Zeta Potential of Escherichia Coli DH5α Grown in Different Growth Media
Wenfa Ng*
Department of Chemical and Biomolecular Engineering, National University of Singapore
*Corresponding Author: Wenfa Ng, Department of Chemical and Biomolecular Engineering, National University of Singapore
Received: 01 December 2021; Accepted: 07 December 2021; Published: 09 December 2021
Article Information
Citation:
Wenfa Ng. Zeta Potential of Escherichia Coli DH5α Grown in Different Growth Media. Journal of Environmental Science and Public Health 5 (2021): 479-489.
View / Download Pdf Share at FacebookAbstract
Proteins and metabolites typically adsorb to bacterial cell surface through a variety of mechanisms such as van der Waals attraction and electrostatic interactions, and forms a layer of nonspecifically adsorbed ions and molecules on the cell surface. Thus, the bacterial cell surface charge comprised the contribution from the cell wall as well as layers of nonspecifically adsorbed ions and molecules on the cell surface. This is the cell surface charge perceived by other bacterial cells in the growth medium. Given that different growth medium comprises different ensemble of proteins and metabolites that could adsorb onto the cell surface of bacteria, it is important to examine the effect of growth in different medium on the cell surface characteristics of bacterial cells using zeta potential as the proxy parameter. Defined at the shear plane, zeta potential provides a comprehensive view of the cell surface charge that include the nonspecifically adsorbed ions and molecules on the cell surface and the intrinsic electric charges in the cell wall. Using Escherichia coli DH5α (ATCC 53868) as model organism, experiments performed with deionized water as wash and resuspension buffer revealed that the zeta potential-pH profiles of cells grown in LB Lennox, and LB Lennox with 2 g/L glucose overlapped each other over the entire pH range from 2 to 9. This suggested that there was little physiological adaptation of the cell envelope of cells grown in LB Lennox supplemented with 2 g/L glucose, which indicated that the medium could be used for increasing biomass yield without affecting cell surface characteristics. Similarly, the zeta potential-pH profiles of E. coli DH5α grown in LB Lennox and LB Lennox buffered with 89 mM potassium hydrogen phosphate buffer also overlapped each other, which highlighted that the buffered medium did not elicit physiological adaptation of the cell envelope. However, supplementation of the LB Lennox (buffered) medium with 6 g/L glucos
Keywords
<p>Bacterial cell surface; Surface charge; Zeta potential; Nonspecific adsorption; Growth medium; Cell envelope; Escherichia coli; LB Lennox; Formulated medium; Tryptic Soy Broth</p>
Article Details
Graphical Abstract
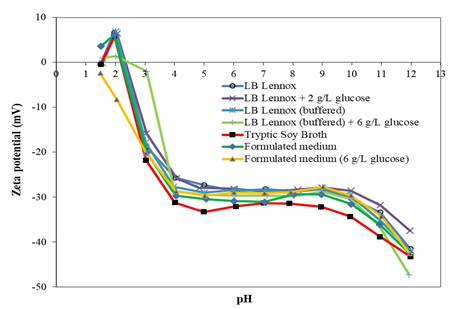
Short Description
Cell surface charge of Escherichia coli DH5α (ATCC 53868), as measured by zeta potential, was found to vary with the type of growth medium used (e.g., LB Lennox, Tryptic Soy Broth, and formulated medium). Specifically, the general shape of the zeta potential-pH profile was the same irrespective of the growth medium used. Similar zeta potential-pH profile was obtained for growth of E. coli DH5α in LB Lennox, LB Lennox + 2 g/L glucose, and LB Lennox (buffered); thereby, highlighting that 2 g/L glucose supplementation, and a high capacity phosphate buffer did not result in physiological adaptation of the cell envelope. On the other hand, zeta potential-pH profiles of E. coli DH5α grown in Tryptic Soy Broth, LB Lennox (buffered) with 6 g/L glucose, formulated medium, and formulated medium with 6 g/L glucose were more negatively charged compared to that grown in LB Lennox medium. This suggested that there might be physiological adaptations to growth in different growth medium at the cell envelope level. Thus, considering that the cell surface charge comprised intrinsic electrical charges of the cell wall and that from a nonspecifically adsorbed layer of ions and molecules, the zeta potential-pH profiles obtained revealed interesting information about how the electrostatic environment of the E. coli DH5α cell surface was perceived by other cells in the growth medium, which holds implications to our understanding of cell-cell interactions in the growth medium.
Significance of Work
Discussion of bacterial cell surface charge would entail an understanding of the real surface charge characteristics. However, given that nonspecific adsorption of ions and molecules would occur on the cell surface from the solution contacting the cell, it is conceptually more relevant to think of cell surface charge as comprising intrinsic cell surface charge (in the cell wall structure), as well as that derived from nonspecific adsorption of ions and molecules on the cell wall. Thus, using deionized water as wash and resuspension buffer, this study elucidated the surface charge characteristics of Escherichia coli DH5α (ATCC 53868) grown in different growth medium, where deionized water was unable to remove the nonspecifically adsorbed ions and molecules from the cell surface. Zeta potential was measured with the microelectrophoresis light scattering method, and served as proxy for the cell surface charge that comprise the contribution from the nonspecifically adsorbed layer of ions and molecules and that intrinsic to the cell surface. Thus, the study illuminated the variation of cell surface charge (zeta potential) with pH, which provided information on the electrostatic environment of the cell surface perceived by other cells in the growth medium. Hence, the experiment results hold relevance to our understanding of cell-cell interactions in the growth medium.
|
Highlights
1. Bacterial cell surface are typically coated with nonspecifically adsorbed ions and molecules from the growth medium, which together with the intrinsic charges in the cell wall, constitutes the cell surface charcteristics of the bacterium. 2. Using deionized water as wash and resuspension buffer, this study aimed to understand how zeta potential varied with pH for Escherichia coli DH5α (ATCC 53868) grown in different growth medium. 3. Zeta potential-pH profiles of cells grown in LB Lennox, LB Lennox with 2 g/L glucose, and LB Lennox (buffered) overlapped each other; thereby; suggesting that physiological adaptation to glucose supplementation and phosphate buffer did not occur at the cell envelope level. 4. On the other hand, supplementation of LB Lennox (buffered) medium with 6 g/L glucose resulted in zeta potential-pH profile more negarively charged than that of cells grown in LB Lennox medium. 5. Similarly, E.coli DH5α grown in Tryptic Soy Broth, formulated medium, and formulated medium with 6 g/L glucose generated zeta potential-pH profile more negatively charged than that of cells grown in LB Lenniox medium. |
1. Introduction
Bacterial cells are typically grown in rich medium for improving biomass formation. However, the diverse proteins and metabolites present in rich medium would naturally find affinity with the negatively charged bacterial cell surface, leading to the adsorption of proteins, ions, and molecules on the cell surface [1]. Thus, the true cell surface charge of bacteria is usually masked by ions, molecules, metabolites and proteins adsorbed to the cell surface [1]. Coupled with loosely bound metabolites and ions, the cell surface of bacteria is coated by myriad proteins, molecules, metabolites and ions. Such a cell surface and its charge characteristics are what are sensed by other bacterial cells in the same growth medium [1].
Different growth media are typically used in the cultivation of bacteria, and thus, different compendium of proteins, molecules, ions and metabolites would be nonspecifically adsorbed on the cell surface. Additionally, metabolites and proteins could also be loosely bound to the cell surface. Hence, different cell surface charge characteristics could be displayed by the same bacterial species during growth in different growth media. More importantly, growth in different media may entail physiological adaptations to a different nutrient composition that translate to the expression of different cell surface proteins and cell wall structure [2]. Thus, the objective of this study was to understand how growth in different growth medium affect the surface charge characteristics of Escherichia coli DH5α (ATCC 53868) through the binding of different proteins and metabolites to the cell surface as well as possible physiological adaptation of the cell envelope to growth in different growth medium.
E. coli DH5α is a recombinant strain capable of growth in a wide variety of growth media except minimal medium [3, 4]. A Gram-negative bacterium with a dense lipopolysaccharide layer on the outer membrane, the E. coli DH5α cell envelope presents a large number of attachment sites for ions and molecules; thereby, allowing the growth medium an opportunity to influence the cell surface charge environment through the nonspecific adsorption of ions and metabolites. Previous studies have sought to understand the cell surface charge of E. coli under different pH conditions, [1, 5, 6] but relatively little is known about the effect of growth medium on the cell surface characteristics of E. coli DH5α across the pH range from 2 to 12.
Using zeta potential as a proxy parameter for cell surface charge, this study attempted to use deionized water wash buffer for removing the loosely bound metabolites and proteins from the cell surface of E. coli DH5α, while preserving the layer of nonspecifically adsorbed ions and molecules on the cell surface. Cells would be resuspended in deionized water prior to zeta potential measurement via the microelectrophoresis light scattering method. The nonspecifically adsorbed ions and molecules provides a record of the type of solution the cells are exposed to, and play a critical role in enabling understanding of how cells are perceived electrostatically by other cells in the growth medium. Thus, this study’s experiment results could reveal the surface charge characteristics of cells as cultured in specific growth medium, which hold implications to understanding how cell-cell interactions in the medium occurred. In addition, the study could also offer clues to the propensity of E. coli DH5α in forming aggregates in various growth media as predicted from the surface charge characteristics profiled.
2. Materials and Methods
2.1 Materials
LB Lennox and Tryptic Soy Broth were purchased from Difco and Merck, respectively, and used as is. Composition of LB Lennox medium was [g/L]: Tryptone, 10.0, Yeast extract, 5.0, NaCl, 5.0. Composition of LB Lennox + 2 g/L glucose medium was [g/L]: Tryptone, 10.0, Yeast extract, 5.0, NaCl, 5.0, D-Glucose, 2.0. Composition of LB Lennox (buffered) medium was [g/L]: Tryptone, 10.0, Yeast extract, 5.0, NaCl, 5.0, K2HPO4, 12.54, KH2PO4, 2.31. Composition of LB Lennox (buffered) + 6 g/L glucose medium was [g/L]: Tryptone, 10.0, Yeast extract, 5.0, NaCl, 5.0, K2HPO4, 12.54, KH2PO4, 2.31, D-Glucose, 6.0.
Composition of Tryptic Soy Broth was [g/L]: Pancreatic digest of casein, 15.0; Papaic digest of soya bean, 5.0; NaCl, 5.0. Composition of formulated medium was in [g/L]: K2HPO4, 12.54; KH2PO4, 2.31; D-Glucose, 4.0; NH4Cl, 1.0; Yeast extract, 12.0; NaCl, 5.0; MgSO4, 0.24. Composition of formulated medium (6 g/L glucose) was in [g/L]: K2HPO4, 12.54; KH2PO4, 2.31; D-Glucose, 6.0; NH4Cl, 1.5; Yeast extract, 12.0; NaCl, 5.0; MgSO4, 0.24.
2.2 Growth of Escherichia coli DH5α in growth medium
Stock cultures of E. coli DH5α were prepared in 40% glycerol and stored at -70 °C before use. One glycerol stock culture was used as inoculum for 100 mL of LB Lennox medium in a 250 mL glass conical flask as seed culture. Incubation conditions were 37 °C and 230 rpm rotational shaking in a temperature controlled incubator.
After 8 hours of cultivation, 1 mL of seed culture was used as inoculum of experiment cultures in 100 mL of respective medium in 250 mL glass conical flask. Incubation conditions were 37 °C and 230 rpm rotational shaking in a temperature controlled incubator (Yih Der LM 570D, Taiwan). Two biological replicate cultures were prepared.
2.3 Sample preparation for zeta potential analysis
After 15 hours of cultivation, 2.5 mL of cell suspension was withdrawn from the culture flask and added to 37.5 mL of non-sterile deionized water in a 50 mL polypropylene centrifuge tube. The mixture was mixed vigorously by shaking and centrifuged at 3300 x g for 10 minutes at 25 °C.
Subsequently, the supernatant was carefully decanted without disturbing the cell pellet. 40 mL of non-sterile deionized water was subsequently added to resuspend the cell pellet, and the mixture vigorously mixed by a combination of shaking by hand and vortexing. The mixture was centrifuged at 3300 x g for 10 minutes at 25 °C, and the supernatant decanted.
The process was repeated one more time before the cell pellet was resuspended in 40 mL of deionized water. pH adjustment was done with nitric acid and sodium hydroxide, with pH measured by an Orion 9156 BNWP pH probe fitted to a Mettler Toledo Delta 322 pH meter.
2.4 Measurement of zeta potential
Samples were added to the microelectrophoretic cell with care taken to avoid bubble formation. After analysis, the microelectrophoretic cell was rinsed with deionized water three times prior to next use. Measurements were done at 25 °C.
4. Results and Discussion
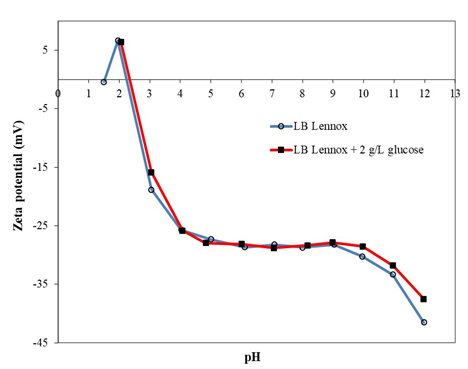
Figure 1: Zeta potential-pH profiles of E. coli DH5α grown in LB Lennox and LB Lennox with 2 g/L glucose medium at 37 °C and 230 rpm rotational shaking. Deionized water was used as wash and resuspension buffer.
- coli DH5α exhibited similar zeta potential-pH profiles for cells grown in LB Lennox and LB Lennox with 2 g/L glucose. Specifically, the zeta potential-pH profiles overlapped each other substantially (Figure 1). This suggested that the surface charge characteristics of E. coli DH5α grown in the two media were similar across the pH range from 2 to 10, which indicated that 2 g/L of glucose supplementation did not elicit significant cellular adaptation of the cell envelope. Thus, from the perspective of cell surface charge characteristics, E. coli DH5α grown in LB Lennox and LB Lennox with 2 g/L glucose were functionally similar, which indicated that LB Lennox with 2 g/L glucose could be used to improve biomass yield without affecting the cell surface characteristics.
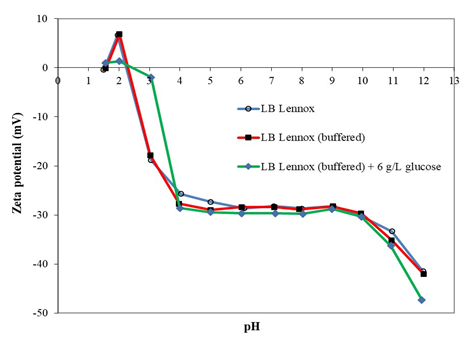
Figure 2: Zeta potential-pH profiles of E. coli DH5α grown in LB Lennox, LB Lennox (buffered), and LB Lennox (buffered) + 6 g/L glucose medium. Deionized water was the wash and resuspension buffer.
Buffer systems are commonly incorporated into medium formulation to help the culture broth maintain a stable pH around neutral to provide a conducive environment for biomass formation [3]. One such buffer system is K2HPO4 and KH2PO4 [3]. Using the potassium hydrogen phosphate buffer system with 89 mM of phosphate, LB Lennox (buffered) growth medium was effective in buffering pH changes that arise due to the secretion of various metabolites by E. coli DH5α during growth in the medium [3]. Experiment results revealed that the zeta potential-pH profile of E. coli DH5α grown in LB Lennox medium and LB Lennox (buffered) medium overlapped each other over the pH range from 2 to 12 (Figure 2). Additionally, the two zeta potential-pH profiles shared the same point-of-zero-charge (pHzpc) which is the pH where the cell surface charge was zero. This highlighted that there was no significant difference in cell surface characteristics of E. coli DH5α grown in LB Lennox and LB Lennox (buffered) medium, which indicated that physiological adaptation of the cell envelope to growth in LB Lennox (buffered) was not likely. Additionally, it also indicated that phosphate ions did not adsorb to the E. coli DH5α cell surface.
On the other hand, the zeta potential-pH profile of E. coli DH5α grown in LB Lennox (buffered) with 6 g/L glucose was more negatively charged in the pH range from 4 to 12 compared to that during growth in LB Lennox and LB Lennox (buffered) medium. This suggested that metabolism of 6 g/L glucose could have generated unique metabolites that nonspecifically adsorbed to the cell surface, or there was physiological adaptation to growth in LB Lennox (buffered) medium with 6 g/L glucose supplementation that resulted in changes to the cell surface characteristics.
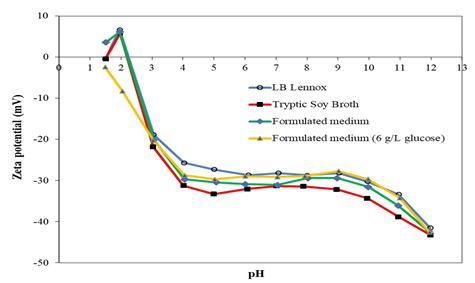
Figure 3: Zeta potential-pH profiles of E. coli DH5α grown at 37 °C and 230 rpm rotational shaking in LB Lennox, Tryptic Soy Broth, formulated medium, and formulated medium (6 g/L glucose). Cells were washed with deionized water and resuspended with the same buffer.
Growth of E. coli DH5α in Tryptic Soy Broth, LB Lennox and formulated medium3 resulted in zeta potential-pH profiles with the same pHzpc, but the profiles exhibited differences in the pH range from 4 to 12 (Figure 3). Specifically, within the pH range from 4 to 12, the zeta potential-pH profile of E. coli DH5α grown in Tryptic Soy Broth was more negatively charged compared to that of cells grown in formulated medium. Additionally, within the same pH range, the zeta potential-pH profile of E. coli DH5α grown in formulated medium was more negatively charged compared to that during growth of the bacterium in LB Lennox medium. This highlighted possible physiological adaptation to growth in different media, as well as nonspecific adsorption of ions and molecules from different growth media onto the cell surface.
In the case of growth of E. coli DH5α in formulated medium with 6 g/L of glucose, the pHzpc was more acidic compared to that during growth of the bacterium in other growth media. Additionally, within the pH range from 1.5 to 5, the zeta potential-pH profile of E. coli DH5α grown in formulated medium with 6 g/L glucose was more negatively charged compared to that during growth in LB Lennox medium. However, from pH 6 to 12, the zeta potential-pH profile of E. coli DH5α grown in LB Lennox and formulated medium with 6 g/L glucose overlapped each other; thereby, highlighting similar cell surface characteristics within the pH range from 6 to 12.
Overall, growth in different media such as LB Lennox, Tryptic Soy Broth and formulated medium likely resulted in differing physiological adaptations that translated to differences in cell envelope structure and surface composition. Additionally, differences in medium composition and types of ions and molecules present also meant that different ensemble of ions and molecules nonspecifically adsorbed to the cell surface of E. coli DH5α during growth in growth media with different compositions.
5. Conclusions
Bacterial cells are inevitably coated with layers of nonspecifically adsorbed and loosely bound ions and molecules during growth in various growth media. And the nonspecifically adsorbed ions and molecules constituted part of the cell surface charge sensed by other nearby bacterial cells. Thus, the goal in bacterial cell surface science should be to understand the cell surface characteristics with the nonspecifically adsorbed ions and molecules layer intact given its possible roles in mediating cell-cell interaction, attachment, agglomeration, and adhesion to different surfaces. This study revealed that the zeta potential-pH profiles of E. coli DH5α cultivated in different growth media shared a similar profile. However, except for growth of the bacterium in LB Lennox, LB Lennox + 2 g/L glucose and LB Lennox (buffered), differences in the zeta potential-pH profiles were observed for cells grown in media with different compositions. This suggested that observed differ-ences in zeta potential-pH profiles could be a combination of physiological adaptation of the cell envelope to growth in different media as well as nonspecific adsorption of ions and molecules from the growth medium onto the cell surface. Given that deionized water wash buffer does not have sufficient ionic strength for removing nonspecifically adsorbed ions and molecules from E. coli DH5α cell surface, the contribution to the cell surface charge from different ensemble of ions and metabolites nonspecifically adsorbed to the cell surface from the contacting growth medium could be significant. Thus, surface charge characteristics of E. coli DH5α were generally different for cells cultivated in different growth medium except during growth in LB Lennox, LB Lennox with 2 g/L glucose and LB Lennox (buffered) where there was no physiological adaptation of the cell envelope to growth in different growth medium.
Conflicts of Interest
The author declares no conflicts of interest.
Funding
The author thank the National University of Singapore for financial support.
References
- Ng W, Ting YP. Bacterial surface charge in layers: revealed by wash buffers of different ionic strength. PeerJ Prepr 6 (2018): e2086v4.
- Kankaanpaa P, Yang B, Kallio H, et al. Effects of polyunsaturated fatty acids in growth medium on lipid composition and on physicochemical surface properties of Lactobacilli. Appl. Environ. Microbiol 70 (2004): 129-136.
- Ng W. High cell density cultivation of Escherichia coli DH5α in shake flasks with a new formulated medium. PeerJ Prepr 6 (2018): e26598v1.
- Ng W. Colourless agar for enhancing colour contrast between microbial colonies and agar. PeerJ Prepr 6 (2018): e89v6.
- Lytle DA, Rice EW, Johnson CH, et al. Electrophoretic mobilities of Escherichia coli O157: H7 and wild-type Escherichia coli Appl. Environ. Microbiol 65 (1999): 3222-3225.
- Zubova SV, Ivanov AY, Prokhorenko IR. The effect of composition of the core region of Escherichia coli K-12 lipopolysaccharides on the surface properties of cells. Microbiology 77 (2008): 293-297.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 