Biological Methods of Polluted Soil Remediation for an Effective Economically-Optimal Recovery of Soil Health and Ecosystem Services
Rafael G. Lacalle1*, José M. Becerril1, Carlos Garbisu2
1Department of Plant Biology and Ecology, University of the Basque Country (UPV/EHU), P.O. Box 644, E-48080 Bilbao, Spain
2Department of Conservation of Natural Resources, NEIKER-Basque Institute for Agricultural Research and Development, Basque Research and Technology Alliance (BRTA), Parque Científico y Tecnológico de Bizkaia, P812, 48160 Derio, Spain
*Corresponding Author: Dr. Rafael G. Lacalle, Department of Plant Biology and Ecology, University of the Basque Country (UPV/EHU), Bilbao, Spain
Received: 17 April 2020; Accepted: 23 April 2020; Published: 05 June 2020
Article Information
Citation:
Rafael G. Lacalle, José M. Becerril, Carlos Garbisu. Biological methods of polluted soil remediation for an effective economically-optimal recovery of soil health and ecosystem services. Journal of Environmental Science and Public Health 4 (2020): 112-133.
View / Download Pdf Share at FacebookAbstract
Soil is one of our most important resources as it supports many critical ecological functions and ecosystem services. Nonetheless, due to a wide variety of environmentally-unsustainable anthropic activities, sadly, our soils are currently contaminated at a global scale with a myriad of potentially toxic inorganic and organic compounds. Regrettably, most, if not all, traditional physicochemical methods of soil remediation are frequently based on economically-infeasible and/or environmentally-destructive techniques. In consequence, in the last years and decades, more sustainable and innovative biological methods of soil remediation (belonging to the sometimes called “gentle remediation options”) are being developed in an attempt to combine: (i) an efficient removal of soil contaminants (in terms of a decrease of total and/or bioavailable contaminant concentrations), (ii) a reduction of soil ecotoxicity, (iii) the legally- and ethically-required minimization of risk for environmental and human health, and, concomitantly, (iv) a recovery of soil health and (v) associated ecosystem services. Ideally, any soil remediation method should not only decrease the concentration of soil contaminants below regulatory limits but should also recover soil health and alongside the provision of essential ecosystem services. Unquestionably, all this must be achieved in full compliance with the binding environmental regulations and, most importantly, via the implementation of economically-feasible (preferably, profitable) strategies of soil remediation.
Keywords
<p>Bioremediation; Contamination; Phytoremediation; Pollution; Soil quality; Vermiremediation</p>
Article Details
1. Soil Contamination
The soil is both a highly complex ecosystem and a non-renewable resource on a human time scale, which harbors a range of physical, chemical and biological processes supporting key functions and essential ecosystem services. Likewise, soil is a dynamic living system that serves as habitat for a myriad of organisms (micro-, meso- and macrofauna) with essential roles in nutrient cycling and the mineralization of organic matter [1]. Lamentably, different anthropic activities are responsible for the current state of soil degradation through erosion, compaction, contamination, sealing, salinization and loss of organic matter and biodiversity [2]. Soil contamination, in particular, is nowadays a serious environmental threat and challenge worldwide. In Europe, the existence of 2.5 million potentially-contaminated sites has been estimated [3]. In these sites, the most common environmental contaminants are metal(oid)s and mineral oils, affecting 35 and 24% of European contaminated soils, respectively. Chlorinated hydrocarbons appear to a lesser extent (8%) but still are a most relevant issue [3]. Frequently, contaminated sites are characterized by the simultaneous presence of different contaminants [4, 5], thus potentially increasing their toxicity and environmental impact, and hampering the application of soil remediation technologies [6]. Soil metal(oid) contamination often results from agricultural, mining and metallurgical activities, while accidental spills and/or industrial activities are recurrently the source of soil organic contaminants. Furthermore, waste discharge and waste treatment processes are a major source of both types of soil contaminants. The U.S Environmental Protection Agency (USEPA) reported that 40% of the hazardous waste sites are contaminated with both organic and metal(oid) contaminants [7].
2. Soil Health
Soil contamination, along with other degradation processes, can negatively affect soil health [8], often defined as “the capacity of a given soil to perform its functions as a living system capable of sustaining biological productivity, promoting environmental quality and maintaining plant and animal health” [9]. But soil is a vastly complex environmental matrix which performs numerous, sometimes conflicting, functions from both an ecocentric and anthropocentric perspective, and, in consequence, many different aspects must be taken into consideration in order to properly assess soil health. Most importantly, to appropriately assess soil health: (i) physical, chemical and biological properties with potential as indicators of soil functioning must always be included in the assessment (after all, physical, chemical and biological processes in the soil ecosystem are not independent but interactive processes); (ii) chemical, (eco)toxicological and ecological approaches must be incorporated to the evaluation; (iii) the intended use for the contaminated site must be taken into close consideration, as the very concept of soil health is somewhat teleological and subjective; (iv) the intrinsic temporal and spatial variability of the system (i.e., spatial heterogeneity, temporal dynamics), as well as the scale of both soil processes and the assessment itself, must be taken into account; and (v) the selection of a suitable (inevitably, often far from perfect) “healthy” reference soil, for comparison and the establishment of target purposes, should be identified.
Soil physicochemical properties such as pH, redox potential, organic matter content, texture, etc., are relevant parameters with potential as indicators of soil health which can strongly alter contaminant bioavailability and, hence, (eco)toxicity in soil. Unfortunately, for most environmental legislations, the total concentration of the contaminants is the key factor for the Environmental Risk Assessment (ERA) of contaminated soils. Nevertheless, such aspect (i.e., total concentration of soil contaminants) is not enough to properly assess or estimate the potential harmful impact of contaminants on soil functioning [10]. As a matter of fact, the mobility and bioavailability of soil contaminants both play a determining role in their uptake by organisms and, therefore, their (eco)toxicity [11, 12]. Contaminant bioavailability is possibly a much more relevant factor, compared to total contaminant concentrations, for a proper soil protection and risk assessment, as it represents the fraction that can be taken up by soil organisms and/or be leached to other environmental compartments. Specifically, metal(oild) bioavailability is mainly conditioned by soil physicochemical properties such as pH, redox potential, moisture content, organic matter content, clay content, the presence of anionic compounds, etc. [13]. Regarding organic contaminants, their bioavailability and mobility depend largely on their solubility, hydrophobicity and interaction, through a variety of physicochemical processes, with the mineral and organic fraction of the soil matrix, e.g. via sorption and complexation mechanisms [12]. Therefore, it is recommended to always include the determination of the bioavailable fraction of the contaminants when assessing soil health and, in particular, during the selection of a soil remediation option and when monitoring the effectiveness of the chosen remediation methodology. Nonetheless, regrettably, there is no consensus about the best way to accurately estimate soil contaminant bioavailability. For metallic contaminants, the most widely accepted methodology is the use of chemical extractants like, for instance, inorganic salts, e.g. NaNO3, (NH4)2SO4 and CaCl2 [14-16]. In any event, for a proper assessment of the impact of soil contaminants on soil health (Figure 1), apart from total and bioavailable contaminant concentrations, biological indicators are required, as they directly reflect the impact of the contaminants on the soil biota [10]. Among them, soil microbial properties are particularly adequate for this purpose, as microorganisms play a key role in many soil functions and the provision of ecosystem services, while quickly delivering ecologically relevant information that integrates many environmental factors [17, 18]. Similarly, standardized (eco)toxicological bioassays with model organisms have been developed and proposed for soil (eco)toxicity studies, including, for instance, Eisenia fetida [19], Vibrio fisheri [20], Lactuca sativa [21] and Cucumis sativus [22].
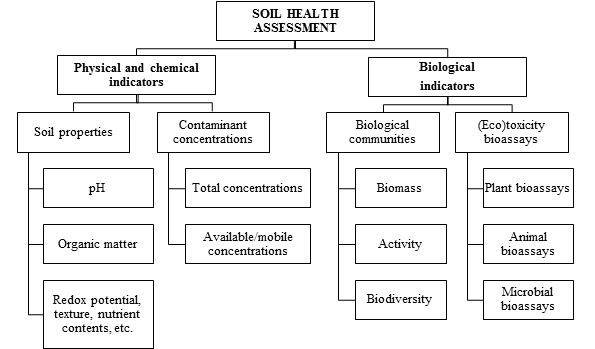
Figure 1: Soil health assessment.
3. From Physicochemical Techniques to Gentle Remediation Options
Traditionally, physicochemical methods, such as excavation and transportation to a controlled landfill, incineration, chemical washing, vitrification, etc. [23], have been used to remediate contaminated soils, However, many of these physicochemical methods of soil remediation have substantial disadvantages and limitations, such as their high-cost, which frequently compromises their applicability [24], and, above all, the fact that they are often environmentally disruptive. Then, although their application can many times effectively remove and/or immobilize the target contaminants, in numerous cases the ecological status of the remediated soil is not improved during the remediation process; on the contrary, the application of these traditional physicochemical methods often leads to a partial or total destruction of the soil biota with concomitant adverse effects on soil processes, functions and health [23]. On the other hand, the interaction between organic and inorganic contaminants in co-contaminated sites makes their remediation by physicochemical techniques more complex [25].
Due to the abovementioned limitations of traditional physicochemical remediation technologies, in the last decades a variety of biological and more sustainable remediation technologies, often termed Gentle Remediation Options (GROs), have emerged (Figure 2). In contraposition to conventional physicochemical remediation techniques, GROs are commonly less invasive and more respectful of the soil environment and its biota [26]. Many of these GROs aim at simultaneously (i) decrease the total and/or bioavailable concentration of soil contaminants; (ii) recover soil functionality; and, sometimes, (iii) produce renewable resources for the bio-based economy [27, 28]. Gentle remediation options often bring social, economic and environmental benefits by integrating sustainable remediation options (e.g., bioremediation, phytoremediation, vermiremediation) with the generation of economic revenues. In particular, the combination of phytoremediation with a profitable crop production, i.e. phytomanagement, has great potential for the recovery of contaminated sites, while providing a range of economic and other (e.g., provision of ecosystem services) benefits.
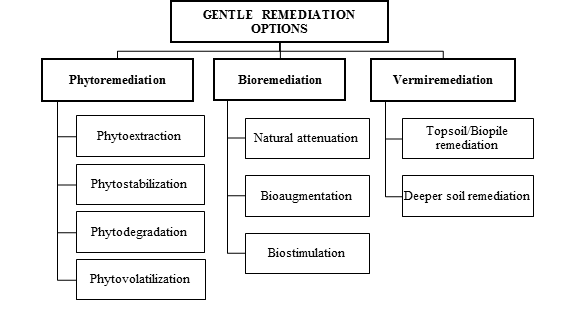
Figure 2: Gentle remediation options.
3.1 Phytoremediation
Phytoremediation has been defined as “the use of plants and associated microbes to reduce the concentration and/or toxic effects of contaminants in the environment” [29]. Due to its low installation and maintenance costs, as well as its many environmental benefits, [30], this phytotechnology can be applied in large field sites in which other remediation options are not cost-effective or practicable [31]. Phytoremediation techniques are suitable for the remediation of soils contaminated with both inorganic and/or organic compounds; however, they are most often applied to soils contaminated with metals. The two most common phytoremediation strategies, i.e. phytoextraction, phytostabilization, are described below.
3.1.1 Phytoextraction: Phytoextraction is a phytotechnology that uses the capacity of some plants to take up and translocate metal contaminants from soil to aboveground plant tissues. Subsequently, the aerial part of the plants can be harvested and, finally, incinerated, with potential benefits in terms of energy production and/or the recovery of high-added value metals [32]. For an effective phytoextraction, the selection of appropriate metal-tolerant plant species is a crucial aspect. Plants can be classified in three categories depending on their strategy to cope with metals: (i) excluders, which actively limit metal uptake and can then immobilize the metal contaminants in the rhizosphere; (ii) indicators, which maintain a metal concentration in their tissues that reflects soil metal concentrations; and (iii) accumulators, which actively take up and translocate metals from soil to their shoots, thus reaching metal concentrations in their aboveground tissues higher than those present in the contaminated soil. Inside this last group, hyperaccumulators are extremely specialized plants that can accumulate heavy metals in their aboveground tissues at remarkably high concentrations (1-10%) [33, 34].
Accumulators and hyperaccumulators have frequently been used for phytoextraction purposes. Noccaea caerulescens (f.k.a. Thlaspi caerulescens), for instance, has been widely studied due to its remarkable capacity to accumulate zinc and/or cadmium in its shoots [35, 36]. Some other commonly studied accumulators are Elsholtzia splendens (copper) [37], Sedum plumbizincicola (cadmium) [38] and Chenopodium spp. (chromium, nickel, cadmium) [39]. (Hyper)accumulators are certainly adapted to environments with high metal concentrations, but their growth rate and biomass are generally low. Therefore, alternatively, non-accumulator plant species but which can produce more aboveground biomass, are easier to cultivate and harvest, and show a better adaptability to prevailing environmental and climatic conditions, have also been used for phytoextraction purposes [23]. After all, the effectiveness of a phytoextraction process is determined not only by contaminant concentrations in aboveground tissues, but also shoot biomass [40]. Due to their faster growth rate, adaptability to environmental stress and high biomass, herbaceous plants are often preferred for phytoextraction purposes, in comparison to shrubs or trees [41]. Examples of plants with potential for phytoextraction strategies are: sunflower (Helianthus annuus), hemp (Cannabis sativa), and several species of the Brassica genus, such as Indian mustard (B. juncea), canola (B. napus) and turnip rape (B. rapa) [42-44].
Unfortunately, phytoextraction has serious limitations when it comes to its practical application in the field. The major drawback for the successful application of this phytotechnology is the great amount of time required to effectively extract the metals from the contaminated soil, particularly from those with medium and high levels of metal contamination [45]. Due to the low biomass characteristic of most hyperaccumulators, as well as the low metal uptake of non-accumulator plants that show high biomass production, a great number of harvests are required for a successful phytoextraction. Other limitations of phytoextraction are: (i) root depth, which narrows the applicability of this phytotechnology to surface soils; (ii) lack of well-known agronomic practices; and (iii) the incapability of most plants to accumulate more than one metal [46].
Another relevant aspect that cannot be neglected when applying phytoextraction strategies is the bioavailability of the metal contaminant. When applying this phytotechnology, it must always be taken into account that only a fraction of the total soil metal will be available for uptake by plants [47], which a priori is the only fraction that can be phytoextracted. Numerous studies have explored the utilization of chelating agents to increase metal bioavailability in order to maximize phytoextraction efficiency by high biomass plants [48-50]. However, this technology, known as chelator-induced phytoextraction, has raised environmental concerns derived from the risk of metal leaching to subsoil and groundwater and/or negative effects of persistent chelants on the soil biota [46,51,52]. In any case, it should be noted that if the goal of a given phytoextraction initiative is only the removal of the bioavailable fraction of the metal contaminant, the time required for the process will be significantly shorter, which is, as previously mentioned, the main critique to phytoextraction [53].
3.1.2 Phytostabilization: Phytostabilization, focused on metal immobilization in the rhizosphere, is a GRO with great potential for those soils with moderate or high levels of metal contamination. Such immobilization can be achieved through metal precipitation or absorption/adsorption in the plant roots [26, 54]. Indeed, besides metal absorption and/or adsorption in the root system, metal phytostabilization can also be achieved through the modification of the soil conditions: for instance, the root exudates of some plants have been reported to modify the rhizosphere pH and redox conditions, thus provoking the precipitation or complexation of potentially toxic metals [8, 55]. Thus, phytostabilization reduces the bioavailable fraction of metals in soil [46]. By reducing the bioavailability and mobility of metals in soil, the risk of contamination of groundwater by metal leaching is reduced, as well as the entry of the metal contaminants to the food chain [56]. Furthermore, a plant cover brings additional benefits to contaminated soils such as an increase in organic matter content, nutrients and soil biological activity; protection from soil erosion; improvement of soil structure; etc. [57, 58].
Apart from being metal tolerant, suitable plants for phytostabilization should have an extensive root system, produce a large amount of biomass, and show a low root-to-shoot metal translocation rate [59]. Many metal excluder plants, such as grasses (Agrostis stolonifera, Lolium perenne) and legumes (Trifolium repens, Medicago sativa, Ulex europaeus), have been effectively used to revegetate metal contaminated soils for phytostabilization purposes [34, 60-62]. Unlike for metal phytoextraction, shrubs and trees are commonly selected as suitable candidates for phytostabilization initiatives. Indeed, due to their capacity to stabilize metals in their massive root systems, tree and shrub species (e.g., Populus spp., Salix spp.) have been widely used for phytostabilization purposes [63, 64]. Interestingly, the use of trees can lower the risk of metal leaching by reducing the downward flow of water due to their high rates of transpiration [63].
The application of phytostabilization can be a challenge in highly degraded soils which, apart from metal contamination, present other problems like erosion, poor physical structure, shortage of essential nutrients and organic matter, etc. [34]. These problems are frequent in mine tailings and dumpsites, hampering the establishment of a healthy plant cover [65]. In this respect, for an effective phytostabilization in highly degraded soils, the use of organic and/or inorganic amendments is often recommended to facilitate plant establishment and growth. This methodology is usually termed assisted phytostabilization, aided phytostabilization or chemophytostabilization [59]. In aided phytostabilization, the promotion of plant growth can be achieved by raising soil pH, enhancing the organic matter content, providing essential nutrients, increasing the water holding capacity, reducing metal bioavailability, etc. [17, 66]. Besides, the utilization of organic and inorganic amendments opens the door to the recycling of wastes, residues and byproducts from diverse origins, in a context of Circular Economy [67]. Some amendments commonly used in aided phytostabilization studies are: animal slurry and manure, paper mill sludge, sewage sludge, urban solid wastes, litter, leonardite, lime (CaCO3), etc. [22, 60, 66, 68]. However, prior to their use, amendments should be thoroughly analyzed. In this respect, an exhaustive physical, chemical and biological characterization of the amendments is required to minimize/avoid the risk of introducing toxic compounds or potential human pathogens into the amended soil, with concomitant hazards for environmental and human health [69].
In any event, it must be emphasized that phytostabilization does not decrease the total concentration of metals in the soil (i.e., the metal contaminants remain in the soil) but only immobilizes them, thereby reducing their mobility and bioavailability. Consequently, there is always the possibility that the metal contaminants are later mobilized due to changes in the soil conditions, with potential adverse consequences in terms of (eco)toxicity and/or metal dispersion. Therefore, phytostabilization processes must always be subjected to long-term monitoring programs regarding metal bioavailability, (eco)toxicity and soil functioning [8]. The main limitation of phytostabilization for its practical application is the fact that current environmental legislations are normally based on total metal concentrations, not on bioavailable metal concentrations, and since this phytotechnology cannot reduce total metal concentrations below the reference critical values established by legislation, it is impractical from a legal point of view.
3.2 Phytomanagement
Despite the number of research papers on phytoremediation, its application at field scale is still limited. Some of the reasons for this phenomenon are, among others, the uncertainty around the required time-scales, the reproducibility of the results, and the current legal frameworks [26, 70]. As a matter of fact, many stakeholders perceive GROs in general, and phytoremediation in particular, as slow technologies which are difficult to apply and suited only for large and marginalized areas with low value [26, 28, 70]. Nonetheless, it must be emphasized that contaminated lands are an extensive and underutilized resource [71] which, when properly managed, can provide economic revenues and valuable ecosystem services. In this respect, phytomanagement encourages the use of plants with phytoremediation potential as part of an integrated site management which pursues, along with the mitigation of the risks derived from the presence of the contaminants, the accomplishment of economic, social and environmental benefits [46]. These benefits include the provision of green space and ecosystem services, the control of soil erosion and, above all, the generation of products and commodities (e.g., bioenergy, wood, biochar, biofortified products) [26, 28, 71]. For that purpose, fast growing, deep rooted and easily propagated high biomass plants are often used, such as agronomical and herbaceous crop plants and trees. Phytomanagement makes site remediation an attractive option for stakeholders due to the environmental, economic and social benefits that can be obtained, while mitigating the risk resulting from the presence of the contaminants. Then, phytomanagement has been proposed as a very appealing “holding strategy” until full site regeneration is possible [26].
3.3 Bioremediation
Bioremediation, or the use of microorganisms (mainly, bacteria and fungi) to clean up contaminated sites, is a sustainable option for the remediation of contaminated soils [72]. Although bioremediation can indeed be used for inorganic contaminants [73], its application is more frequent for organic contaminants such as mineral oils, petroleum hydrocarbons, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), pesticides, etc. [12]. There are three main approaches for the bioremediation of contaminated areas [74]: (i) natural attenuation, which is naturally carried out by the native microbial populations present in the contaminated area; (ii) bioaugmentation, which is based on the inoculation of selected microbial strains with the capacity to degrade the target contaminants at a fast rate; and (iii) biostimulation, which is focused on the modification of the environmental conditions (e.g., moisture, pH, nutrients, oxygen), in order to stimulate the biodegradation of the target contaminants.
In natural attenuation processes, the degradation of the contaminants is strongly determined by the (i) metabolic capacity of the native microbial populations; (ii) the physicochemical properties of the contaminated soil; and (iii) the chemical properties of the target contaminants. Under favorable conditions, some contaminants (e.g., short-chain petroleum hydrocarbons) show high levels of degradation by natural attenuation [22, 74]. However, the efficiency of natural attenuation for more recalcitrant compounds is very low or null, especially for aged contaminants [12].
In order to maximize the efficiency of bioremediation processes, the degrading capacity of indigenous microbial populations can be stimulated, via biostimulation strategies, by adjusting the supply of essential macro- and/or micronutrients, temperature, available oxygen, soil pH, redox potential, moisture, etc. [75]. However, the most common practice is probably the addition of nutrients, either in inorganic form [76] or as organic amendments such as sewage sludge, manure, compost, etc. [73, 77, 78]. Again, it must be taken into consideration that the rate of contaminant degradation will depend on the (i) physicochemical characteristics of the soil; (ii) specific degrading microbial populations present in the contaminated soil; and (iii) chemical nature of the contaminants themselves. Therefore, it is not surprising than the type and dose of the amendments (inorganic and/or organic) must always be carefully selected considering these three aspects [12]. Other practices include the addition of surfactants to increase contaminant availability [79], the application of biochar [80], and the growth of plants for phytostimulation purposes [22].
Bioaugmentation is focused on the inoculation of previously isolated and cultivated microbial strains, individually or as a consortium, to stimulate the biodegradation of the target organic contaminants. The inoculation of “cocktails” of degrading strains is more frequent, compared to the inoculation of individual strains, as microorganisms in consortium can combine different metabolic activities that complement each other from a bioremediation point of view [76, 81, 82]. The microbial strains used for bioaugmentation are commonly isolated from similarly contaminated soils, sometimes from the same soil to be remediated, in order to increase the probability of their survival and their capacity to express their biodegrading activity when inoculated in the contaminated soil [83, 84]. The genetic modification of bacterial strains to improve their biodegradation performance, by means of genetically optimizing the production of enzymes and metabolic pathways relevant for the biodegradation of the target contaminants, has also been studied [85, 86]. However, when selecting the different strains for the bioaugmentation consortium, their compatibility with each other and their ecological fitness in the soil under remediation must be taken into consideration. Actually, bioaugmentation initiatives fail quite often, mostly due to the incapability of the inoculated strains to compete and properly develop in the contaminated soil [12, 87].
3.4 Vermiremediation
Vermiremediation has been described as the use of earthworms for the removal of contaminants from soil [88]. Earthworms are known to burrow through the soil, mixing it in their guts [89], and, consequently, they are capable of changing the physicochemical and biological properties of the soil, such as nutrient availability, aeration, soil structure and, hence, the activity of soil microbial communities [90]. In addition, earthworms can increase the interaction between soil microbial communities and contaminants, thus facilitating the biodegradation of the target contaminants [91]. Some studies have investigated the interaction between earthworms and metal contaminants [92, 93], but vermiremediation is more commonly used for organic contaminants. Indeed, organic contaminants such as herbicides, PCBs, PAHs or, in general, petroleum-derived hydrocarbons have been successfully remediated through vermiremediation using a variety of earthworm species [94-97]. A particularly interesting earthworm species, Eisenia fetida, has been used for vermiremediation purposes [98], as well as bioindicator of metal ecotoxicity in soil [19, 99].
In order to successfully apply vermiremediation, several aspects need to be considered, such as the behavior of the earthworms, their nutritional requirements, the characteristics of the soil, and the nature of the contaminants themselves. According with their location in the soil, earthworms can be cataloged as epigeic, endogeic and anecic: epigeic earthworms, such as E. fetida, require high amounts of organic matter and, then, they live at or near the soil surface where they feed on leaf litter, decaying roots and dung. In consequence, they can (i) be used to remediate topsoil; and (ii) be inoculated in biopiles employed for bioremediation purposes, where little burrowing is necessary. Endogeic and anecic earthworms, e.g. Lumbricus terrestris, on the other hand, are better suited for the vermiremediation of deeper soil [91]. In order to guarantee the survival of the inoculated earthworms, sometimes it is required to first ameliorate soil contamination levels [100]. Certainly, extreme conditions (in terms of soil pH, salinity, contaminant bioavailability) or a lack of organic matter can complicate the establishment of the inoculated earthworms [92]. Organic amendments can then be used to reduce contaminant bioavailability, while adding organic matter and improving soil structure [101].
4. Mixed remediation technologies for mixed contamination
Mixed contamination, when inorganic and organic contaminants appear together, is present in many contaminated soils. Soils with mixed contamination combine the individual challenges from each individual class of contaminant with those derived from the combination of two or more types of contaminants with different properties. Regarding (eco)toxicity, synergistic effects on soil biota can occur from that combination. Likewise, from a remediation point of view, the efficiency of the applied techniques may be reduced by the presence of other types of contaminants or by the interaction between them [22]. For instance, in mixed contaminated soils, it has been reported that co-contamination with metals and organic compounds can cause metal immobilization [102] or, by contrast, an increase in metal mobility [25]. Therefore, the outcome of the interaction between different types of contaminants is site-specific, as it depends on the specific properties of the contaminated soil, as well as on the type and concentration of the contaminants themselves [103].
Regarding the effectiveness of remediation technologies, metals can provoke toxic effects on soil microbial communities, decreasing their abundance and/or activities [104] and, consequently, reducing their capacity to degrade the target organic contaminants [105]. In a phytoremediation experiment, it was observed that co-contamination with copper and pyrene decreased plant growth and the removal of pyrene, compared to the experiments performed with each contaminant individually [106]. As a result, the application of only one remediation technology might not be effective for soils with mixed contamination. Regrettably, many remediation studies are focused on just one type of contaminant, probably due to the complexity of tackling mixed contamination cases. Nevertheless, in the last years, more and more remediation studies have dealt with mixed-contaminated soils. For instance, bioaugmentation with a bacterial consortium has been reported to be effective for the remediation of soils simultaneously contaminated with Cr (VI) and lindane [107] or pyrene [108].
The combination of plants and bacteria for phytoremediation purposes offers great potential for soils with mixed contamination [109]. The presence of plants can enhance the activity and functional diversity of soil microbial communities by releasing root exudates and improving the conditions for microbial growth in the rhizosphere [62, 110]. Root exudates create a nutrient-rich environment which can influence the behavior of metals [111] and enhance the biodegradation of organic contaminants [112]. The combination of vermiremediation with phytoremediation and bioremediation has been successfully tested for the remediation of soils contaminated with metals and organic contaminants [85, 113-115]. The combination of these biological remediation technologies appears promising for mixed-contaminated soils. Expectedly, factors such as type of soil, chemical properties of the contaminants, and the biological species selected for the remediation (e.g., the specific species of plants, bacteria and earthworms) will strongly modify the outcome of the remediation process.
As mentioned above, the use of amendments is very common during the implementation of GROs. In particular, organic amendments provide soil nutrients and organic matter, improve soil structure, enhance water holding capacity, alter contaminant bioavailability, etc. In consequence, organic amendment can alleviate toxicity for the species involved in the biological remediation of mixed-contaminated soils. Many studies have reported the benefits of using organic amendments during the implementation of GROs [22, 114, 116, 117]. Other authors have combined the application of nanoremediation (with, for instance, nanoscale zero valent iron) with GROs with mixed results [118-121]. The term nanoremediation refers to the application of metallic nanoparticles (<100 nm) for the remediation of contaminated sites [122]. In particular, the use of zero-valent iron nanoparticles (nZVI) has caught the attention of the scientific community for the remediation of contaminated waters and soils [123]. Zero-valent iron nanoparticles have an iron core and a shell of iron oxide and, due to their small size, show a very high surface/volume ratio [124]. The iron core acts as the electron donor, while the shell plays coordination and electrostatic functions, attracting and adsorbing charged ions [125]. Zero-valent iron nanoparticles have been applied for the remediation of both organic and inorganic contaminants. Regarding inorganic contaminants, nanoparticles can form complexes with soil metals, thus decreasing their bioavailability [122]. Besides, by changing the redox potential of the soil, nZVI can alter the speciation of the metal contaminants, decreasing their bioavailability and (eco)toxicity. For instance, nZVI can reduce Cr (VI) to Cr (III), a less toxic and bioavailable form [126]. On the other hand, nZVI have been reported to effectively remediate soils contaminated with organic compounds, especially those contaminated with organochlorinated compounds [127, 128]. Nevertheless, the use of nanoparticles for soil remediation has been questioned due to their potential negative impact on soil biota. In any case, there is still a lack of information regarding the mobility, bioaccumulation, dynamics and (eco)toxicity of nZVI in the soil environment [129, 130]. Zero-valent iron nanoparticles have been described to provoke toxicity through two mechanisms: (i) physical damage by direct contact, disrupting cell membrane architecture and increasing permeability; and (ii) oxidative stress, leading to molecular and biochemical destruction [131]. Adverse effects of nZVI have been reported in plants [132], animals [133] and microbial communities [134]. As expected, the potential effects of nZVI on soil biota are highly conditioned by the soil type and environmental conditions [121,131]. Therefore, it is essential to perform an assessment of the potential effects of nZVI on soil biota prior to their application under real field conditions, in order to first establish a safe, non-toxic and effective concentration for nanoremediation purposes [130]. Indeed, despite their proven effectiveness for remediation, the effect of nanoparticles on soil biota, including the biological species used for remediation, is yet full of uncertainties. Then, the potential adverse impact of nZVI on soil organisms must be tested prior to their use, alone or in combination with GROs.
In conclusion, when facing a mixed-contaminated soil, it is essential to first take into account a variety of aspects, such as soil type, nature of the contaminants, compatibility of the remediation technologies, etc. in order to then be able to apply a tailor-made strategy for each case. Besides, as indicated above, it is crucial to assess the efficiency of the remediation methods not only in terms of the decrease in contaminant concentrations but also according to the recovery of soil health and ecosystem services [135-137].
5. Conclusions
Economically-feasible sustainable biological methods of soil remediation (e.g., phytoremediation, phytomanagement, bioremediation, vermiremediation) are being developed to: (i) efficiently remove contaminants from soil; (ii) decrease their bioavailability, mobility, (eco)toxicity and potential risks for environmental and human health; and, simultaneously, (iii) recover soil health and the provision of ecosystem services. The remediation of mixed-contaminated soils is particularly challenging, as it combines the individual challenges for each individual contaminant with those derived from their combination. Interestingly, the combination of biological and non-biological methods offers great potential for the remediation of mixed-contaminated soils.
Acknowledgements
This research was financially supported by projects AGL2015-64481-C2-1-R and AGL2016-76592-R from MINECO, PhytoSUDOE-SOE1/P5/E0189 and GV ITO18-16. RGL received a pre-doctoral grant from the Basque Government.
Conflict of Interest
The authors declare no competing interests.
References
- Brevik EC, Cerdà A, Mataix-Solera J, et al. The interdisciplinary nature of Soil. Soil 1 (2015): 117-129.
- Epelde L, Ma Becerril J, Alkorta I, et al. Heavy metal phytoremediation: microbial indicators of soil health for the assessment of remediation efficiency. In: Singh A, Kuhad CR, Ward PO, editors. Advances in Applied Bioremediation, Springer Berlin Heidelberg (2009): 299-313.
- Van Liedekerke M, Prokop G, Rabl-Berger S, et al. Progress in the management of contaminated sites in Europe. Publications Office of the European Union (2014).
- Mansour SA. Evaluation of residual pesticides and heavy metals levels in conventionally and organically farmed potato tubers in Egypt. Sustainable Potato Production: Global Case Studies, Springer Netherlands, Dordrecht (2012): 493-506.
- Khan AB, Kathi S. Evaluation of heavy metal and total petroleum hydrocarbon contamination of roadside surface soil. International Journal of Environmental Science and Technology 11 (2014): 2259-2270.
- Agnello AC, Bagard M, Van Hullebusch ED, et al. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Science of the Total Environment 563 (2016): 693-703.
- SUPERFUND: EPA’s estimated costs to remediate existing sites exceed current funding levels, and more sites are expected to be added to the national priorities list. United States Government Accountability Office (2010).
- Gómez-Sagasti MT, Alkorta I, Becerril JM, et al. Microbial monitoring of the recovery of soil quality during heavy metal phytoremediation. Water, Air, Soil Pollution 223 (2012): 3249-3262.
- Doran JW, Zeiss MR. Soil health and sustainability: Managing the biotic component of soil quality. Applied Soil Ecology 15 (2000): 3-11.
- Alvarenga P, Ferreira C, Mourinha C, et al. Chemical and ecotoxicological effects of the use of drinking-water treatment residuals for the remediation of soils degraded by mining activities. Ecotoxicology and Environmental Safety 161 (2018): 281-289.
- Vamerali T, Bandiera M, Mosca G. Field crops for phytoremediation of metal-contaminated land. A review. Environmental Chemistry Letters 8 (2010): 1-17.
- Megharaj M, Ramakrishnan B, Venkateswarlu K, et al. Bioremediation approaches for organic pollutants: A critical perspective. Environment International 37 (2011): 1362-1375.
- Vangronsveld J, Cunningham SD. Metal-contaminated soils: In situ inactivation and phytorestoration. Springer-Verlag, Georgetown (1998).
- Madejón E, de Mora AP, Felipe E, et al. Soil amendments reduce trace element solubility in a contaminated soil and allow regrowth of natural vegetation. Environmental Pollution 139 (2006): 40-52.
- Menzies NW, Donn MJ, Kopittke PM. Evaluation of extractants for estimation of the phytoavailable trace metals in soils. Environmental Pollution 145 (2007): 121-130.
- Vázquez S, Moreno E, Carpena RO. Bioavailability of metals and As from acidified multicontaminated soils: Use of white lupin to validate several extraction methods. Environmental Geochemistry and Health 30 (2008): 193-198.
- Epelde L, Becerril JM, Mijangos I, et al. Evaluation of the efficiency of a phytostabilization process with biological indicators of soil health. Journal of Environment Quality 38 (2009): 2041.
- Jeffery S, Gardi C, Jones A, et al. European atlas of soil biodiversity. Publication Office of the European Union, Luxembourg (2010).
- Irizar A, Rodríguez MP, Izquierdo A, et al. Effects of soil organic matter content on cadmium toxicity in Eisenia fetida: implications for the use of biomarkers and standard toxicity tests. Archives of Environmental Contamination and Toxicology 68 (2015): 181-192.
- Abbas M, Adil M, Ehtisham-ul-Haque S, et al. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Science of the Total Environment 626 (2018): 1295-1309.
- Valerio ME, García JF, Peinado FM. Determination of phytotoxicity of soluble elements in soils, based on a bioassay with lettuce (Lactuca sativa). Science of the Total Environment 378 (2007): 63-66.
- Lacalle RG, Gómez-Sagasti MT, Artetxe U, et al. Brassica napus has a key role in the recovery of the health of soils contaminated with metals and diesel by rhizoremediation. Science of The Total Environment 618 (2018): 347-356.
- Ali H, Khan E, Sajad MA. Phytoremediation of heavy metals-Concepts and applications. Chemosphere 91 (2013): 869-881.
- Houben D, Pircar J, Sonnet P. Heavy metal immobilization by cost-effective amendments in a contaminated soil: Effects on metal leaching and phytoavailability. Journal of Geochemical Exploration 123 (2012): 87-94.
- Dubé J-S, Galvez-Cloutier R, Winiarski T. Heavy metal transport in soil contaminated by residual light non-aqueous phase liquids (LNAPLs). Canadian Geotechnical Journal 39 (2002): 279-292.
- Cundy AB, Bardos RP, Puschenreiter M, et al. Brownfields to green fields: Realising wider benefits from practical contaminant phytomanagement strategies. Journal of Environmental Management 184 (2016): 67-77.
- Kumpiene J, Bert V, Dimitriou I, et al. Selecting chemical and ecotoxicological test batteries for risk assessment of trace element-contaminated soils (phyto)managed by gentle remediation options (GRO). Science of The Total Environment 496 (2014): 510-522.
- Kidd P, Mench M, Álvarez-López V, et al. Agronomic practices for improving gentle remediation of trace element-contaminated soils. International Journal of Phytoremediation 17 (2015): 1005-1037.
- Greipsson S. Phytoremediation. Nat Educ Knowl 2 (2011): 7.
- Van Aken B. Transgenic plants for enhanced phytoremediation of toxic explosives. Current Opinion in Biotechnology 20 (2009): 231-236.
- Garbisu C, Alkorta I. Basic concepts on heavy metal soil bioremediation. Eur J Min Proc Environ Protect 3 (2003): 58-66.
- Chaney RL, Baker AJM, Morel JL. The long road to developing agromining/phytomining (2018): 1-17.
- Baker AJM. Accumulators and excluders - strategies in the response of plants to heavy metals. Journal of Plant Nutrition 3 (1981): 643-654.
- Barrutia O, Artetxe U, Hernández A, et al. Native Plant Communities in an Abandoned Pb-Zn Mining Area of Northern Spain: Implications for Phytoremediation and Germplasm Preservation. International Journal of Phytoremediation 13 (2011): 256-270.
- Hernández-Allica J, Becerril JM, Zárate O, et al. Assessment of the efficiency of a metal phytoextraction process with biological indicators of soil health. Plant and Soil 281 (2006): 147-158.
- Jacobs A, Noret N, Van Baekel A, et al. Influence of edaphic conditions and nitrogen fertilizers on cadmium and zinc phytoextraction efficiency of Noccaea caerulescens. Science of The Total Environment 665 (2019): 649-659.
- Chen Y, Wang Y, Wu W, et al. Impacts of chelate-assisted phytoremediation on microbial community composition in the rhizosphere of a copper accumulator and non-accumulator. Science of The Total Environment 356 (2006): 247-255.
- Cui H, Fan Y, Yang J, et al. In situ phytoextraction of copper and cadmium and its biological impacts in acidic soil. Chemosphere 161 (2016): 233-241.
- Bhargava A, Shukla S, Srivastava J, et al. Chenopodium: a prospective plant for phytoextraction. Acta Physiologiae Plantarum 30 (2007): 111-120.
- Li JT, Liao B, Lan CY, et al. Cadmium tolerance and accumulation in cultivars of a high-biomass tropical tree (Averrhoa carambola) and its potential for phytoextraction. Journal of Environment Quality 39 (2010): 1262.
- Malik RN, Husain SZ, Nazir I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak J Bot 42 (2010): 291-301.
- Meers E, Ruttens A, Hopgood M, et al. Potential of Brassica rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere 61 (2005): 561-572.
- Zalewska M, Nogalska A. Phytoextraction potential of sunflower and white mustard plants in zinc-contaminated soil. Chilean Journal of Agricultural Research 74 (2014) 485-489.
- Solhi M, Shareatmadari H, Hajabbasi MA. Lead and zinc extraction potential of two common crop plants, Helianthus annuus and Brassica napus. Water, Air, and Soil Pollution 167 (2005): 59-71.
- Zhao FJ, Lombi E, McGrath SP. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant and Soil 249 (2003): 37-43.
- Burges A, Alkorta I, Epelde L, et al. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. International Journal of Phytoremediation 20 (2018): 384-397.
- Lasat MM. Phytoextraction of Toxic Metals. Journal of Environment Quality 31 (2002): 109.
- Salt DE, Blaylock M, Kumar NPBA, et al. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Nature Biotechnology 13 (1995): 468-474.
- Barrutia O, Garbisu C, Hernández-Allica J, et al. Differences in EDTA-assisted metal phytoextraction between metallicolous and non-metallicolous accessions of Rumex acetosa Environmental Pollution 158 (2010): 1710-1715.
- Bian X, Cui J, Tang B, et al. Chelant-induced phytoextraction of heavy metals from contaminated soils: a review. Polish Journal of Environmental Studies 27 (2018): 2417-2424.
- Marques APGC, Rangel AOSS, Castro PML. Remediation of heavy metal contaminated soils: phytoremediation as a potentially promising clean-up technology. Critical Reviews in Environmental Science and Technology 39 (2009): 622-654.
- Yang L, Wang G, Cheng Z, et al. Influence of the application of chelant EDDS on soil enzymatic activity and microbial community structure. Journal of Hazardous Materials 262 (2013): 561-570.
- Vangronsveld J, Herzig R, Weyens N, et al. Phytoremediation of contaminated soils and groundwater: lessons from the field. Environmental Science and Pollution Research 16 (2009): 765-794.
- Hrynkiewicz K, Zloch M, Kowalkowski T, et al. Efficiency of microbially assisted phytoremediation of heavy-metal contaminated soils. Environmental Reviews 26 (2018): 316-332.
- Wu G, Kang H, Zhang X, et al. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. Journal of Hazardous Materials 174 (2010): 1-8.
- Raskin I, Ensley BD, Burt D. Phytoremediation of toxic metals: using plants to clean up the environment. J. Wiley (2000).
- Mench M, Bussière S, Boisson J, et al. Progress in remediation and revegetation of the barren Jales gold mine spoil after in situ treatments. Plant and Soil 249 (2003): 187-202.
- Arienzo M, Adamo P, Cozzolino V. The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Science of The Total Environment 319 (2004): 13-25.
- Alkorta I, Becerril JM, Garbisu C. Phytostabilization of metal contaminated soils. Reviews on Environmental Health 25 (2010): 135-146.
- Pérez-de-Mora A, Burgos P, Madejón E, et al. Microbial community structure and function in a soil contaminated by heavy metals: effects of plant growth and different amendments. Soil Biology and Biochemistry 38 (2006): 327-341.
- Bidar G, Garçon G, Pruvot C, et al. Behavior of Trifolium repens and Lolium perenne growing in a heavy metal contaminated field: Plant metal concentration and phytotoxicity. Environmental Pollution 147 (2007): 546-553.
- Barrutia O, Garbisu C, Epelde L, et al. Plant tolerance to diesel minimizes its impact on soil microbial characteristics during rhizoremediation of diesel-contaminated soils. Science of The Total Environment 409 (2011): 4087-4093.
- Pulford ID, Watson C. Phytoremediation of heavy metal-contaminated land by trees: a review. Environment International 29 (2003): 529-540.
- Vamerali T, Bandiera M, Coletto L, et al. Phytoremediation trials on metal- and arsenic-contaminated pyrite wastes (Torviscosa, Italy). Environmental Pollution 157 (2009): 887-894.
- Burges A, Epelde L, Benito G, et al. Enhancement of ecosystem services during endophyte-assisted aided phytostabilization of metal contaminated mine soil. Science of the Total Environment 562 (2016): 480-492.
- Alvarenga P, Gonçalves APP, Fernandes RMM, et al. Organic residues as immobilizing agents in aided phytostabilization: (I) Effects on soil chemical characteristics. Chemosphere 74 (2009): 1292-1300.
- Míguez F, Gómez-Sagasti MT, Hernández A, et al. In situ phytomanagement with Brassica napus and bio-stabilised municipal solid wastes is a suitable strategy for redevelopment of vacant urban land. Urban Forestry and Urban Greening 47 (2020): 126550.
- Galende MA, Becerril JM, Barrutia O, et al. Field assessment of the effectiveness of organic amendments for aided phytostabilization of a Pb–Zn contaminated mine soil. Journal of Geochemical Exploration 145 (2014): 181-189.
- Goss MJ, Tubeileh A, Goorahoo D. A review of the use of organic amendments and the risk to human health. Advances in Agronomy 120 (2013): 275-379.
- Mench M, Lepp N, Bert V, et al. Successes and limitations of phytotechnologies at field scale: outcomes, assessment and outlook from COST Action 859. Journal of Soils and Sediments 10 (2010): 1039-1070.
- Evangelou MWH, Papazoglou EG, Robinson BH, Schulin R. Phytomanagement: phytoremediation and the production of biomass for economic revenue on contaminated land. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L, editors. Phytoremediation, Springer International Publishing, Cham (2015): 115-132.
- Fingerman M, Nagabhushanam R. Bioremediation of aquatic and terrestrial ecosystems. Science Publishers Inc, Enfield (2016).
- Park JH, Lamb D, Paneerselvam P, et al. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. Journal of Hazardous Materials 185 (2011): 549-574.
- Bento FM, Camargo FAO, Okeke BC, et al. Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresource Technology 96 (2005): 1049-1055.
- Carberry JB, Wik J. Comparison of ex situ and in situ bioremediation of unsaturated soils contaminated by petroleum. Journal of Environmental Science and Health Part A, Toxic/Hazardous Substances and Environmental Engineering 36 (2001): 1491-1503.
- Ramadass K, Megharaj M, Venkateswarlu K, et al. Bioavailability of weathered hydrocarbons in engine oil-contaminated soil: Impact of bioaugmentation mediated by Pseudomonas on bioremediation. Science of The Total Environment 636 (2018): 968-974.
- Ros M, Rodríguez I, García C, et al. Microbial communities involved in the bioremediation of an aged recalcitrant hydrocarbon polluted soil by using organic amendments. Bioresource Technology 101 (2010): 6916-6923.
- Lee S-H, Oh B-I, Kim J. Effect of various amendments on heavy mineral oil bioremediation and soil microbial activity. Bioresource Technology 99 (2008): 2578-2587.
- Zeng Z, Liu Y, Zhong H, et al. Mechanisms for rhamnolipids-mediated biodegradation of hydrophobic organic compounds. Science of The Total Environment 634 (2018): 1-11.
- Kong L, Gao Y, Zhou Q, et al. Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. Journal of Hazardous Materials 343 (2018): 276-284.
- Fuentes MS, Raimondo EE, Amoroso MJ, et al. Removal of a mixture of pesticides by a Streptomyces consortium: Influence of different soil systems. Chemosphere 173 (2017): 359-367.
- Shen T, Pi Y, Bao M, et al. Biodegradation of different petroleum hydrocarbons by free and immobilized microbial consortia. Environmental Science: Processes and Impacts 17 (2015): 2022-2033.
- Alisi C, Musella R, Tasso F, et al. Bioremediation of diesel oil in a co-contaminated soil by bioaugmentation with a microbial formula tailored with native strains selected for heavy metals resistance. Science of The Total Environment 407 (2009): 3024-3032.
- Tahir U, Yasmin A, Khan UH. Phytoremediation: Potential flora for synthetic dyestuff metabolism. Journal of King Saud University - Science 28 (2016): 119-130.
- Fernández-Luqueño F, Valenzuela-Encinas C, Marsch R, et al. Microbial communities to mitigate contamination of PAHs in soil - possibilities and challenges: a review. Environmental Science and Pollution Research 18 (2011): 12-30.
- Pieper DH, Reineke W. Engineering bacteria for bioremediation. Current Opinion in Biotechnology 11 (2000): 262-270.
- Polti MA, Aparicio JD, Benimeli CS, et al. Simultaneous bioremediation of Cr(VI) and lindane in soil by actinobacteria. International Biodeterioration and Biodegradation 88 (2014): 48-55.
- Sinha RK, Bharambe G, Ryan D. Converting wasteland into wonderland by earthworms-a low-cost nature’s technology for soil remediation: a case study of vermiremediation of PAHs contaminated soil. The Environmentalist 28 (2008): 466-475.
- Eijsackers H, Van Gestel CAM, De Jonge S, et al. Polycyclic aromatic hydrocarbon-polluted dredged peat sediments and earthworms: a mutual interference. Ecotoxicology 10 (2001): 35-50.
- Rodriguez-Campos J, Dendooven L, Alvarez-Bernal D, et al. Potential of earthworms to accelerate removal of organic contaminants from soil: A review. Applied Soil Ecology 79 (2014): 10-25.
- Hickman ZA, Reid BJ. Increased microbial catabolic activity in diesel contaminated soil following addition of earthworms (Dendrobaena veneta) and compost. Soil Biology and Biochemistry 40 (2008): 2970-2976.
- Eijsackers H. Earthworms as colonisers: primary colonisation of contaminated land, and sediment and soil waste deposits. Science of The Total Environment 408 (2010): 1759-1769.
- Kavehei A, Hose GC, Gore DB. Effects of red earthworms (Eisenia fetida) on leachability of lead minerals in soil. Environmental Pollution 237 (2018): 851-857.
- Schaefer M, Juliane F. The influence of earthworms and organic additives on the biodegradation of oil contaminated soil. Applied Soil Ecology 36 (2007): 53-62.
- Kersanté A, Martin-Laurent F, Soulas G, et al. Interactions of earthworms with atrazine-degrading bacteria in an agricultural soil. FEMS Microbiology Ecology 57 (2006): 192-205.
- Natal-da-Luz T, Lee I, Verweij RA, et al. Influence of earthworm activity on microbial communities related with the degradation of persistent pollutants. Environmental Toxicology and Chemistry 31 (2012): 794-803.
- Luepromchai E, Singer AC, Yang C-H, et al. Interactions of earthworms with indigenous and bioaugmented PCB-degrading bacteria. FEMS Microbiology Ecology 41 (2002): 191-197.
- Chachina SB, Voronkova NA, Baklanova ON. Biological remediation of the petroleum and diesel contaminated soil with earthworms Eisenia fetida. Procedia Engineering 152 (2016): 122-133.
- Shin K-H, Kim J-Y, Kim K-W. Earthworm toxicity test for the monitoring arsenic and heavy metal-containing mine tailings. Environmental Engineering Science 24 (2007): 1257-1265.
- Tejada M, Masciandaro G. Application of organic wastes on a benzo(a)pyrene polluted soil. Response of soil biochemical properties and role of Eisenia fetida. Ecotoxicology and Environmental Safety 74 (2011): 668-674.
- Elliston T, Oliver IW. Ecotoxicological assessments of biochar additions to soil employing earthworm species Eisenia fetida and Lumbricus terrestris. Environmental Science and Pollution Research (2019).
- Galvez-Cloutier R, Dubee J-S. Impact of tesidual NAPL on water flow and heavy metal transfer in a multimodal grain size soil under saturation conditions: implications for contaminant mobility. In: Sara M, Everett L, editors. Evaluation and remediation of low permeability and dual porosity environments, ASTM International, West Conshohocken (2002): 126-137.
- Chirakkara RA, Cameselle C, Reddy KR. Assessing the applicability of phytoremediation of soils with mixed organic and heavy metal contaminants. Reviews in Environmental Science and Bio/Technology 15 (2016): 299-326.
- Khan S, El-Latif Hesham A, Qiao M, et al. Effects of Cd and Pb on soil microbial community structure and activities. Environmental Science and Pollution Research 17 (2010): 288-296.
- Sandrin TR, Maier RM. Impact of metals on the biodegradation of organic pollutants. Environmental Health Perspectives 111 (2003): 1093-1101.
- Chigbo C, Batty L, Bartlett R. Interactions of copper and pyrene on phytoremediation potential of Brassica juncea in copper–pyrene co-contaminated soil. Chemosphere 90 (2013): 2542-2548.
- Aparicio JD, Raimondo EE, Gil RA, et al. Actinobacteria consortium as an efficient biotechnological tool for mixed polluted soil reclamation: Experimental factorial design for bioremediation process optimization. Journal of Hazardous Materials 342 (2018): 408-417.
- Wang C, Gu L, Ge S, et al. Remediation potential of immobilized bacterial consortium with biochar as carrier in pyrene-Cr(VI) co-contaminated soil. Environmental Technology 40 (2019): 2345-2353.
- Batty LC, Dolan C. The potential use of phytoremediation for sites with mixed organic and inorganic contamination. Critical Reviews in Environmental Science and Technology 43 (2013): 217-259.
- Balseiro-Romero M, Gkorezis P, Kidd PS, et al. Use of plant growth promoting bacterial strains to improve Cytisus striatus and Lupinus luteus development for potential application in phytoremediation. Science of The Total Environment 581-582 (2017): 676-688.
- Kidd P, Barceló J, Bernal MP, et al. Trace element behaviour at the root–soil interface: Implications in phytoremediation. Environmental and Experimental Botany 67 (2009): 243-259.
- Kuiper I, Lagendijk EL, Bloemberg GV, et al. Rhizoremediation: a beneficial plant-microbe interaction. Molecular Plant-Microbe Interactions 17 (2004): 6-15.
- Sivaram AK, Logeshwaran P, Lockington R, et al. Phytoremediation efficacy assessment of polycyclic aromatic hydrocarbons contaminated soils using garden pea (Pisum sativum) and earthworms (Eisenia fetida). Chemosphere 229 (2019): 227-235.
- Elyamine A, Moussa M, Ismael M, et al. Earthworms, rice straw, and plant interactions change the organic connections in soil and promote the decontamination of cadmium in soil. International Journal of Environmental Research and Public Health 15 (2018): 2398.
- Martinkosky L, Barkley J, Sabadell G, et al. Earthworms (Eisenia fetida) demonstrate potential for use in soil bioremediation by increasing the degradation rates of heavy crude oil hydrocarbons. Science of The Total Environment 580 (2017): 734-743.
- Marchand C, Mench M, Jani Y, et al. Pilot scale aided-phytoremediation of a co-contaminated soil. Science of The Total Environment 618 (2018): 753-764.
- Reddy KR, Amaya-Santos G, Yargicoglu E, et al. Phytoremediation of heavy metals and PAHs at slag fill site: three-year field-scale investigation. International Journal of Geotechnical Engineering (2017): 1-16.
- Su H, Fang Z, Tsang PE, et al. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environmental Pollution 214 (2016): 94-100.
- Huang D, Xue W, Zeng G, et al. Immobilization of Cd in river sediments by sodium alginate modified nanoscale zero-valent iron: Impact on enzyme activities and microbial community diversity. Water Research 106 (2016): 15-25.
- Huang D, Qin X, Peng Z, et al. Nanoscale zero-valent iron assisted phytoremediation of Pb in sediment: Impacts on metal accumulation and antioxidative system of Lolium perenne. Ecotoxicology and Environmental Safety 153 (2018): 229-237.
- Gómez-Sagasti MT, Epelde L, Anza M, et al. The impact of nanoscale zero-valent iron particles on soil microbial communities is soil dependent. Journal of Hazardous Materials 364 (2019): 591-599.
- Gil-Díaz M, Pinilla P, Alonso J, et al. Viability of a nanoremediation process in single or multi-metal(loid) contaminated soils. Journal of Hazardous Materials 321 (2017): 812-819.
- Medina-Pérez G, Fernández-Luqueño F, Vazquez-Nuñez E, et al. Remediating polluted soils using nanotechnologies: Environmental benefits and risks. Polish Journal of Environmental Studies 28 (2019): 1013-1030.
- Li S, Wang W, Liang F, et al. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. Journal of Hazardous Materials 322 (2017): 163-171.
- Li XQ, Zhang WX. Sequestration of metal cations with zerovalent iron nanoparticles - A study with high resolution x-ray photoelectron spectroscopy (HR-XPS). Journal of Physical Chemistry C 111 (2007): 6939-6946.
- Singh R, Misra V, Singh RP. Synthesis, characterization and role of zero-valent iron nanoparticle in removal of hexavalent chromium from chromium-spiked soil. Journal of Nanoparticle Research 13 (2011): 4063-4073.
- Elliott DW, Lien H-L, Zhang W-X. Degradation of lindane by zero-valent iron nanoparticles. Journal of Environmental Engineering 135 (2009): 317-324.
- Yang S-C, Lei M, Chen T-B, et al. Application of zerovalent iron (Fe0) to enhance degradation of HCHs and DDX in soil from a former organochlorine pesticides manufacturing plant. Chemosphere 79 (2010): 727-732.
- Machado S, Stawinski W, Slonina P, et al. Application of green zero-valent iron nanoparticles to the remediation of soils contaminated with ibuprofen. Science of The Total Environment 461-462 (2013): 323-329.
- Patil SS, Shedbalkar UU, Truskewycz A, et al. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environmental Technology and Innovation 5 (2016): 10-21.
- Xie Y, Dong H, Zeng G, et al. The interactions between nanoscale zero-valent iron and microbes in the subsurface environment: A review. Journal of Hazardous Materials 321 (2017): 390-407.
- Ma X, Gurung A, Deng Y. Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species. Science of The Total Environment 443 (2013): 844-849.
- Stefaniuk M, Oleszczuk P, Ok YS. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chemical Engineering Journal 287 (2016): 618-632.
- Fajardo C, Ortíz LT, Rodríguez-Membibre ML, et al. Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: A molecular approach. Chemosphere 86 (2012): 802-808.
- Epelde L, Burges A, Mijangos I, et al. Microbial properties and attributes of ecological relevance for soil quality monitoring during a chemical stabilization field study. Applied Soil Ecology 75 (2014): 1-12.
- Burges A, Epelde L, Blanco F, et al. Ecosystem services and plant physiological status during endophyte-assisted phytoremediation of metal contaminated soil. Science of the Total Environment 584-585 (2017): 329-338.
- Epelde L, Becerril JM, Alkorta I, et al. Adaptive long-term monitoring of soil health in metal phytostabilization: ecological attributes and ecosystem services based on soil microbial parameters. International Journal of Phytoremediation 16 (2014): 971-981.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 