and 10.0 mg.L-1concentrations for 28 days (PND 25 to PND 53, sub-chronic) and 90 days (PND 25 to PND 114, chronic).
2.3 Procurement of blood plasma and tissue samples
On PND 54 and PND 115, animals were weighed, and venous blood was collected immediately from the heart and centrifuged at 926 g for 10 min to prepare plasma samples. Plasma was stored at -20 °C for determination of hormone concentrations. Mice were then quickly decapitated, and following the dissection, reproductive organs were weighed and separated out. One testis and epididymis of each mouse were rinsed with saline and stored at -20 °C for biochemical analyses. For sperm collection, cauda epididymis tissue was sliced into pieces in 5 ml Human Tubal Fluid (HTF) medium (Merck, UK) and sperms were allowed to swim out in petri dish. These were left for 30 min in an incubator at 37 °C with 5% CO2 in air. For histology, the other testis and epididymis of each animal were quickly rinsed with saline and fixed in 10% formalin.
2.4 Hormone determination
Determination of gonadotropins (LH and FSH)
Luteinizing hormone (LH): Plasma LH was quantitatively determined through competitive enzyme linked immunosorbent assay (ELISA) using a commercial kit (Mybiosource, USA) following the manufacturer’s procedure provided for this bioassay. Fifty microliters of standard, blank, and samples were added into wells coated with a biotinylated LH antibody. To each coated well, 50 μl of reagent A was added. The plate was then slightly shaken to clear the cloudiness, then covered and incubated for 60 min. Washing was done three times with 350 μl of washing solution. Wells were decanted and allowed to dry. Reagent B (100 μl) was then added to each well and incubation was carried out for 60 min. After 1 h, wells were washed five times with the washing solution, 90 μl of horseradish peroxidase (HRP) enzyme was then added to each well and incubated again for 15-25 min at 37 °C until blue color developed. Fifty microliters of stop solution were then added into each well and mixed thoroughly till blue color turned into yellow. Absorbance was recorded at 450 nm in triplicate within 1 min on an ELISA plate reader (Via Medical Pro reader-96, Germany). The detection range of the kit was 50 mIU/mL to 0.78 mIU/mL, while the lowest detection limit was 0.08 mIU/mL. Inter- and intra-assay coefficient of variations were 8.0% and 12.0% respectively.
Follicle stimulating hormone (FSH): Plasma FSH was quantitatively measured using a commercial kit and following the manufacturer’s protocol (Mybiosource, USA). To each of the wells, 100 mL of standard and samples were added. Wells were incubated for 90 min at 37 °C. Subsequently, washing was done twice and 100 µl of biotinylated FSH antibody was added and samples were incubated for 1 h at 37 °C. Washing of the plate was done three times. Leaving out the blank wells, 100 µl of enzyme conjugate was added and incubated for 30 min. After 30 min, five additional washes were given, followed by addition of 100 µl of color reagent and incubation was carried out at 37 °C in dark. After appearance of dark color, 100 µl of color reagent C was added to stop the reaction. The contents were mixed thoroughly, and absorbance was recorded at 450 nm using an ELISA plate reader as above. Three readings were taken within 10 min. Detection range of the kit was 0.25 ng/mL to 50 ng/mL, sensitivity was 0.125 ng/mL, while the intra- and intra assay coefficient of variations were <15%. Concentration is given in mIU/mL.
2.5 Testicular homogenate preparation
Testes were homogenized in 1 mL phosphate buffered saline (PBS) and centrifuged at 926 g for 15 min. Supernatant was collected for antioxidant bioassays.
2.6 Plasma and testicular testosterone
Intra-testicular and blood plasma testosterone: Testosterone concentrations were determined in whole blood plasma and testicular homogenates through quantitative ELISA as mentioned above following the method provided by the manufacturer (Amgenix, USA). Determination of testosterone was based on competitive binding of sample testosterone and testosterone conjugated enzyme (Horseradish peroxidase), whereby, 50 µL of control, standard, and testicular homogenate or blood plasma were added into streptavidin coated wells. To each of the wells, 100 µL of testosterone-enzyme conjugate reagent and 50 µL of biotin reagent were then added. After gently mixing for 30 sec, the ELISA plate was incubated for 1 h at room temperature. Wells were washed three times after incubation with the washing buffer for the removal of any unbound enzyme conjugate. Following this, TMB substrate reagent (100 µL) was added to the wells and another incubation was done for 30 min. Stop solution (50 µL) was added to each of each the wells and mixed for 20 sec. Absorbance was read 450 nm on an ELISA plate reader as above. Sensitivity of the kit was 0.05ng/mL, while the inter- and intraassay coefficient of variations were 5.2% and 7.4%. respectively.
2.7 Oxidative parameters
Reactive oxygen species (ROS): ROS production in the testicular homogenates was determined according to [32]. One milligram of N.N-Diethyl para phenylene diamine sulfate (DEPPD) was dissolved in 10 ml distilled water for the preparation of reagent 1. FeSO4 stock solution contained 50 mg ferrous sulfate dissolved in 10 ml sodium acetate buffer (pH 4.8). Fifty microliters of FeSO4 stock solution were mixed with 100 ml of sodium acetate buffer to prepare reagent 2. Both reagents were mixed in a 1:25 ratio and placed in dark for 2 min. Sodium acetate buffer (1200 µl), above mixed reagents (1680 µl), and 60 µl of testis homogenate were mixed in a cuvette. Absorbance was read on UV-Visible Spectrophotometer (Agilent 8453, USA) at 505 nm. For each sample, three readings were taken at an interval of 15 sec intervals and then averaged.
Thiobarbituric acid reactive substance (TBARS) assay: TBARS is an indirect measurement of oxidative stress produced due to lipid peroxidation, whereby malondialdehyde formation in testicular homogenates through its reaction with thiobarbituric acid (TBA) was determined according to the method as described [33]. The reaction mixture was prepared in a test tube by adding 0.1 mL of 1.5 mM ascorbic acid, 0.1 mL of 50 mM Tris -HCL, 0.1 mL of 1mM FeSO4, 0.6 mL of distilled water, and 0.1mL of testis homogenate. After vigorous mixing, the contents were incubated for 15 min at 37 °C. After adding 1 mL of thiobarbituric acid and tricholoroacetic acid (0.375% and 10% respectively), the contents were thoroughly mixed and boiled in a water bath for 15 min at 100 °C and centrifuged at 2500 rpm for 10 min. Absorbance of the supernatant was recorded at 535 nm. For each sample, three readings were quickly taken and then averaged.
2.8 Antioxidant enzymes
Catalase (CAT) assay: CAT activity in the samples was determined as described [34] with some modifications. Reaction mixture was prepared by adding 1.99 ml of potassium phosphate buffer (pH 7.0), 1000 µl H2O2 (5.9 mM), and 100 µl testicular homogenate. Three absorbance readings were recorded after 1min interval at 240 nm. One unit CAT activity was 0.01 absorbance change unit/min.
Superoxide dismutase (SOD) assay: The enzyme activity was determined according to the method as described [35]. Briefly, reaction mixture was prepared by adding 1.5 mL of L-Mehionine, 0.75 of Triton X-100, and 1mL of nitroblue tetrazolium (NBT). The final volume was brought to 30 ml by adding 50 mM PBS (pH 7.8). One milliliter of this mixture was added to test tubes and 20 µl of sample was added into each one of them. Sample tubes were illuminated with a fluorescent lamp for 7 min and the contents were incubated at 37 °C for 5 min. For initiation of reaction, 10 µl of riboflavin were added and the contents were incubated again at 40 °C for 8 min. Absorbance was recorded in triplicate during 1 min at 560 nm.
Peroxidase (POD) assay: Activity of POD in the testis homogenate was determined according to the method as described [34]. The reaction mixture was prepared by mixing 75 µl hydrogen peroxide (40 mM), 625 µl of 50 mM potassium phosphate buffer (50 mM pH 5.0), 25 µl guaiacol (20 mM), and 25 µl of testis homogenate. Absorbance was recorded spectrophotometrically after 1 min at 470 nm. One unit of POD activity was reflected as 0.01absorbance change as unit/min.
Reduced glutathione (GSH) assay: Non-enzymatic reduced glutathione was determined in testicular homogenates according to a previous method [36]. The reaction mixture was prepared by adding 1ml of sodium phosphate buffer (0.4 M), 0.5 ml DTNB, and 0.1 mL of sample in a cuvette. After the development of yellow color, the absorbance was taken at 412 nm. Values were recorded in triplicate and then averaged.
2.8 Sperm parameters
Epididymal sperm count: For sperm count, 10 µl of sperm mixture was mixed with 190 µl of distilled water to prepare 1:20 dilution. Ten microliters were then taken and suspended into an improved Neubauer’s chamber. Sperms were counted under a photomicroscope (BH2 Nikon, Japan) at 40x magnification as described by [37].
Sperm motility: For the evaluation of sperm motility, a preheated slide was taken and 10 µl of sperm suspension were placed and covered with a cover slip. Epididymal sperm motility was evaluated at 200x magnification.
Sperm viability: Sperm viability was assessed according to a previous protocol [38]. Sperm suspension (20 µl) was combined with the same volume of Eosin-nigrosin stain (0.05%). Following 2 min of incubation, the slides were observed at 40x magnification on a light microscope. Alive sperms carried no color while the dead sperms in the suspension turned pink. Percentage of sperm viability was counted after observing at least 200 sperms in each sample.
Sperm DNA damage: Comet assay was carried out on mice epididymal sperm suspension [39]. Glass microscopic slides were coated with 1% normal melting agarose, covered, and left to solidify at 4 °C for 20 min. Covers were removed gently, and 85 mL of low-melting agarose was spread over the first layer containing 20 µl of sperm suspension and 65 µl of 1% low-melting agarose. After solidification, cell lysis was allowed to complete by plunging slides into freshly prepared chilled buffer and keeping there for 2 h. Electrophoresis was performed at 24 V for 30 min. Slides were stained with 80 µl ethidium bromide and observed on a fluorescent microscope at 40 x magnification (Nikon AFX-1 Optiphot, Japan). The Casplab software was used for scoring comets (version 1,2.3b2, Poland). Around 50 to 100 cells were observed in each sample. Percent tail and head DNA (%) and tail length of (µm) sperm DNA comet were determined.
2.9 Histology and light microscopy
Standard procedure of Hematoxylin and Eosin (H&E) was followed for histology of testes and epididymis. Prepared slides were observed using a photomicroscope (Leica, Germany). Images were captured using a digital camera (Canon, Japan) attached to the microscope. Image analysis was done on Image J software (Ver. 1.53t Microsoft Inc. USA). Area was measured in µm2 for seminiferous tubules, interstitial space, and epididymal tubules. The percent area was then calculated by using the following formula: %A(st)= (A (st)×100)/T
While, A(st) is the area of seminiferous tubules, and T is the total area of the section.
Different types of cells (spermatogonia, spermatocytes, and spermatids) were counted by observing 50 seminiferous tubules for each animal and average values per seminiferous tubule were calculated.
Statistical analyses
Data are shown as mean ± S.E. Mean values of treatment groups were compared with the control values through one-way ANOVA followed by Dunnet’s post-hoc test using the GraphPad Prism (5.01, CA USA). Probability was P < 0.01.
3. Results
3.1 Body and testis weight
In the sub-chronic exposure to NaAsO2, no statistically significant difference was noticeable at any dose in the body weight or testicular weight between control and treated groups of mice on PND 54 (Table 1). In contrast, chronic exposure to NaAsO2 had caused significant reductions (P<0.001) in the body weight and as well testis weight at all doses when determined on PND 114 (Table 1).
|
Sub-chronic exposure |
Chronic exposure |
|||
|
Treatments |
Body weight (g) |
Testis weight (mg) |
Body weight (g) |
Testis weight (mg) |
|
Control |
27.5±0.44 |
98.7±1.33 |
37.7±0.32 |
100.7±0.60 |
|
0.01 mg.L-1 |
26.1±0.56 |
97.4±0.76 |
34.2±0.40** |
96.4±0.90** |
|
5 mg.L-1 |
26.5±0.51 |
97.1±0.62 |
33.5± 0.90** |
93.0±0.70*** |
|
10 mg.L-1 |
26.2±0.83 |
96.2±0.70 |
32.3±0.90*** |
91.2± 1.00*** |
**, *** show significant difference at P < 0.01 and P < 0.001 respectively as compared to control
Table1: Body weight and testis weight of postnatal prepubertal mice following sub-chronic and chronic exposure to different doses of NaAsO2. Values are expressed as mean ± S.E.
3.2 Hormone concentrations
Sub-chronic exposure to NaAsO2
Table 2 represents hormonal changes. In the sub-chronic NaAsO2 exposure, plasma LH and FSH concentrations were significantly reduced at 5.0 (P<0.01) and 10.0 mg.L-1 (P<0.001) doses of NaAsO2. Low dose NaAsO2 (0.01 mg.L-1) treatment did not cause any change in plasma LH or FSH concentrations. Similarly, significant reduction (P<0.001) occurred in both the plasma testosterone and intratesticular testosterone concentrations at 5 and 10 mg.L-1doses of NaAsO2, while low dose of 0.01 mg.L-1did not cause any statistically significant difference when compared with the control mice (Table 2).
Table 2: Hormone concentrations in postnatal prepubertal mice following exposure to NaAsO2 in drinking water. Data are expressed as mean ± S.E
|
Groups |
LH |
FSH |
Plasma Testosterone (ng/ml) |
Intratesticular Testosterone (ng/g) |
|
(mIU/mL) |
(mIU/mL) |
|||
|
Sub-Chronic |
||||
|
Control |
2.89±0.14 |
2.80±0.07 |
54.96±1.48 |
3.33±0.10 |
|
0.01 mg. L-1 |
2.76±0.13 |
2.68±0.06 |
52.92±0.80 |
3.10±0.06 |
|
5.0 mg. L-1 |
2.20±0.07** |
2.52±0.02** |
49.17±0.94** |
2.86±0.05** |
|
10.0 mg. L-1 |
2.33±0.11** |
2.37±0.07*** |
47.15±0.79*** |
2.76±0.10*** |
|
Chronic |
||||
|
Control |
3.08±0.03 |
2.85±0.05 |
55.2±1.15 |
3.33±0.13 |
|
0.01 mg. L-1 |
2.72±0.09** |
2.53±0.07** |
48.6±0.57*** |
2.92±0.07** |
|
5.0 mg. L-1 |
2.35±0.07*** |
2.19±0.06*** |
45.93±0.69*** |
2.39±0.10*** |
|
10.0 mg. L-1 |
2.47±0.02*** |
1.90±0.08*** |
43.77±1.00*** |
2.49±0.09*** |
**, *** indicate significant difference at P< 0.01 and P < 0.001 respectively compared to control
Chronic exposure to NaAsO2
In the chronic set up, NaAsO2 treatment led to significantly reduction (P<0.001) in plasma LH and FSH concentrations (Table 2). Similarly, both the plasma testosterone and intra-testicular testosterone concentrations were reduced in all treatment groups as compared to control animals (Table 2). Treatment with 5 and 10 mg.L-1 NaAsO2 led to a greater reduction (P<0.001) in testosterone concentration as compared to 0.01 mg.L-1 dose (P<0.01).
3.3 Testicular biochemical parameters
Sub-chronic exposure to NaAsO2
Following the sub-chronic arsenic exposure, ROS and TBARS increased significantly (P<0.001) at 5 and 10 mg.L-1 NaAsO2 doses, while significant reduction occurred in the levels of antioxidant enzymes, the CAT, SOD and POD. Similar trend was noticeable in GSH concentrations (Table 3). Sight increases (P<0.05) in ROS levels and decrease (P<0.01) in CAT, SOD and POD were also noticeable at 0.01 mg.L-1concentration of NaAsO2 (Table 3).
Chronic exposure to NaAsO2
In the chronic exposure to NaAsO2, ROS and TBARS increased significantly (P<0.001) at all doses of NaAsO2. Levels of antioxidant enzymes CAT, SOD, POD and non-enzymatic GSH were, in contrast, decreased significantly (P<0.001) at all NaAsO2 doses (Table 3).
|
Groups |
ROS (Absorbance) |
TBARS (nM.min/mg protein) (unit/min) |
CAT (unit/mg) |
SOD (unit/min) |
POD (µM/g) |
GSH (µM/g) |
|
Sub-Chronic |
(PND 25 - 53) |
|||||
|
Control |
3.27±0.25 |
1.40±0.13 |
17.7±0.70 |
80.8±1.33 |
15.8±0.42 |
18.6±0.66 |
|
0.01 mg.L-1 |
4.00±0.18* |
1.87±0.16* |
14.8±0.49** |
73.5±1.28** |
13.5±0.56* |
17.9±0.60 |
|
5.0 mg.L-1 |
4.16±0.11** |
2.72±0.17*** |
14.6±0.47** |
48.7±2.07*** |
10.6±0.53*** |
14.3±0.73** |
|
10.0 mg.L-1 |
4.5±0.12*** |
2.99±0.02*** |
13.7±0.57*** |
45.0±0.66*** |
8.4±0.60*** |
14.4±1.03** |
|
Chronic |
(PND 25 - 114) |
|||||
|
Control |
2.97±0.21 |
1.13±0.02 |
18.5±0.52 |
83.8±1.2 |
15.5±0.6 |
19.2±0.5 |
|
0.01 mg.L-1 |
4.11±0.18*** |
2.40±0.11*** |
12.3±9.9*** |
38.7±1.98** |
9.9±0.7*** |
13.8±0.6** |
|
5.0 mg.L-1 |
4.48±0.12*** |
3.62±0.21*** |
11.0±7.1*** |
34.1±0.98*** |
7.1±0.2*** |
11.1±0.4** |
|
10.0 mg.L-1 |
5.07±0.21*** |
4.21±0.17*** |
8.2±0.55*** |
31.2±1.0*** |
6.4±0.5*** |
9.91±0.6** |
*, **, *** indicate significant difference at P < 0.05, P < 0.01 and P < 0.001 compared to control.
Table3: Oxidative stress parameters and levels of antioxidant enzymes in testicular homogenates of postnatal prepubertal mice after sub-chronic and chronic oral exposure to NaAsO2 in drinking water. Values are mean ± S.E.
Sperm parameters
Sub-chronic exposure to NaAsO2
Sperm count (Fig. 1), motility (Fig. 2), and viability (Fig. 3) were reduced (P<0.001) at 5 and 10 mg.L-1 doses of NaAsO2 when compared with the control group of mice. Low dose (0.01 mg.L-1) treatment with NaAsO2 did not induce any alteration in these parameters.
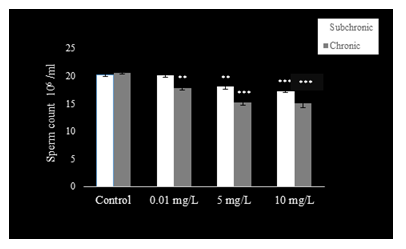
Figure 1: Sperm count decreased in PND 54 and PND 115 mice after sub-chronic and chronic exposure to oral doses of NaAsO2. Data are mean ± S.E. ** P<0.01 *** P<0.001
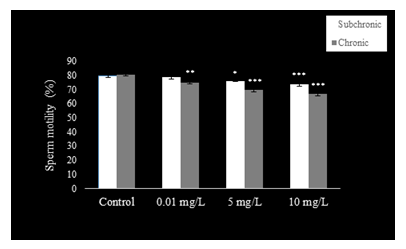
Figure 2: Sperm motility decreased in PND 54 and PND 115 mice after sub-chronic and chronic exposure to oral doses of NaAsO2. Data are mean ± S.E. ** P<0.01 *** P<0.001
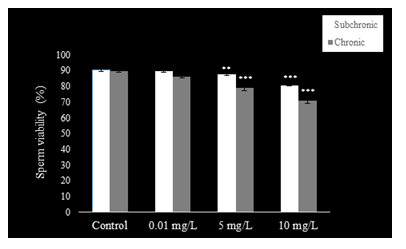
Figure 3: Sperm viability decreased in PND 54 and PND 115 mice after sub-chronic and chronic exposure to oral doses of NaAsO2. Data are mean ± S.E. ** P<0.01 *** P<0.001
Chronic exposure to NaAsO2
Compared to control mice, significant decline (P<0.001) was observed in sperm count (Fig. 1) with all doses of NaAsO2. Sperm motility (Fig. 2) decreased significantly in all treatment groups (P<0.001), whereas sperm viability (Fig. 3) also decreased significantly (P<0.001) in the 5 and 10 mg.L-1treatment groups as compared to the control group.
3.4 Histology and morphometry of testes
Sub-chronic NaAsO2 exposure
Figure 4 shows the testicular morphology following sub-chronic NaAsO2 exposure. In the control group, seminiferous tubules were compactly arranged. Type A and type spermatogonia, spermatids and spermatozoa were readily visible. The tubular lumen contained many spermatozoa while germinal epithelium was normal and thick. Leydig cells showed a normal appearance (Fig. 4A). In the 0.01 mg.L-1 NaAsO2 treatment group, slight degeneration of germinal epithelium was noticeable. Cells showed fragmentation and detachment from the germinal epithelium and gathered in the lumen (Fig. 4B). At higher doses of 5 and 10 mg.L-1NaAsO2, greater cell damage was noticeable in the germinal epithelium. Cells showed degeneration, detachment from the germinal epithelium, degeneration of type A and Type B spermatogonia and loss of spermatocyte population. Loss of spermatids and spermatozoa were also evident (Fig. 4C & D respectively). Leydig cells also showed degeneration.
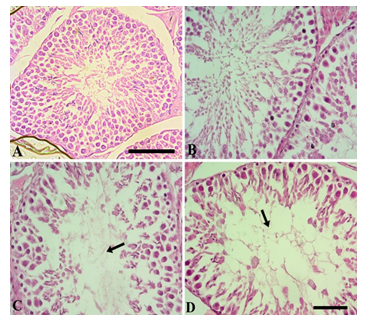
Figure 4: Photomicrograph of postnatal prepubertal mice testes after sub-chronic (PND 25 to PND 54) exposure to NaAsO2 in drinking water. (A) control testis (B) 0.01 mg/L (C) 5 mg/L (D) 10 mg/L NaAsO2. Arrows show degenerated spermatozoa. Scale bar= 20 µm.
Table 4 represents morphometry of mice testis following the sub-chronic and chronic exposure to NaAsO2. At low dose, area of seminiferous tubules and interstitial space although demonstrated statistically non-significant alterations but these were noticeable. The diameter of seminiferous tubules however remained unaffected. Epithelial cell height reduced significantly at 5 mg.L-1 (P<0.01) and 10 mg.L-1 (P<0.001) doses of NaAsO2. Diameter of seminiferous tubular lumen showed significant increase (P<0.01) only with 10 mg.L-1 treatment. Number of spermatocytes decreased significantly in the testes at 10 mg.L-1NaAsO2 dose (P<0.01), while spermatid numbers also decreased (P<0.001) in the testis at highest test dose of NaAsO2 (10 mg.L-1).
Chronic exposure to NaAsO2
Figure 5 shows testicular morphology in the chronic exposure to of mice to NaAsO2. Control testes showed compact seminiferous tubules, normal germinal epithelium, compact presence of type A and type B spermatogonia and spermatocytes. Lumen contained spermatozoa, while the Leydig cells showed normal appearance (Fig. 5A). Conversely, at 0.01 mg.L-1dose of NaAsO2, although the germinal epithelium was near normal, the luminal space increased and a reduction in spermatozoa was readily noticeable (Fig. 5B). At high dose concentrations of NaAsO2 (5 and 10 mg.L-1), excessive degeneration of germinal epithelium and germ cell loss was observed. Type A, type B spermatogonia, spermatocytes and spermatid populations were significantly reduced. Spermatozoa declined significantly (Fig. 5C &D). The Leydig cells also showed structural alterations.

Figure 5: Photomicrograph of postnatal prepubertal mouse testes after chronic (PND 25 to PND 115) exposure to NaAsO2 in drinking water. (A) control testis (B) 0.01 mg/L (C) 5 mg/L (D) 10 mg/L NaAsO2. Arrows show degenerated germinal epithelia and degenerated spermatozoa. Scale bar=20 µm.
Morphometry of mice testis demonstrated significant alterations following chronic exposure to NaAsO2 (Table 4), whereby percent area of seminiferous tubules and interstitium were significantly altered but these changes were non-significant at low dose of 0.01 mg.L-1, while seminiferous tubular diameter and epithelial height decreased significantly (P<0.001), and a significant increase was noticeable in the lumen diameter at low NaAsO2 dose. In contrast, at high NaAsO2 doses, significant decrease occurred in percent seminiferous tubular area (P<0.05 at 5 mg.L-1and P<0.01 at 10 mg.L-1doses). Interstitial area on the other increased significantly (P<0.05 at 5 mg/L and P<0.01 at 10 mg.L-1doses). Seminiferous tubular diameter and epithelial height decreased significantly at both low and high arsenic doses (P<0.001) but lumen diameter of seminiferous tubules was significantly increased (P<0.001), in comparison to the control group (Table 4). Compared to control testes, spermatocyte and spermatid numbers showed noticeable dose dependent decrease (P<0.001) at low, moderate and high NaAsO2 doses.
Table 4: Morphometric parameters of postnatal prepubertal mice testes show significant alterations after sub-chronic and chronic oral exposure to NaAsO2 in drinking water. Values are mean ± S.E.
|
Groups |
Area of Seminiferous tubules (%) |
Area of Interstitium (%) |
Seminiferous Tubule Diameter (µm) |
Epithelial Height (µm) |
Lumen Diameter (µm) |
|
Sub-Chronic |
|||||
|
Control |
89.76±1.56 |
10.23±1.56 |
201.78±1.85 |
56.91±0.92 |
31.57±0.76 |
|
0.01 mg. L-1 |
89.69±0.95 |
10.30±0.95 |
199.45±0.95 |
56.57±0.82 |
31.38±0.83 |
|
5.0 mg. L-1 |
88.44±2.75 |
11.55±2.75 |
197.78±0.65 |
53.62±0.57* |
33.23±0.67 |
|
10.0 mg. L-1 |
85.14±2.04 |
14.85±2.04 |
197.87±0.86 |
51.83±0.80*** |
34.64±0.80* |
|
Chronic |
|||||
|
Control |
88.66±1.03 |
11.33±1.03 |
201.6±1.62 |
59.19±0.96 |
29.93±0.32 |
|
0.01 mg. L-1 |
84.97±2.06 |
15.02±2.06 |
190.1±1.25*** |
45.45±1.84*** |
36.55±0.56*** |
|
5.0 mg. L-1 |
79.48±2.53* |
20.51±2.53* |
185.9±1.89*** |
40.73±1.42*** |
40.00±0.52*** |
|
10.0 mg. L-1 |
76.26±3.49** |
23.7±3.49** |
183.6±1.29*** |
39.08±1.26*** |
40.56±0.54*** |
*, **, *** indicate significant difference at P < 0.05, P < 0.01 and P < 0.001 respectively compared to control
3.5 Sperm DNA damage
Sub-chronic NaAsO2 exposure
Exposure of postnatal mice to sub chronic NaAsO2 doses revealed significant sperm DNA damage at high dose concentrations (5 and 10.0 mg.L-1) (Fig. 6B-D) as compared to control (Fig. 6A) and low dose treatment (Fig. 6B). Sperm tail DNA and tail length were significantly increased (P<0.05), while percent head DNA decreased significantly (P<0.05) after 10 mg.L-1NaAsO2 treatment as compared to control mice (Table 5).
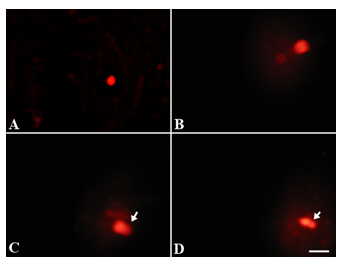
Figure 6: Sperm DNA damage (A) in postnatal prepubertal mice upon sub-chronic (PND 25 to PND 54) exposure to NaAsO2 in drinking water. Control (B) 0.01 mg/L (C) 5 mg/L (D) 10 mg/L. Arrows show increased tail length with 10 mg/L NaAsO2 treatment. Scale bar=20 µm.
Table 5: Sperm DNA damage in postnatal prepubertal mice after sub-chronic and chronic oral exposure to NaAsO2 in drinking water. Values are mean ± S.E.
|
Groups |
% Tail DNA |
Tail length (µm) |
% Head DNA |
|
Sub-Chronic |
|||
|
Control |
5.14 ± 2.29 |
3.26 ± 0.37 |
94.84 ± 2.29 |
|
0.01 mg. L-1 |
7.06 ± 0.74 |
3.66 ± 0.33 |
92.92 ± 0.75 |
|
5.0 mg. L-1 |
8.70 ± 1.72 |
4.30 ± 1.35 |
91.2 ± 1.72 |
|
10.0 mg. L-1 |
15.6 ± 2.37* |
10.6 ± 1.67* |
84.36 ± 2.38* |
|
Chronic |
|||
|
Control |
6.79 ± 0.95 |
3.06 ± 0.06 |
93.1 ± 0.96 |
|
0.01 mg. L-1 |
16.10 ± 2.75 |
9.66 ± 2.66 |
83.8 ± 2.75 |
|
5.0 mg. L-1 |
41.27 ± 6.24** |
19.0 ± 3.21* |
58.8 ± 6.25** |
|
10.0 mg. L-1 |
48.01 ± 8.8** |
20.6 ± 3.93** |
51.98 ± 8.8** |
*, ** indicate significant difference at P < 0.01, P<0.001 and P < 0.001 respectively compared to control.
Chronic exposure to NaAsO2
Upon chronic exposure of postnatal mice to NaAsO2, a significant DNA damage was noticeable at all NaAsO2 doses (Fig. 7B-D) when compared with the control (Fig. 7A). Percent tail DNA and tail length increased significantly (P<0.01) at 5 and 10 mg.L-1 doses of NaAsO2, while percent head DNA decreased significantly (P<0.01) at these doses (Table 5).
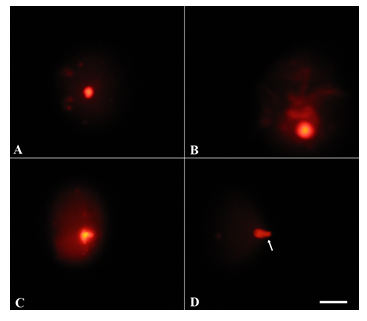
Figure 7: Sperm DNA damage (A) in postnatal prepubertal mice upon chronic (PND 25 to PND 115) exposure to NaAsO2 in drinking water. (A) Control (B) 0.01 mg/L (C) 5 mg/L (D) 10 mg/L. Arrow shows increased tail length after 5 and 10 mg/L NaAsO2 treatments. Scale bar= 20 µm.
3.6 Histology and morphometry of epididymes
Sub-chronic NaAsO2 exposure
The epithelium of the cauda epididymis showed no morphological alterations in NaAsO2 treated groups (Fig. 8B-D) as compared to the control group (Fig. 8A). Epididymal sperms however demonstrated significant decrease in the lumen at 5 and 10 mg.L-1concentrations of NaAsO2 (Fig. 8C &D). Epithelial cell height of epididymal cells decreased significantly (P<0.001) at 5 and 10 mg/L NaAsO2 doses in the sub-chronic set up (Fig. 10). In contrast, luminal diameter of epididymis increased significantly only at 10 mg.L-1 dose of NaAsO2 (Fig. 11). whereas tubular diameter of the epididymis decreased significantly at high dose (Fig. 12).
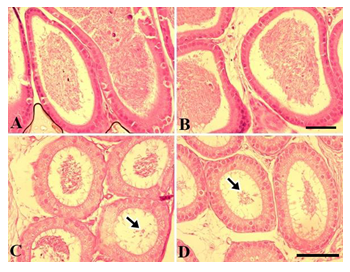
Figure 8: Photomicrographs of postnatal prepubertal mouse epididymal tissue sub-chronic (PND 25 to PND 54) exposure to NaAsO2 in drinking water. (A) control tissue (B) 0.01 mg/L (C) 5 mg/L (D) 10 mg/L NaAsO2. Arrows show disrupted epithelia and reduction in spermatozoa. Scale bar=20 µm.
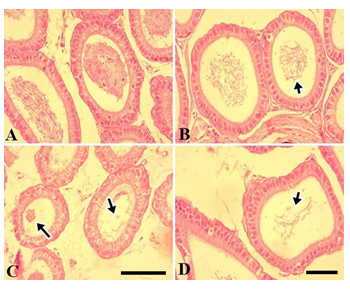
Figure 9: Photomicrographs of postnatal prepubertal mouse epididymal tissue following chronic (PND 25 to PND 54) exposure to NaAsO2 in drinking water. (A) control tissue (B) 0.01 mg/L (C) 5 mg/L (D) 10 mg/L NaAsO2. Arrows show highly reduced spermatozoa and wider lumen. Scale bar=20 µm.
Chronic exposure to NaAsO2
In the chronic setup, epididymal sperms decreased slightly at 0.01 mg.L-1dose of NaAsO2 (Fig. 6B) as compared to the control (Fig. 9A). In contrast, highly significant reduction was noticeable in the epididymal sperms at 5 mg.L-1and 10 mg.L-1doses of NaAsO2 (Fig. 9C &D). Moreover, epididymal epithelia also showed noticeable structural abnormalities (Fig. 9C &D), whereby a significant decrease (P<0.001) was noticeable in the epididymal epithelial cell height at all arsenic concentrations (Fig. 10). In contrast, luminal diameter of cauda epididymis increased significantly (P<0.001) at all doses (Fig. 11), while the tubular diameter of cauda epididymis decreased significantly (P<0.001) in all treatment groups in comparison to control (Fig. 12).
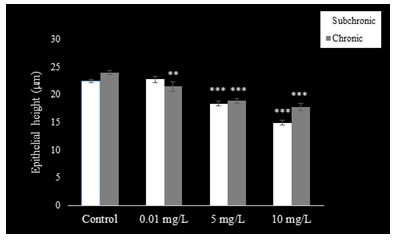
Figure 10: Epididymal epithelial height (µm) decreased in PND 54 and PND 115 mice after sub-chronic and chronic exposure to oral doses of NaAsO2. Data are mean ± S.E. ** P<0.01 *** P<0.001
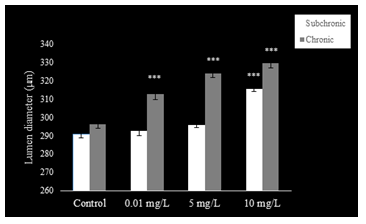
Figure 11: Epididymal luminal diameter (µm) increased in PND 54 and PND 115 mice after sub-chronic and chronic exposure to oral doses of NaAsO2. Data are mean ± S.E. ** P<0.01 *** P<0.001
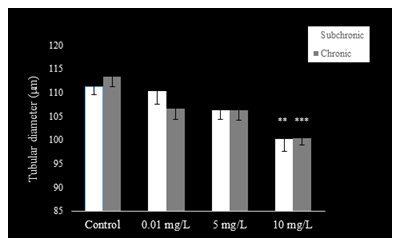
Figure 12: Epididymal tubular diameter (µm) decreased in PND 54 and PND 115 mice after sub-chronic and chronic exposure to oral doses of NaAsO2. Data are mean ± S.E. ** P<0.01 *** P<0.001
4. Discussion
In the present study, postnatal mice (PND 25) were exposed orally to NaAsO2 in drinking water sub-chronically up to PND 54 and chronically to PND 114 at three different dose concentrations, 0.01 mg.L-1, 5.0 mg.L-1and 10.0 mg.L-1. Dose selection criteria were: the lowest dose of 0.01 mg.L-1equals 10 ppb or 10 mg.L-1, a concentration that has been declared by the WHO as safest permissible limit of arsenic in drinking water [7,9], whereas the highest dose used in the present experiments (10 mg.L-1) is equivalent to 10,000 ppb, taking into consideration accidental exposure of humans to high arsenic concentration, and also because in several countries of the world, arsenic levels in the ground water exceed well above the safe level up to 50 mg.L-1 and exceeding well above in few other countries to 4500 mg.L-1 [7,9]. Currently, oral exposure of prepubertal mice to NaAsO2 caused significant reductions in body weight and testicular weight, plasma LH, FSH and testosterone and as well as intratesticular testosterone were decreased, whilst causing significant elevations in ROS and TBARS levels and reductions in the levels of antioxidant enzymes the SOD, POD and CAT and as well as non-enzymatic glutathione reductase (GSH). Histologically, degeneration of serminiferous tubular germinal epithelium occurred. Disintegration of primary and secondary spermatogonia, spermatocytes, spermatids and spermatozoa were readily noticeable, which indicated severe germ cell loss. The percentage area of seminiferous tubules, percent interstitial area, seminiferous tubular diameter and epithelial cell height all decreased, while lumen diameter increased. Sperm count, motility and viability decreased as well. Comet assay further demonstrated significant damage to sperm DNA whereby percent tail DNA and tail length increased, while the percent head DNA was decreased. More severe effects were observed in the chronic set up. Of note, in the chronic set up, 0.01 mg.L-1 dose, which was lowest, also caused severe alterations in all above parameters. This showed that chronic exposure of developing male gonad to even lower doses of NaAsO2 can indeed be very harmful to the prepubertal reproductive system which may lead to sever consequences later in the adult life. In the sub-chronic set up, high arsenic doses of 5 mg.L-1and 10.0 mg.L-1were found to be highly damaging.
While some studies demonstrate decline in the body weight of young rats upon chronic exposure to arsenic [40] but others show no effects of arsenic on body weight in adult animals [15, 41,42]. However, present dose dependent decline in the body weight and testis weight upon exposure to NaAsO2 exposure is similar to as reported in adult rats [43,44].
Besides causing weakness, tiredness, inflammation of colon and stomach linings chronic exposure to arsenic induces heart diseases, disruption of the nervous system, and several renal and hepatic diseases [45], weight loss, malabsorption of food and anorexia which is reported to appear after arsenic exposure [46]. It can therefore be safely predicted that indirect systemic and metabolic factors could have played a contributing role in causing a decrease in the body weights and testicular weight in the current in vivo experiments in prepubertal mice.
Decrease in the pituitary gonadotropins, the LH and FSH and gonadal sex hormone testosterone following exposure to NaAsO2 indicates direct suppression of the HPG-axis, most likely via inhibition of the central neuronal pathway for release of gonadotropins. Alternatively, inhibition of steroid biosynthesis by the gonads might also be one of the reasons. In the sub-chronic arsenic exposure, testosterone (intratesticular and plasma), LH, and FSH concentrations were found significantly reduced in the highest dose groups (5 and 10 mg.L-1), but in the chronic exposure to NaAsO2 from PND 25-114, low dose of 0.01 mg.L-1also caused considerable decrease in the hormonal profile. Similar reductions in the plasma testosterone and LH after arsenic exposure have been reported in rats [15, 41]. A significant reduction has been reported in plasma testosterone levels after 1-month exposure to 20 and 40 mg.L-1NaAsO2 in adult mice, but the LH concentrations remained unaffected [14]. Since the testosterone levels are critical for sustaining the normal functioning of the seminiferous tubules and consequently, any reduction in its concentration may cause disintegration of the germinal epithelium [47]. Under normal circumstances, germinal cells are protected from degeneration by FSH, and hence any decline in its concentration might promote the disruption of germinal epithelium of the seminiferous tubules [15,48]. Presently, excessive degeneration of the germinal epithelium, primary and secondary spermatogonia, spermatocytes, spermatids and spermatozoa that was observed in the seminiferous tubules following the exposure of mice to sub-chronic and chronic NaAsO2 treatment was quite possibly due to significantly lower levels of gonadotropins and testosterone.
Moreover, the sperm parameters like the sperm count, motility and viability were found to be significantly reduced with NaAsO2 treatment. Sub-chronic exposure to a low concentration of NaAsO2 (0.01 mg.L-1) did not induce any alterations in the sperm parameters, whereas chronic exposure led to significant toxic effects of arsenic at all concentrations of NaAsO2. Furthermore, alterations in sperm parameters can be attributed to low levels of testosterone but alterations in the levels of other seminal plasma factors may also account for, revealing that the toxicity of arsenic was time and dose dependent. Corroborating with the present results in postnatal mice, exposure of postnatal rats to arsenic has been shown to cause significant reduction in the sperm count [48]. Similar results have been demonstrated in adult mice, whereby arsenic exposure induced alterations in sperm morphology and number [17]. As is evident from present histology also, a decline in sperm count was most likely due to germ cell loss. This decrease in sperm count is also related to a reduced production in the seminiferous tubules, and any blockage or phagocytosis occurring in the ducts [49].
Present NaAsO2 treatment in both the sub-chronic and chronic treatments induced severe morphological alterations in mouse testis and as well as epididymis. In general, arsenic treatment induced shrinking of seminiferous tubule diameter, decrease in epithelial cell height, loss of germ cells and mature sperms. Luminal space increased considerably with respect to the arsenic dose. A substantial reduction in the number of sperms stored in the epididymis was also evident in both set of experiments. Low dose arsenic treatment (0.01 mg.L-1) although did not induce morphological changes in the testis and epididymis after sub-chronic exposure but chronic treatment with this dose initiated damaging effects on the reproductive organ’s histology. Numerous studies have also demonstrated parallel findings in rats [15, 50] and male goats [51] with arsenic treatment. Similar germ cells diminution and reduction in seminiferous tubule diameter has been indicated in other studies [52,53]. Presently, epididymal tissue also demonstrated significant histological alterations, severe reduction in spermatozoa and increased lumen.
Increased ROS and TBARS with an accompanying reduction in antioxidant enzymes were observed in both sub-chronic and chronic treatments. It is already known that ROS formation due to arsenic exposure can damage proteins, lipids, and interstitial tissues [14]. It can improve collagen synthesis and in response the testis interstitial tissue becomes fibroid [54]. Glutathione plays a fundamental role in the mammalian antioxidant defense system. Marked increase in lipid peroxidation, ROS generation, and GSH depletion in rat brain, liver, and RBCs after 12 weeks arsenic exposure has been reported [55]. Concomitant decline that was observed presently in the antioxidant enzyme levels, the SOD, POD, CAT and non-enzymatic reduced glutathione, and as well as gross degenerations and alterations that occurred in the structure of seminiferous tubules indicate that destructive effects to developing gonads quite possibly occurred due to overproduction of ROS and excessive lipid peroxidation inflicted by NaAsO2.
Generalized DNA damage in rats has been strongly linked with the free radicals generated by arsenic [56]. Presently also, chronic exposure of postnatal mice to NaAsO2 revealed considerable DNA damage to developing male mice testes as demonstrated by the Comet assay. This was most likely due to the generation of ROS and a disruption of cellular membranes due to lipid peroxidation. The study overall demonstrated that sub-chronic or chronic exposure to NaAsO2 is detrimental to postnatal male mice reproductive system.
5. Conclusion
In conclusion, the present study indicated that NaAsO2 induces severe toxic effects in postnatal day 25mice exposed to sub-chronic and chronic doses. Arsenic intake is capable of inducing considerable morphological, biochemical, hormonal, and DNA disruptions during the postnatal reproductive development. The most significant finding of the present study is that even a low dose of 0.01 mg.L-1 (10 ppb) of arsenic, which did not cause any significant damage in the sub-chronic set up, the same dose given to mice in the chronic exposure was capable of inducing considerable physiological and cellular damage to developing reproductive organs. Thus, long-term human exposure to even lower concentrations of arsenic is not safe for male reproduction especially in the childhood.
Declaration of interest
The authors declare no conflict of interest.
Funding
This research was funded by Quaid-i-Azam University, Islamabad, Pakistan through university research fund (no specific grant No.)
References
- Mukherjee M K, Sengupta M A, Hossain S, et al. Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario, J. Health Popul. Nutr 10 (2006) 142-163.
- W Ali, A Rasool, M Junaid, et al. A comprehensive review on current status, mechanism, and possible sources of arsenic contamination in groundwater: a global perspective with prominence of Pakistan scenario, Environ. Geochem. Health 41 (2019): 737-760.
- R Nickson, J McArthur, W Burgess, et al. Arsenic poisoning of Bangladesh groundwater, Nature 395 (1998): 338.
- A K Sharma, Chr Tjell Jens, JJ Sloth, et al. Review of arsenic contamination, exposure through water and food and low-cost mitigation options for rural areas. Appl. Geochem 41 (2014): 11-33.
- S Shankar, U Shanker. Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation, Sci. World J 9(2014).
- Arsenic in Drinking-water. Background Document for Preparation of WHO Guidelines for Drinking-water Quality. World Health Organization, Geneva. Switzerland 11 (2011).
- SB Adeloju, S Khan, FP Antonio. Arsenic contamination of groundwater and its implications for drinking water quality and human health in under-developed countries and remote communities—A Review, Sci 4(2021): 1926.
- E Shaji, M Santosh, KV Sarath, et al. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula, Geosci. Front 12(3) (2021): 1-18.
- D Chakraborti, SK Singh, M.M Rahman, et al. Groundwater arsenic contamination in the Ganga River Basin: a future health danger, Int. J. Environ. Res. Public Health 15 (2018): 180.
- D Saha, RK Ray. Groundwater resources of India: potential, challenges and management, in: Sikdar, P.K. (Eds.), Groundwater Development and Management. Springer, Cham 4(2019): 19-42.
- S Bhowmick, S Pramanik, P Singh, et al. Arsenic in groundwater of West Bengal, India: a review of human health risks and assessment of possible intervention options, Sci. Total Environ. 612 (2018): 148-169.
- JF Holson, JM Desesso, C F Jacobson, et al. Appropriate use of animal models in the assessment of risk during prenatal development: an illustration using inorganic arsenic, Teratology 62 (2000): 51-71.
- J Das, J Ghosh, P Manna, et al. Taurine protects rat testes against NaAsO2-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol. Lett 187 (2009): 201-210.
- S I Chang, SH Kim, JD Park, et al. Decreased levels of plasma testosterone/LH ratio in male mice exposed to sodium arsenite, Biomol. Therap 18 (2010): 257-261.
- M Sarkar, GR Chaudhuri, A Chattopadhyay, et al. Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats, Asian J. Androl 5 (2003) 27-32.
- MP Waalkes, JM Ward, J Liu, et al. Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice, Toxicol. Appl. Pharmacol. 186 (2003): 7-17.
- N Pant, RC Murthy, SP Srivastava. Male reproductive toxicity of sodium arsenite in mice, Hum Exp. Toxicol. 23 (2004): 399-403.
- M E Dávila-Esqueda, ME Jiménez-Capdeville, JM Delgado, et al. Effects of arsenic exposure during the pre-and postnatal development on the puberty of female offspring. Exp. Toxicol. Path 64 (2012): 25-30.
- S Khan, AG Telang, J K Malik. Arsenic-induced oxidative stress, apoptosis and alterations in testicular steroidogenesis and spermatogenesis in wistar rats: ameliorative effect of curcumin, WJPP 2 (2013): 33-48.
- H Shen, W Xu, J Zhang, et al. Urinary metabolic biomarkers link oxidative stress indicators associated with general arsenic exposure to male infertility in a Han Chinese population, Environ. Sci. Technol 47 (2013): 8843-8851.
- TR Kumar, K Doreswamy, B Shrilatha. Oxidative stress associated DNA damage in testis of mice: induction of abnormal sperms and effects on fertility, Mutat. Res 513 (2002): 103-111.
- MS Golub, MS Macintosh, N Baumrind. Developmental and reproductive toxicity of inorganic arsenic: animal studies and human concerns, J. Toxicol. Environ. Health, Part B Crit. Rev 1 (1998): 199-237.
- AO Morakinyo, P U Achema, OA Adegoke. Effect of Zingiber officinale (Ginger) on sodium arsenite-induced reproductive toxicity in male rats, African J. Biomedic. Res 13 (2010): 39-45.
- K Sudha. Effect of arsenic induced toxicity in testis of male rats, Indian J. Fund. Appl. Life Sci 3 (2012): 126-130.
- AM Jalaludeen, R Lee, WY Lee, et al. Ameliorating effect of selenium against arsenic induced male reproductive toxicity in rats, Reprod. Dev. Biol 38 (2014): 107-114.
- W He, R J Greenwell, DM Brooks, et al. Arsenic exposure in pregnant mice disrupts placental vasculogenesis and causes spontaneous abortion, Toxicol. Sci 99(2007): 244-253.
- DS Hill, BJ Wlodarczyk, RH Finnell. Reproductive consequences of oral arsenate exposure during pregnancy in a mouse model, Birth Defects Res. B Dev. Reprod. Toxicol 83(2008): 40-47.
- EJ Martinez-Finley, AMS Ali, A M Allan. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: consequence of lower corticosterone receptor levels, Pharmacol. Biochem. Behav 94(2009): 271-277.
- RC Lantz, B Chau, P Sarihan, et al. In utero and postnatal exposure to arsenic alters pulmonary structure and function, Toxicol. Appl. Pharmacol 235(2009): 105-113.
- CD Kozul-Horvath, F Zandbergen, BP Jackson, et al. Effects of low-dose drinking water arsenic on mouse fetal and postnatal growth and development, PloS one 7 (2012): e38249.
- MP Reilly, JC Saca, A Hamilton, et al. Dearth, Prepubertal exposure to arsenic (III) suppresses circulating insulin-like growth factor-1 (IGF-1) delaying sexual maturation in female rats, Reprod.Toxicol 44 (2014): 41-49.
- I Hayashi, Y Morishita, K Imai, et al. High-throughput spectrophotometric assay of reactive oxygen species in serum, Mut. Res./Genetic Toxicol. Environ. Mutagen 631 (2007): 55-61.
- M Iqbal, SD Sharma, H Rezazadeh, et al. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury, Redox Report 2(1996): 385-391.
- A Maehly, B. Chance, The assay of catalase and peroxidases, Meth. Enzymol 2 (1955): 764-775.
- P Kakkar, B Das, PN Viswanathan. A modified spectrophotometric assay of superoxide dismutase, Indian J. Biochem. Biophys 21(1993): 130-132.
- DJ Jollow, JR Mitchell, NA Zampaglione, et al. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite, Pharmacology 11 (1974): 151-169.
- F Zobeiri, R A Sadrkhanlou, S Salami, et al. The effect of ciprofloxacin on sperm DNA damage, fertility potential and early embryonic development in NMRI mice, Vet. Res. Forum 3(2012): 131-135.
- AJ Wyrobek, LA Gordon, J G Burkhart, et al. An evaluation of the mouse sperm morphology test and other sperm tests in nonhuman mammals: A report of the US Environmental Protection Agency Gene-Tox Program, Mut. Res 115(1983): 1-72.
- CM Hughes, S Lewis, VJ McKelvey-Martin, et al. The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity, Human Reprod 13(1998): 1240-1247.
- VM Rodr?guez, L Carrizales, MS Mendoza, et al. Giordano, Effects of sodium arsenite exposure on development and behavior in the rat, Neurotoxicol. Teratol 24(2002): 743-750.
- K Jana, S Jana, P K Samanta. Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action, Reprod. Biol. Endocrinol 4 (2006).
- TJ Chiou, ST Chu, WF Tzeng, et al. Arsenic trioxide impairs spermatogenesis via reducing gene expression levels in testosterone synthesis pathway, Chem. Res. Toxicol 21(2008): 562-1569.
- M Soleimani Mehranjani, M Hemadi. The effects of sodium arsenite on the testis structure and sex hormones in vasectomised rats, Intern. J. Reprod. BioMed 5(2007): 127-134.
- P Dhar, M S Somesh, P Kaushal, et al. Effects of arsenic exposure during early postnatal period on Leydig cells of rat testis, Toxicol. Environ. Chem 92(2010): 1157-1168.
- AH Hall. Chronic arsenic poisoning, Toxicol. Lett 128 (2002): 69-72.
- HH Goebel, PF Schmidt, J Bohl, et al. Polyneuropathy due to acute arsenic intoxication: biopsy studies, J. Neuropath. Exp. Neurol 40(1990): 137-149.
- YJ Kim, JY Chung, S G Lee, et al. Arsenic trioxide-induced apoptosis in TM4 Sertoli cells: the potential involvement of p21 expression and p53 phosphorylation, Toxicology 285(2011): 142-151.
- P Kaushal, P Dhar, SM Shivaprasad, et al. Postnatal exposure to sodium arsenite (NaAsO2) induces long lasting effects in rat testes, Toxicol. Int 19(2012): 215-222.
- HO Goyal, T D Braden, M Mansour, et al. Diethylstilbestrol-treated adult rats with altered epididymal sperm numbers and sperm motility parameters, but without alterations in sperm production and sperm morphology, Biol. Reprod 64(2001): 927-934.
- AA Fouad, WH Albuali, I Jresat. Protective effect of thymoquinone against arsenic-induced testicular toxicity in rats. in: Kuala Lumpur, Malaysia: International Conference on Pharmacology and Pharmaceutical Medicine (ICPPM) 8(2014).
- MA Wares, MA Awal, S K Das, et al. Chronic natural arsenic exposure affecting histoarchitecture of gonads in Black Bengal goats (Capra aegagrushircus), J. Adv. Vet. Anim. Res 2 (2018): 128-133.
- I Ahmad, KM Akthar, T Hussain. Arsenic induced microscopic changes in rat testis, Prof. Med. J 15 (2008): 287-291.
- G Sharma, M Kumar. Morphometrical study of seminiferous tubules of mice after using arsenic and Chlorophytum borivilianum, Pharmacol. Online 2 (2011): 348-359.
- K Yamanaka, M Fujisawa, H Tanaka, et al. Significance of human testicular mast cells and their subtypes in male infertility, Human Reprod 15(2000): 1543-1547.
- SJ Flora. Arsenic-induced oxidative stress and its reversibility following combined administration of n-acetylcysteine and meso 2, 3-dimercaptosuccinic acid in rats, Clin. Exp. Pharmacol. Physiol 26(1999): 865-869.
- R Kadirvel, K Sundaram, S Mani, et al. Supplementation of ascorbic acid and α-tocopherol prevents arsenic-induced protein oxidation and DNA damage induced by arsenic in rats, Human Exp.Toxicol 26(2007): 939-946.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 