HPLC-MS/MS Method for Quantification of Pharmaceuticals in Subtropical Rivers and Water Treatment Plants in Brazil
BÖGER Beatriz1, VILHENA Raquel de O1, FACHI Mariana M1, CONCENTINO Victor1, JUNKERT Allan M1, SANTOS Josiane MMF1, DOMINGOS Eric L1, ZAMORA Patrício Guillermo-Peralta2, PONTAROLO Roberto1,*
1Department of Pharmacy, Pharmaceutical Sciences Post-Graduate Program, Federal University of Paraná, Curitiba, Brazil
2Department of Chemistry, Chemistry Post-Graduate Program, Federal University of Paraná, Curitiba-PR, Brazil
*Corresponding Author: Roberto Pontarolo PhD, Department of Pharmacy, Federal University of Paraná, 632 Lothário Meissner Avenue, 80210-170, Curitiba, Paraná, Brazil
Received: 07 October 2020; Accepted: 13 October 2020; Published: 08 December 2020
Article Information
Citation:
BÖGER Beatriz, VILHENA Raquel de O, FACHI Mariana M, CONCENTINO Victor, JUNKERT Allan M, SANTOS Josiane MMF, DOMINGOS Eric L, ZAMORA Patrício Guillermo-Peralta, PONTAROLO Roberto. HPLC-MS/MS Method for Quantification of Pharmaceuticals in Subtropical Rivers and Water Treatment Plants in Brazil. Journal of Environmental Science and Public Health 4 (2020): 390-408.
View / Download Pdf Share at FacebookAbstract
A multiclass analytical method for the investigation of 25 pharmaceuticals in the main rivers of the four largest hydrographic basins and an industrial water treatment plant in Curitiba, Brazil was developed and validated. The analysis of the pharmaceuticals was done using SPE-HPLC-MS/MS. The LoD and LoQ concentrations were 10–100 ng L–1 and 20–200 ng L–1, respectively. The extraction and quantitation of the 25 compounds proved to be precise and accurate. Surface water samples collected during the spring of 2019 (n=16) were analyzed. Caffeine, lidocaine, sulfamethoxazole, and carbamazepine were the main compounds present in the river samples. Sulfamethoxazole was the antibiotic found in the highest concentrations. Other antibiotics, psychiatric drugs, anti-inflammatories, analgesics, antiretrovirals, and antidiabetics were also frequently detected and quantified. It was also observed that the treatment employed at the treatment station did not efficiently remove all pharmaceutics investigated. The results of this study indicate an intense anthropogenic influence in the Iguaçu basin, mainly due to the presence of domestic sewage. The lack of sanitation has a negative impact on the water quality of the Iguaçu River, which is highly degraded due to the influence of the tributaries of the Metropolitan Region of Curitiba.
Keywords
<p>Environmental; Pharmaceuticals; Micropollutants; River water; Water treatment plant</p>
Article Details
1. Introduction
In recent years, the occurrence and disposal of compounds of pharmaceutical origin in aquatic environments has gained more attention. With this, scientific interest in the effects and detection of these micropollutants in surface waters has increased significantly (Alves et al., [1]; Donnachie et al., [2]; Rivera-Utrilla et al., [3]; Torres et al., [4]; Castiglioni et al., [5]). These contaminants are chemically active molecules with different organic functions, physical–chemical properties, and biological properties, and they are applied to produce therapeutic effects in humans and animals (Kümmerer [6]; Torres et al., [4]). Therefore, when discarded in the environment, either in unaltered form or as metabolites, they can negatively impact the aquatic ecosystem, affect water quality, modify health, and cause unwanted effects in wildlife (Rivera-Utrilla et al., [3]; Torres et al., [4]).
Such products are continuously introduced into the environment and persist in small concentrations (in the range of ng L–1 or μg L–1). They can reach surface waters by several routes, with sewage effluents being the main source, followed by residues from agriculture and livestock, hospital effluents, and other non-specific sources (Alves et al., [1]; Das et al., [7]; Donnachie et al., [2]). The therapeutic classes most found in rivers are anti-inflammatories and analgesics, antidepressants, anti-epileptics, beta-blockers, steroidal hormones, antibiotics, antifungals, and other substances (such as caffeine, plasticizers, insect repellents, and abused drugs) (Ebele et al., [8]; Tran et al., [9]; Castiglioni et al., [5]).
Even though there is no current regulation that regulates the presence of drugs in surface waters, the monitoring of these substances and their metabolites is essential to ensure the quality, biodiversity, and health of organisms that depend on water (Bila and Dezotti [10]; Ebele et al., [8]; Rivera-Utrilla et al., [3]). Although the concern with the investigation of products of pharmaceutical origin in surface waters has grown worldwide, there are few published studies that have evaluated the occurrence of these micropollutants in urban rivers in Brazil since it is a country with a vast quantity of watersheds (Aus der Beek et al., [11]; Elliott et al., [12]; Starling et al., [13]).
However, in general, monitoring data for pharmaceutical products in surface waters in Brazil is quite scarce if we consider the dimension of the country (Starling et al., [13]). This may reflect the absence of monitoring legislation or awareness of the importance of this pollution. For this study, the Upper Iguaçu Basin and its main tributaries were selected due to their presentation of several fountains with high potential for human supply. The Iguaçu River is the longest river in the state of Paraná (Brazil), with a length of 1275 km and 98 tributaries (Ide et al., [14]; Leite et al., [15]). At the headwaters of the basin, where the metropolitan area of Curitiba is located, there is a large concentration of the population and industrial, commercial, and service activities. Agriculture predominates in the interior, with a greater emphasis on soybeans, wheat cultivation, and pastures (Ide et al., [14]; Setti et al., [16]). However, this basin has been showing high levels of degradation and increasing human occupation, mainly on the margins of its tributaries.
Therefore, the aims of this study were (1) to investigate the occurrence of pharmaceutical residues in the Iguaçu River and its major tributaries through a validated method; and (2) to evaluate the removal of these micropollutants in a Brazilian water treatment plant (WTP).
2. Materials and Methods
2.1 Compounds analyzed
The choice of pharmaceutical products to be studied was based on a list of pharmaceuticals prioritized for their ecological risks to the environment from some authors (Besse and Garric [17]; De Voogt et al., [18]; Han and Lee [19]; Ji et al., [20]). The 25 selected pharmaceuticals were amoxicillin, azithromycin, acetaminophen, acyclovir, atenolol, ciprofloxacin, caffeine, carbamazepine, captopril, cimetidine, diazepam, doxycycline, enalapril, fluoxetine, glibenclamide, glimepiride, ketoprofen, lidocaine, loratadine, metformin, norfloxacin, propranolol, sulfamethoxazole, sibutramine, and sulfadiazine.
2.2 Chemicals and reagents
High-performance liquid chromatography (HPLC) grade acetonitrile and methanol were obtained from Merck (Darmstadt, Germany). Formic acid (98%) and ammonium formate (99%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was obtained using a Milli-Q water purification system (Merck Millipore, Bedford, MA, USA). Chemical standards of norfloxacin (99.8%) and ciprofloxacin (93.4%) were purchased from Zhejiang Langhua Pharmaceutical Co. (Zhejiang, China). Doxycycline (98.7%), amoxicillin (98%), and sulfamethoxazole (99.5%) were acquired from Dr. Ehrenstorfer (Bavaria, Germany). Azithromycin (94.4%) was purchased from Alembic Pharmaceuticals Limited (Gujarat, India). Acetominophen (99.8%), sibutramine (94.4%), acyclovir (<98%), metformin (97.5%), atenolol (99.9%), caffeine (99.96%), carbamazepine (99.96%), diazepam (99.96%), and fluoxetine (99.9%) were supplied by the United States Pharmacopeia (Maryland, USA). Standards of captopril (<98%), ketoprofen (<98%), cimetidine (<99.5%), enalapril (<98%), glibenclamide (<99.6%), glimepiride (<98%), lindocaine (<98%), loratadine (<98%), propranolol (<98%), and sulfadiazine (<98%), which were used as internal standards (ISs), were obtained from Brazilian Pharmacopeia (Rio de Janeiro, Brazil). Standard stock solutions of these compounds were prepared with different compositions of methanol, water, and acetonitrile, with formic acid and ammonium formate depending on the solubility, and stored at −40°C in the dark at a concentration of 1000 μg mL−1 for a maximum of 1 month. Work solutions were freshly prepared in water and acetonitrile (50:50, v/v) and contained 0.1% formic acid and 5 µM ammonium formate at final concentrations of 100, 10, and 1 μg mL−1.
2.3 Sample collection
Surface water samples were collected during the spring of 2019 (n=16) in main rivers of the 4 largest hydrographic basins in the city of Curitiba, Brazil. In addition, they are the major tributaries of the Iguaçu River. The six sampling sites selected were the following: 3.8 km away from source of the Belém River (25°23'17.1"S 49°15'59.9"W); 9.2 km away from source of the Belém River (25°25'13.1"S 49°16'09.5"W); the Barigui River (25°25'54.7"S 49°18'49.9"W); the Atuba River (25°22'50.4"S 49°12'26.2"W); the Iguaçu River (25°35'59.8"S 49°23'53.7"W); and at the effluent of the Iguaçu Industrial WTP (25°35'59.8"S 49°23'53.7"W) (Figure 1). Sampling was performed at sites with high river flow. One-liter samples of water were collected in amber glass bottles. Samples were kept at 4°C until arrival to the laboratory. After collection, the samples were vacuum filtered through a glass fiber membrane with a 0.45 μm pore size and 47 mm diameter (Millipore). The pH of the samples was measured and adjusted to 6.5 after the filtration process.
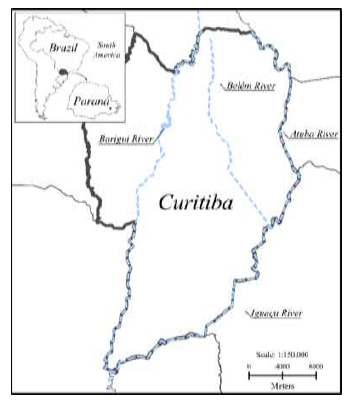
Figure 1: Surface water sampling locations along the Iguaçu River and its major tributaries in Curitiba, PR, Brazil.
2.3.1 Sample preparation: Solid-phase extraction (SPE) was performed on a Waters Vacuum Manifold (Milford, Massachusetts, USA) with 200 mg, 3 mL–1 Phenomenex Strata-X® cartridges (Torrance, California, USA) allowing a 500-fold concentration factor. SPE cartridges were conditioned with 4 mL of methanol followed by 6 mL of ultrapure water. A 250 mL water sample was pre-concentrated through the cartridge at a constant flow rate of 10 mL min–1. Afterwards, cartridges were air dried for 2 min, and analytes were eluted in 4 mL of methanol. Extracts were evaporated to dryness in a thermostatic bath at 30°C under a gentle stream of nitrogen. The residues were dissolved in 0.5 mL of mobile phase (water and acetonitrile at 50:50 v/v) containing 0.1% formic acid and 5 µM ammonium formate before being transferred to HPLC vials. Pre-concentration was conducted with a simple and fast protocol allowing for a 500-fold concentration factor.
2.4 Instrumentation and analytical conditions
The analyses were carried out using an Agilent 1200 HPLC System (Wilmington, USA) with a G1312B binary pump, G1379B degasser, and G1316B column oven interfaced with an Applied Biosystems API 3200 triple quadrupole equipped with an electrospray ionization (ESI) source operated in positive mode (ABSciex, Toronto, Canada). The HPLC system was connected to a CTC Sample Manager (Model 2777 Waters Corporation, Milford, USA) operated at 5°C. High-purity nitrogen and zero-grade air, used as the curtain gas (CUR), nebulizer gas (GS1), turbo gas (GS2), and collision gas (CAD) were produced using a high-purity nitrogen generator from Peak Scientific Instruments (Chicago, USA). Initially, for multiple reaction monitoring (MRM), standard working solutions of all analytes were prepared at a concentration of 500 ng mL–1 using different concentrations of ammonium formate and formic acid additives in water/acetonitrile (50:50, v/v) and they were directly infused into a mass spectrometer. After establishing the concentration of the additives, MRM and flow injection analysis (FIA) were performed in order to optimize the conditions of the analyzer and the ionization source, respectively. The chromatographic conditions were based on the study by Ferrer and Thurman [21]. The stationary phase used was one Zorbax Eclipse XDB-C8 column with 4.6 mm × 150 mm, 5 μm particle size (Agilent, Milford, USA). Different mobile phase compositions and gradient elution modes were tested, with a flow rate ranging from 200 to 400 μL min−1. Injection volumes between 10 μL and 20 μL were evaluated, and column temperatures of 30, 35, and 40°C were tested. The optimum condition was achieved with water (A) and acetonitrile/water (95:5 v/v) (B), both containing 0.1% formic acid and 5 mM of ammonium formate using gradient elution (10% B initial and ramped linearly from 10% B to 100% B for 0–35 min) and a column temperature of 30°C. The flow rate was 400 μL min−1, and the injection volume was 20 μL. The needle was washed with acetonitrile/methanol (50:50, v/v). Data acquisition and processing were performed with MS Workstation with Analyst software version 1.4.2 (ABSciex, Toronto, Canada).
2.5 Method validation
The method was validated according to the requirements of the European Commission [22] and ICH recommendations [23]. The validation parameters were selectivity, linearity, limit of detection (LoD), limit of quantitation (LoQ), recovery, precision, and accuracy. All validation parameters were evaluated using a mixture of matrix from all sites of collection spiked with known concentrations of the pharmaceuticals. The selectivity was determined by comparing the slopes of the analytical curves prepared in the matrix and solvent. The results were compared with the Student’s t test (p<0.05). Diluted (5:1) extracts fortified with standards and fortified ultra-pure water were used to evaluate the matrix effect. This is because no similar matrix was found without the analytes. The linearity of the response was determined using a linear regression model obtained through external standardization. One calibration curve was built each day, with five calibration levels (in triplicate) in the general concentration ranges of 10–60 µg L−1 and 100–500 µg L−1 for the pharmaceuticals. All concentrations that were above the highest point in the calibration curve were diluted and reanalyzed. A variation of up to 20% in relative standard deviation (RSD) for the precision and at each level was allowed, for limit of quantification too. The obtained data were then submitted to linear regression analysis. The homoscedasticity of the variances (Brown-Forsythe test), the residual normality (Anderson-Darling test), and the t-test for the significance of the slopes were evaluated. The LoD and LoQ for the instrumentation were calculated using a signal/noise ratio of 3:10.
In order to measure the SPE efficiency, recoveries were calculated by analyzing samples for each analyte at a medium level (40 µg L−1 or 350 µg L−1) of fortification. The accuracy was calculated as the relative error (RE), also at medium level. The intra-day precision was established by analyzing six replicates at three levels on one day, and the inter-day precision was verified by determinations on different days and for two different analysts. The results were evaluated by the RSD, Student’s t test, and F test. The development and validation of the method were done to determine the precision and accuracy of the quantification of the pharmaceuticals in the studied rivers. Improving the parameters of the methods found in the literature was not an objective (Ferrer andThurman [21]; Kasprzyk-Hordern et al., [24]; Monteiro et al., [25]; Tuc et al., [26]).
3. Results and Discussions
3.1 Optimization of HPLC-MS/MS conditions
The ionization intensity of the compounds was varied according to the structures of the molecules. The fragments established for quantification and identification are demonstrated in (Table 1).
The selected fragments agree with the literature data. The most intensive fragment ion from each precursor ion was selected for quantification. The ion-source parameters for positive electrospray ionization (ESI) mode were set as follows: CUR, 18 psi; CAD, 6 psi; GS1, 45 psi; GS2, 50 psi; ion spray voltage, 5500 V; source temperature, 650°C.
In order to optimize, the selection of chromatographic parameters was performed according to the results of the chromatographic profile (reduction of peak tailing and better resolution). Figure 2 shows the extracted-ion chromatogram for the 25 pharmaceuticals studied at maximum concentration levels under optimized conditions.
Notes: *Transitions used for quantification of the compounds. a) DP, declustering potential. b) EP, entrance potential. c) CXP, collision cell exit potential. d) CEP, collision cell entrance potential. e) CE, collision energy.
Table 1: Ion transitions of the analytes used for quantification and qualification, as well as compound-dependent parameters.
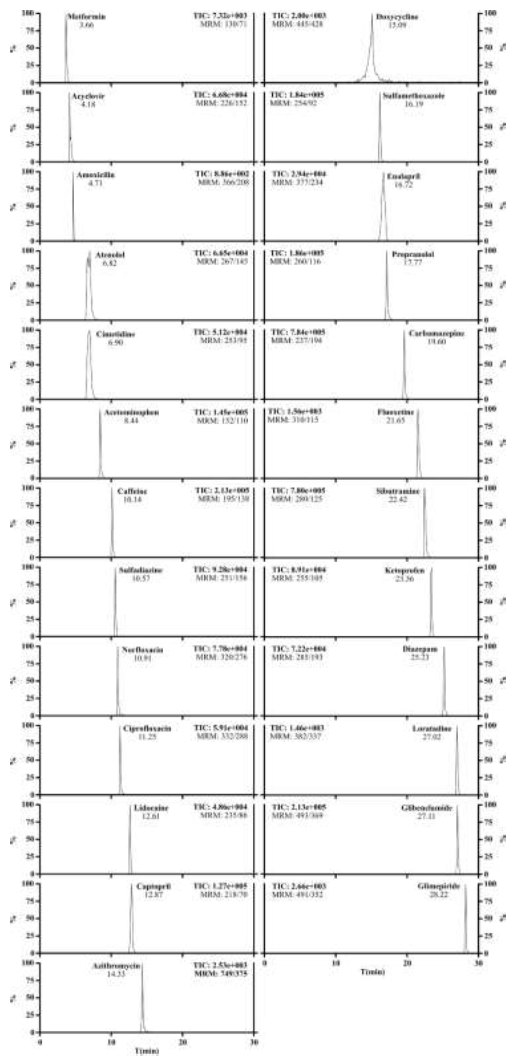
Figure 2: Extracted-ion chromatograms corresponding to the analysis of pharmaceuticals by liquid chromatography tandem mass spectrometry (LC-MS/MS).
3.2 Method validation
The present method was shown to be selective. The comparison of the slopes of both curves (ultrapure water and mixture of the matrix) presented no statistically significant differences (RSD <5%; Student’s t-test, p>0.05). The results of linearity to ranges evaluated are shown in Table 2.
According to ICH recommendations [23] and Commission Decision 2002/657/EC, the correlation coefficients for linearity were greater than 0.99 for all pharmaceuticals studied. Through regression analysis, the method was found to be linear for all compounds of interest. The LoD and LoQ concentrations were 10–100 ng L–1 and 20–200 ng L–1, respectively. These concentrations agree with the values found in other studies (Dinh et al., [27]; Monteiro et al., [25]).
For environmental samples, recovery is usually determined from the extraction efficiency and was found to vary according to the extraction conditions (Ferrer andThurman [21]). The extraction recoveries (23.77 to 123.49%) were reproducible (RSD<7.34) for all pharmaceuticals studied. Acyclovir, amoxicillin, azotomycin, captopril, ciprofloxacin, and norfloxacin showed the worst recovery of <70% (Table 3), but these recoveries were reproducible (RSD<8.41%) and were similar to that found in the literature (Dinh et al., [27]; Dinsdale and Guwy [28]; Monteiro et al., [25]).
Furthermore, the accuracies describe the yield of analytes obtained after all analytical steps, including pre-treatment and instrumental analysis (Table 3). According to the Directive 2002/657/EC, for this concentration range, the acceptable range to the relative errors is –50 to 20%.
Therefore, the method was accurate for all compounds. Intra-day and inter-day precisions were within the range of 2.25–19.20% and 0.87–22.39%, respectively (Table 3). In addition, the inter-day precision results showed equality of variances (F-test, p>0.05), and the averages were not statistically different (Student t-test, p>0.05). Therefore, the present method was reliable and reproducible for the quantitative analysis of all studied compounds.
Note: Regression coefficients shown as mean ± standard deviation. a) Anderson-Darling (p>0.05). b) Brown-Forsythe test (p>0.05). c)tR, retention time. d)r, correlation coefficient. e) LoD, limit of detection. f) LoQ, limit of quantification. g) LoD and LoQ with concentration factor.
Table 2: Linearity parameters for validation of the LC-MS/MS method.
Note: RSD, relative standard deviation. RE, relative error. Recovery shown as mean ± standard deviation.
Table 3: Precision, accuracy, and recovery data.
3.3 Environmental application
3.3.1 Global analysis of the levels of pharmaceuticals in the river water samples: Some authors have reported the occurrence of drug residues in surface waters in Brazil, but no study has covered several therapeutic classes in different rivers in a Brazilian capital and comparing the removal efficiency of a WTP (Ebele et al., [8]; Locatelli et al., [29]; Monteiro et al., [25]; Osawa et al., [30]; Sodré et al., [31). This study demonstrated the minimum, maximum concentrations and the frequency of quantification of drugs in all the studied sites (four samples were collected on different days from the Belém river and Barigui river and one sample on the Atuba, Iguaçu rivers and the water treatment station industrial). As shown in Table 4, many of the studied substances were frequently quantified in the different rivers.
Of the antibiotics studied, sulfamethoxazole was found with a greater frequency (n=14) and concentration (1.85 µg L–1 in the Barigui river). Amoxicillin and azithromycin were the antibiotics present (n=12) with the highest concentrations (6.35 µg L–1 and 0.65 µg L–1, respectively), both in the Barigui River. Ciprofloxacin (0.34 µg L–1), norfloxacin (0.13 µg L–1), sulfadiazine (0.02 µg L–1), and doxycycline (<0.2) were the least detected antibiotics in the samples, probably because tetracyclines (doxycycline) and fluoroquinolones (ciprofloxacin and norfloxacin) are more easily absorbed in sediments than macrolides (azithromycin), sulfonamides, and β-lactams (amoxicillin) (Nikolaou et al., [32]). In addition to strong adsorption in sediments, the non-detection of doxycycline in water samples may be due to mechanisms such as complexation with metals (Monteiro et al., [25]). Studies conducted by Locatelli et al., [29], in rivers located in São Paulo, Brazil showed the presence of amoxicillin (2.42 µg L–1), ciprofloxacin (0.11 µg L–1), norfloxacin (0.05 µg L–1), and sulfamethoxazole (0.1 µg L–1). In a study conducted in Rio de Janeiro, Brazil, the presence of azithromycin, amoxicillin, and sulfamethoxazole was found, with concentrations of up to 35 ng L–1, 287.5 ng L–1, and 287.5 ng L–1 respectively (Monteiro et al., [25]).
Like sulfamethoxazole, carbamazepine was also frequently (n=13) found in the samples since the study at levels up to 1.59 µg L–1. Carbamazepine and sulfamethoxazole are compounds that have been widely described in the literature for their consumption, occurrence, persistence in the environment, and resistance to treatment (De Voogt et al., [18]; Han and Lee, [19]; Madikizela et al., [33]). In addition, like propranolol, these drugs are resistant to photodegradation, favoring their permanence in the environment (Nikolaou et al., [32). Other psychiatric drugs were also found, such as diazepam (n=14) and fluoxetine (n=10). Diazepam had a higher concentration in the first sampling point of the Belém river (0.23 µg L–1), whilst fluoxetine had a higher concentration in the Barigui river (0.62 µg L–1). In studies on African and European continents, diazepam and fluoxetine are generally detected in aquatic environments at concentration levels below 100 ng L–1 and 109.2 ng L–1, respectively (Fekadu et al., [34]).
Note: a) Reported data considering only positive results. b) Number of samples with concentrations reported above method. nd, not detected.
Table 4: Concentration of the pharmaceuticals investigated in surface waters of the rivers and water treatment plant (WTP) in Curitiba-PR, Brazil.
Analgesics and anti-inflammatories are classes of pharmaceutical substances most frequently detected in the environment (Ebele et al., [8]; Fekadu et al., [34]; Madikizela et al., [33]). In the present study, acetaminophen (n=16) and ketoprofen (n=10) were present in most samples analyzed, with concentrations ranging from 0.03–1.69 µg L–1 and 0.02–1.10 µg L–1, respectively. Antihypertensives such as enalapril and atenolol were quantified in all rivers, with the highest concentration found in the Barigui river (0.12 µg L–1 and 5.3 µg L–1, respectively). Propranolol was found in concentrations of up to 0.03 µg L–1 (n=8). Captopril was found in greater concentrations in the Barigui river (2.61 µg L–1), while in the Atuba river it was not detected. As for antidiabetics, metformin had the highest frequency (n=15), and glibenclamide had the highest concentration in the first point of the Belém river (1.26 µg L–1). Metformin is a drug commonly found in surface waters in Europe. In a study carried out on the Elbe river in Germany, the metformin concentration was 1.7 µg L–1, which is higher than that found in the present study (<0.83 µg L–1) (Scheurer et al., [35]).
The antiretroviral acyclovir had a maximum concentration of 0.99 µg L–1. When compared with results found in Germany, the concentration found in surface water was lower than that of this study, presenting 0.19 µg L–1 of acyclovir in surface water and 1.78 µg L–1 in sewage (Prasse et al., [36]). There have been few studies that have reported the presence of antiretrovirals in the literature, despite being a class of dangerous drugs in relation to toxicity (Madikizela et al., [33]; Prasse et al., [36]). Lidocaine and caffeine were quantified in all samples. It is interesting to note that caffeine had the highest concentration and frequency in all rivers studied, contributing to its role as an anthropogenic pollution marker (Ide et al., [14]). Different concentrations of caffeine have been found in rivers, indicating different levels of pollution. Despite the high values found for this substance, it presents a low risk for aquatic and human life (Sodré et al., [37]). Similar data were obtained by Sodré et al., [31], who found caffeine in all samples collected in the Atibaia river (São Paulo, Brazil).
Overall, the concentrations of drugs in the rivers of Curitiba-PR were higher than those found in the international literature for surface waters (Ebele et al., [8]; Ferrer and Thurman, [21]; Kasprzyk-Hordern et al., [24]; Kim and Zoh, [38]; Lin et al., [39]; Madikizela et al., [33]; Sodré et al., [37]; Zhang et al., [40]). Still, the concentrations vary according to the therapeutic class, such as higher concentrations of analgesics in relation to antibiotics (Ebele et al., [8]; Wille et al., [41]). During the study period, it was also possible to observe large variations, showing a continuous scale of pollution. Since pharmaceutical substances are generally not very volatile, distribution in the environment will occur mainly by aqueous transport (Nikolaou et al., [32]). Although not all medicines have high persistence, they are omnipresent in the environment because the release rates are higher than their self-cleaning rates in water (Nikolaou et al, [32]).
3.4 Pharmaceutical dispersion along the Belém river
In Table 4, it is possible to see the results of the samples collected at two points along the Belém River (3.8 km and 9.2 km), showing that some concentrations of drugs decrease along the river (acetaminophen, amoxicillin, atenolol, caffeine, ketoprofen, diazepam, glibenclamide, glimepiride, metformin, norfloxacin, and propranolol). The collection point closest to the source is located near a city park where people are often fishing. The collection point furthest from the source is located in the city center. Although the highest concentration of human activity is at the center, lower concentrations of most substances were found at this point compared to the first collection point. This may indicate a greater presence of housing and irregular sewage connections at the first point, which was confirmed by the determination of caffeine (5.65 at point 1 and 0.9 µg L–1 at point 2) (Ide et al., [14]), thus indirectly showing that there are better sanitation conditions in the central region of the city. Other drugs remained relatively constant along the river, such as enalapril, fluoxetine, carbamazepine, and sulfamethoxazole.
Acyclovir, azithromycin, captopril, ciprofloxacin, lidocaine, sibutramine, and sulfadiazine increased in concentration along the Belém river. The physical properties of the micropollutants can affect the movement of contaminants from one stage to another (e.g., soil–water movement). The mobility of the compounds is determined by transport/retention factors that depend on related chemical properties, such as the acid dissociation constant (pKa) and octanol–water partition coefficient (Kow) (Kim and Zoh [38]; Nikolaou et al., [32]).
3.5 Removal of medicaments in an industrial water treatment plant
Table 4 shows the values of the drugs found in the surface waters of the Iguaçu river and their removal rate after the water had passed through the Industrial Water Treatment Station. Even though the effluent of this WTP is only used for industries in the region, unitary operations (coagulation/sedimentation, sand filtration, and chlorination) employed at the stations are similar to those of the water that serves the population. Since existing water treatment systems cannot completely remove medicines from water, knowledge of how to remove medicines through WTPs contributes to the production of improved drinking water. There were high removal rates (~100%) for azithromycin, captopril, glibenclamide, glimepiride, propranolol, sulfamethoxazole, and sulfathiazine after the water treatment process. However, the removal rate was low for medications such as caffeine (40%), carbamazepine (9%), enalapril (33%), and metformin (25%).
Drug removal rates may be different in studies published in the literature due to the use of different measurement methods and treatment process settings (Kim and Zoh [38]). Nam et al., [42], reported that the efficiency of removing micropollutants detected in a WTP ranged from 6% to 100%. Acetaminophen, caffeine, and carbamazepine were removed (>80%) in the WTP. In the process studied by Jiang et al., [43], the following removal rates were found: ketoprofen (62.84%), carbamazepine (4.14%), caffeine (8.49%), sulfadiazine (68.53%), sulfamethoxazole (44.37%), and acetaminophen (89.19%). However, in the present study the concentrations of acetaminophen, ketoprofen, diazepam, and norfloxacin increased, resulting in negative removal. Negative removal rates have been observed in other works (Lin et al., [39]; Zhang et al., [40]). These negative removal rates may occur due to the release of pharmaceutical products adsorbed on organic particles, conjugated metabolites that can become their precursors by enzymatic processes, the physical–chemical properties of the contaminants, and/or the applied treatment processes (Jiang et al., [43]).
4. Conclusion
The developed method was validated and proven to be reliable for the intended application. Caffeine, lidocaine, sulfamethoxazole, and carbamazepine were the main compounds present in the river samples. Sulfamethoxazole was the antibiotic found in the highest concentrations. Other antibiotics, psychiatric drugs, anti-inflammatories, analgesics, antiretrovirals, and antidiabetics were also frequently detected and quantified. It was also observed that the treatment employed at the treatment station did not efficiently remove all pharmaceutics investigated. It was possible to observe this reduction for azithromycin, captopril, glibenclamide, glimepiride, propranolol, Sulfamethoxazole, and sulfadiazine. However, it was not effective in removing drugs such as caffeine, carbamazepine, enalapril, and metformin. The results of this study indicate intense anthropogenic influence in the Iguaçu basin, mainly due to the presence of domestic sewage. Therefore, it is important to carry out more studies like this in order to identify the presence of other micropollutants since the Alto Iguaçu Basin has economic relevance for the region. Although the Belém, Barigui and Atuba rivers are not used to collect water due to pollution, there is a concern to improve the quality of these rivers since they are inside in the urban landscape and human recreation. Therefore, comprehensive understanding of the occurrence and destination of drugs in surface water and drinking water is essential to effectively predict the effects of pharmaceutical-based micropollutants on the recipient environment.
Acknowledgments
The authors express their gratitude for research funding to the CAPES (Brazilian Federal Agency for Support and Evaluation of Graduate Education within the Ministry of Education of Brazil) - Finance Code 001. The authors would like to thank the Secretariat of Science, Technology and Higher Education (SETI-PR) for the financial support of laboratory infrastructure.
Conflict of Interest
The authors have declared no conflict of interest.
References
- Alves T, Girardi R, Pinheiro A. Micropoluentes orgânicos: ocorrência, remoção e regulamentação. REGA, 14 (2017): 1-20.
- Donnachie RL, Johnson AC, Sumpter JP. A rational approach to selecting and ranking some pharmaceuticals of concern for the aquatic environment and their relative importance compared with other chemicals. Environ. Toxicol. Chem 35 (2016): 1021-1027.
- Rivera-Utrilla J, Sánchez-Polo M, Ferro-García MÁ, et al. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 93 (2013): 1268-1287.
- Torres NH, de Salles Pupo MM, Ferreira LFR, et al. Spatial and seasonal analysis of antimicrobials and toxicity tests with Daphnia magna, on the sub-basin of Piracicaba river, SP, Brazil. J. Environ. Chem. Eng 5 (2017): 6070-6076.
- Castiglioni S, Zuccato E, Fattore E, et al. Micropollutants in Lake Como water in the context of circular economy: a snapshot of water cycle contamination in a changing pollution scenario. Journal of Hazardous Materials (2019): 121441.
- Kümmerer K. The presence of pharmaceuticals in the environment due to human use - present knowledge and future challenges. J. Environ. Manage 90 (2009): 2354-2366.
- Das S, Ray NM, Wan J, et al. Micropollutants in Wastewater: Fate and Removal Processes. in: R. Farook, Z. Ahmad (Eds.), Phys.-Chem. Wastewater Treat. Resour. Recovery. IntechOpen, London, UK (2017): 75-107.
- Ebele AJ, Abou-Elwafa Abdallah M, Harrad S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerging Contam 3 (2014): 1-16.
- Tran NH, Reinhard M, Gin KYH. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res 133 (2018): 182-207.
- Bila DM, Dezotti M. Fármacos no Meio Ambiente. Nova 26 (2003): 523-530.
- Aus der Beek T, Weber FA, Bergmann A, et al. Pharmaceuticals in the environment-Global occurrences and perspectives. Environmental Toxicology and Chemistry 35 (2016): 823-835.
- Elliott SM, Erickson ML, Krall AL, et al. Concentrations of pharmaceuticals and other micropollutants in groundwater downgradient from large on-site wastewater discharges. PLoS ONE 13 (2018): 1-17.
- Starling MCVM, Amorim CC, Leão MMD. Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. Journal of Hazardous Materials 372 (2019): 17-36.
- Ide AH, Osawa RA, Marcante LO, et al. Occurrence of Pharmaceutical Products, Female Sex Hormones and Caffeine in a Subtropical Region in Brazil. Clean - Soil, Air, Water (2017): 45.
- Leite NF, Peralta-Zamora P, Grassi MT. Distribution and origin of polycyclic aromatic hydrocarbons in surface sediments from an urban river basin at the Metropolitan region of Curitiba, Brazil. J Environ Sci (China) 23 (2011): 904-911.
- Setti AA, Lima JEFW, Chaves AGM, et al. Introdução ao Gerenciamento de Recursos Hídricos. Brasília, BR: ANEEL (2001).
- Besse JP, Garric J. Médicaments à usage humain: risque d'exposition et effets sur less milieux récepteurs. Proposition d'une liste de médicaments à usage humain à surveiller dans les eaux de surface continentales. Cemagref (2007): 1-241.
- De Voogt P, Janex-Habibi ML, Sacher F, et al. Development of a common priority list of pharmaceuticals relevant for the water cycle. Water Sci. Technol 59 (2009): 39-46.
- Han EJ, Lee DS. Science of the Total Environment Significance of metabolites in the environmental risk assessment of pharmaceuticals consumed by human. Sci. Total Environ 592 (2017): 600-607.
- Ji K, Han EJ, Back S, et al. Prioritizing human pharmaceuticals for ecological risks in the freshwater environment of Korea. Environmental Toxicology and Chemistry 35 (2016): 1028-1036.
- Ferrer I, Thurman EM. Analysis of 100 pharmaceuticals and their degradates in water samples by liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 1259 (2012): 148-157.
- Community E. The performance of analytical methods and the interpretation of results. Off. J. Eur. Communities: Legis (2002): 221-228.
- Validation of Analytical Procedures: Text and Methodology Q2 (R1). London (2005).
- Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. Multi-residue method for the determination of basic/neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra performance liquid chromatography-positive electrospray ionisation tandem mass spectrometry. Chromatogr. A 1161 (2007): 132-145.
- Monteiro MA, Spisso BF, Ferreira RG, et al. Development and validation of liquid chromatography-Tandem mass spectrometry methods for determination of beta-lactams, macrolides, fluoroquinolones, sulfonamides and tetracyclines in surface and drinking water from Rio de Janeiro, Brazil. Braz. Chem. Soc 29 (2018): 801-813.
- Tuc Q, Alliot F, Moreau-guigon E, et al. Measurement of trace levels of antibiotics in river water using on-line enrichment and triple-quadrupole LC – MS / MS. Talanta 85 (2011): 1238-1245.
- Dinh QT, Alliot F, Moreau-Guigon E, et al. Measurement of trace levels of antibiotics in river water using on-line enrichment and triple-quadrupole LC-MS/MS. Talanta 85 (2011): 1238-1245.
- Dinsdale RM, Guwy AJ. Multi-residue method for the determination of basic / neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra performance liquid chromatography – positive electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 1161 (2007): 132-145.
- Locatelli MA, Sodre FF, Jardim WF. Determination of antibiotics in Brazilian surface waters using liquid chromatography-electrospray tandem mass spectrometry. Environ. Contam. Toxicol 60 (2011): 385-393.
- Osawa R, Ide A, Sampaio N, et al. Determinação de fármacos anti-hipertensivos em águas superficiais na região metropolitana de Curitiba /Determination of antihypertensive drugs in surface water in the metropolitan region of Curitiba. RBRH 20 (2015): 2318-2331.
- Sodré FF, Dutra PM, Dos Santos VP. Pharmaceuticals and personal care products as emerging micropollutants in Brazilian surface waters: A preliminary snapshot on environmental contamination and risks. Braz. Soc. Ecotoxicol 2 (2007): 22-34.
- Nikolaou A, Meric S, Fatta D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Bioanal. Chem 387 (2007): 1225-1234.
- Madikizela LM, Tavengwa NT, Chimuka L. Status of pharmaceuticals in African water bodies: Occurrence, removal and analytical methods. J. Environ. Manage 193 (2017): 211-220.
- Fekadu, S., Alemayehu, E., Dewil, R., Van der Bruggen, B., 2019. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ., 654, 324-337.
- Scheurer, M., Michel, A., Brauch, H. J., Ruck, W., Sacher, F., 2012. Occurrence and fate of the antidiabetic drug metformin and its metabolite guanylurea in the environment and during drinking water treatment. Water Res., 46, 4790-4802.
- Prasse, C., Schlüsener, M. P., Schulz, R., Ternes, T. A., 2010. Antiviral drugs in wastewater and surface waters: A new pharmaceutical class of environmental relevance? Sci. Technol., 44, 1728-1735.
- Sodré, F. F., Dutra, P. M., Santos, V. P. d., 2018. Pharmaceuticals and personal care products as emerging micropollutants in Brazilian surface waters: a preliminary snapshot on environmental contamination and risks. Eclética Quim., 43, 22-34.
- Kim, M. K., Zoh, K. D., 2016. Occurrence and removals of micropollutants in water environment. Environ. Eng. Res., 21, 319-332.
- Lin, T., Yu, S., Chen, W., 2016. Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere, 152, 1-9.
- Zhang, Y., Wang, B., Cagnetta, G., Duan, L., Yang, J., Deng, S., Huang, J., Wang, Y., Yu, G., 2018. Typical pharmaceuticals in major WWTPs in Beijing, China: Occurrence, load pattern and calculation reliability. Water Res., 140, 291-300.
- Wille, K., Noppe, H., Verheyden, K., Vanden Bussche, J., De Wulf, E., Van Caeter, P., Vanhaecke, L., 2010. Validation and application of an LC-MS/MS method for the simultaneous quantification of 13 pharmaceuticals in seawater. Anal. Bioanal. Chem., 397, 1797-1808.
- Nam, S. W., Jo, B. I., Yoon, Y., Zoh, K. D., 2014. Occurrence and removal of selected micropollutants in a water treatment plant. Chemosphere, 95, 156-165.
- Jiang, X., Qu, Y., Liu, L., He, Y., Li, W., Huang, J., Yu, G., 2019. PPCPs in a drinking water treatment plant in the Yangtze River Delta of China : Occurrence , removal and risk assessment. Front. Environ. Sci. Eng., 13, 27.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 