Assessment of the Level of Heavy Metals in Tap Water Network System of Riyadh, Saudi Arabia
Yousef J Alanazi1, Fahad Ibraheem Al-Masoud2,3 *, Zaid Q Ababneh4,5
1Nuclear and Radiological Regulatory Commission (NRRC) Riyadh, Kingdom of Saudi Arabia
2Nuclear Science Research Institute (NSRI), King Abdulaziz City for Science and Technology (KACST), Riyadh, Saudi Arabia
3Department of Soil Sciences, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
4Physics Department, Faculty of Science, Yarmouk University, Irbid, Jordan
5College of Applied Medical Sciences, King Saud Bin Abdulaziz University for Health Sciences, Al-Ahsa, Saudi Arabia
*Corresponding Author: Dr. Fahad Ibraheem Al-Masoud, PhD., Nuclear Science Research Institute (NSRI), King Abdulaziz City for Science and Technology (KACST), P.O. Box 6086, Riyadh, 11441, Saudi Arabia, Department of Soil Sciences, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
Received: 18 February 2020; Accepted: 25 February 2020; Published: 05 March 2021
Article Information
Citation:
Yousef J Alanazi, Fahad Ibraheem Al-Masoud, Zaid Q Ababneh. Assessment of the Level of Heavy Metals in Tap Water Network System of Riyadh, Saudi Arabia. Journal of Environmental Science and Public Health 5 (2021): 137-154.
View / Download Pdf Share at FacebookAbstract
Water pollution due to the presence of heavy metals may affect the health of millions of people around the world. Therefore, the awareness of water quality and its continuous monitoring is essential for human safety. This work aimed to investigate the presence of heavy metals in the water for human consumption distributed in the network of Riyadh, Kingdom of Saudi Arabia, potentially affected by industries, agricultural, and chemical treatments. Water samples were collected from tap water and water treatment plant stations in Riyadh. All samples were tested for the physical parameters: such as total dissolved solids (TDS), pH, and electric conductivity (EC), as well as the concentration of eight trace metals (Al, Cr, Fe, Ni Cu, Zn, Cd, and Pb). The results showed that, with the exception of Fe, the heavy metal concentrations and the physical parameters in the investigated water samples satisfied the drinking water guidelines established by national and international organizations (WHO, EU, and SASO). Fe average concentration was found to be 833 µg L-1, which is higher than the permissible limit (300 µg L-1, SASO) in more than 95 % of the investigated samples. Thus, this research suggests that further investigation and assessment should be implemented for human safety by governmental agencies to public water distribution networks and the water treatment plant stations' product water.
Keywords
<p>Heavy metals; Water network; Water quality standard; Physicochemical; Heavy pollution index (HPI); Riyadh</p>
Article Details
1. Introduction
Water is the primary source of life for humankind, plants, and animals. Therefore, water resources have been given the most attention in terms of its safety and quality. Water quality varies from source to another, and it is mostly influenced by pollution-induced by either natural and anthropogenic sources (WHO [1]). One of the major natural causes of water pollution is the high concentration of metals, which caused by geological, geographical, and chemical treatment factors that successively might affect human health (Apollaro et al., [2]; EU [3]; Humans [4]; Sastre et al., [5]; Schroeder [6] et al., 2019; WHO [1]; Wiesenberger [7]; Zhou et al., [8]). At the same time, anthropogenic pollution sources in water are due to the mining, smelting, and other alternative industrial disposal activities, which end up in increasing the level of heavy metals in the water. Consequently, it will create severe health hazards to humans and the environment (Lee et al., [9]). Furthermore, rapid and unorganized urbanization, which associated with economic and industrial development, has contributed to elevated heavy metals in water resources in some Middle Eastern and Asian countries such as Saudi Arabia, Egypt, Iran, China, and India (Radwan and Salama [10]; Wong et al., [11]). This caused an impairment of water quality and various ecosystem services.
Water pollution has become one of the challenging global problems. Thus, for safe human water consumption, several national and international organizations published recommendations for water quality and set up regulations for the maximum permissible levels of heavy metals in water. These organizations are the World Health Organization (WHO [1]), European Commission (EU [3]), Gulf Standardization Organization (GSO [12]), and Saudi Standards, Metrology and Quality Organization (SASO [13]). Furthermore, several researchers worldwide have investigated the pollution in various types of waters (tap water, groundwater, bottled water) due to the presence of heavy metals and its associated health effect on humans (Apollaro et al., [2]; Bhaskar et al., [14]; Fallahzadeh et al., [15]; Reimann et al., [16]; Saleh et al., [17]; Vardè et al., [18]; Veschetti et al., [19]; Wulan et al., [20]). Most of the reports recommended the need for periodic and systematic monitoring of the water quality to minimize the human health risk.
In Riyadh, Saudi Arabia, the water is sourced from groundwater, desalinated water, and treated wastewater, which is mainly used in industry, irrigation, and public water supplies (Al-Bassam and Al-Rumikhani [21]; MOEP [22]). However, the major water supply portion comes from groundwater reserves within the deep sedimentary rock layer. Groundwater is essential in Saudi Arabia because it is extensively used for several purposes, such as in drinking, agriculture, and industry.
Although the heavy metals in water are generally present in low concentrations (ppb), they do have an effective harmful action in human life, crops, and water bodies. If above the limit, it may cause a severe hazard for humans, animals, and plants health. Thus, identifying and quantifying heavy metals are essential for assessing water quality because of its importance to all living organisms (Zhou et al., [8]). In Saudi Arabia, several studies were performed concerning the heavy metals content in various types of water (groundwater, bottled water, tap water, cooler water) in different parts of the country, particularly in Riyadh (Al-Hammad and El-Salam [23]; Al-Saleh [24]; Alabdula’aly et al., [25]; Alabdula’aly and Khan [26]; Alfadul and Khan [27]). To a large extent, the results of the investigated samples showed that the Fe presence was at a significant level exceeding the recommended level stated by EU and SASO standards (EU [3]; SASO [13]). In this study, water samples from different districts of Riyadh city were tested for Al, Cr, Fe, Ni, Cu, Zn, Pb, and Cd using a coupled plasma mass spectrometry (ICP-MS) technique. The measured heavy elements concentration values were compared with the corresponding values set up by the national and international organizations for drinking water. Moreover, the overall water quality was assessed by calculating the Heavy metal Pollution Index (HPI). Therefore, continuous monitoring and systematic assessment of the heavy elements in the water distribution system is essential for public health. The data presented in this work may help in establishing baseline data that can serve as a reference for future monitoring.
2. Materials and Methods
2.1 Study area
The study area is located in Riyadh city (24° 38′ 27″ N, 46° 46′ 22″ E), the capital of Saudi Arabia, and the largest city in the country. It has an area of 380,497,8 km2 and a population of more than seven million (Statistics [28]). The region has an arid climate with an average high temperature of 42.6 oC in July and warm winter. The area receives a median annual rainfall between 41 to 230 mm (Shepherd [29]).
Riyadh is located on the sedimentary Nejd Plateau about 600 m above the sea level and surrounded by a desert. The Nejd Plateau includes a sequence of mountains formation called the Tuwayq mountains, which extends in an arc-shaped ridge from the southwest to the northeast after which to the northwest with a length of 1100 km. However, Riyadh topography is flat, composed of sand, silt, and associated fine sediments.
The majority of the water in Riyadh is desalinated from seawater (60%) with the remaining being pumped from groundwater (40%) (from numerous wells dispensed in and around the city). The groundwater sources are from the Wasia and Manjur aquifers (Sharaf and Hussein [30]). Minjur aquifer provides up to 75% of Riyadh water requirements. The Minjur belong to the upper Triassic period age and composed of sandstone and some shale. The thickness of the formation is varied from one place to another, depending on the geological formation. The wells drilled in this formation ranged from depth 1200 meters to 1500 meters (Powers et al., [31]). Wasia aquifer is considered a vast groundwater reservoir; it appears in the central Najd and extended as far as the Arabian Gulf. It belongs to the upper Cretaceous age and is composed of sandstone and shale (Sharief et al., [32]).
The city consumes almost 1.5 million m3 per day, provided from several water treatment plants distributed within the city. The water produced from treatment plants is mixed with desalination seawater before it pumped through the pipes to different sites in the city (SASO [13]).
2.2 Water sampling and preparation
Tap water samples were collected from twenty-nine sites located in the Riyadh region. In addition, water samples were collected directly from three main water treatment plant stations, which are used for comparison purposes. These stations are Al-Shamaisi, Salbouk, and Manfuha, and these samples were given the ID numbers (30-32). The specific location of each sampling site is listed in Table 1 and shown in Figure 1.
Riyadh's eastern regions receive their water from the Wasia Water Treatment Plant Station, which is extracted from the Wasia aquifer. The pumped water is mixed with desalinated seawater before being distributed to the local residences, and this includes the following samples (1-11). The northern and northeastern Riyadh regions are supplied by the Salbouk water treatment plant station, which is extracted from the Minjur aquifer, and this includes the following samples (12-19). Southern Riyadh gets its water from the Manfuha Water Treatment Plant Station, which, in turn, is fed by the Minjur aquifer. This includes the subsequent samples ( 20-27), while samples (28-29) are provided by the Al-Shamaisi Water Treatment Plant Station, which is fed by the Minjur aquifer.
All the collected water samples were placed in cleaned plastic buckets during March of 2018. All samples were filtered with 0.45 µm pore size nitrocellulose membrane, then acidified with 2% HNO3 and stored in cleaned polyethylene bottles after the triplicate measurements of pH, electrical conductivity (EC), and total dissolved solids (TDS). The samples were subsequently kept at 5 oC before further analysis.
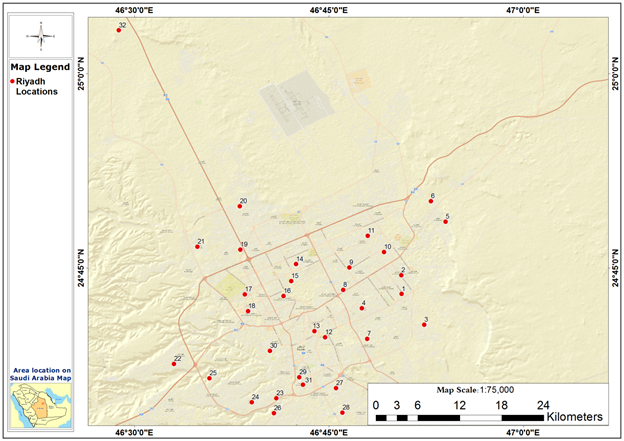
Figure 1: Water sampling locations in the study area.
STD: Standard deviation; GSO: Gulf Standardization Organization; WHO: World Health Organization; SASO: Saudi Standards; Metrology and Quality Organization
Table 1: Specific location and levels of TDS, EC, and pH in Riyadh water network, Kingdom of Saudi Arabia.
2.3 Elemental concentrations measurements
Water samples were analyzed for eight heavy metals (Al, Cr, Fe, Ni Cu, Zn, Cd, and Pb) using a Perkin Elmer NexION 300 ICP-MS type equipped with PE AS93 Plus autosampler. The ICP-MS analytical technique was used to detect trace metals because of its high sensitivity and simultaneous detection of multi-element with low concentrations (Allen et al., [33]; Allen et al., [34]; Banks et al., [35]; Gießmann and Greb [36]; Hall et al., [37]; Moens [38]; Reimann et al., [39]; Riondato et al., [40]). Each analyzed sample was repeated three times, and the results were reported as the mean value ± SD. Relative standard deviation (% RSD) values were found to be less than 10% for all analyzed heavy metals, reflecting the high accuracy of the analyzed method.
2.4 Heavy Pollution Index (HPI)
Heavy metal Pollution Index (HPI) has been used to assess the water quality due to the presence of total heavy metals in each sample. The mathematical model of HPI is proposed by (Mohan et al., [41]).
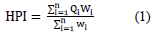
Where Qi refers to the ith parameter's sub-quality index, Wi refers to the unit weight of the ith heavy element, and n represents the number of investigated elements. The calculation of Qi parameter is as follows
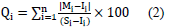
where Mi is the measured value of the ith heavy element, Ii indicates the ideal value of the ith heavy element in drinking water, and Si refers to the highest permissible value of the ith element in drinking water. Based on (Horton [42]; Mohan et al., [41]), Wi value is inversely proportional to the ith element's standard value in drinking water.
2.5 Method validation
The method validation was performed by analyzing the linearity, limit of detection (LOD), and accuracy using certified reference material (NIST-SRM 1643d) for corresponding elements. The instrument's linearity was performed by generation calibration curves between the intensity and the concentration for each element using standard solutions with different known concentrations. The calibration curves regression analysis are presented in Table 2. The results showed that the correlation coefficients (r2) for the calibration curves were (r2 > 0.997), indicating an excellent linear response through the range of studied concentrations.
STD: Standard deviation; GSO: Gulf Standardization Organization; WHO: World Health Organization; SASO: Saudi Standards; Metrology and Quality Organization
Table 2: Linearity range of ICP- MS calibration curves.
The detection limit for each investigated element was determined by using triplicate measurements of a blank sample of deionized water with 2% HNO3. Table 3 listed the certified and the measured values for triplicate measurements, besides both the recovery and the detection limits values. The results showed that the detection limits for the studied elements ranged from 0.042 to 8.742 mg L-1, and the measured values were found to be within the range of the certified values. Besides, the results showed that the recovery values for the studied elements in SRM1643d ranged from 96.3% to 103.13%, indicating a good agreement with the certified values.
STD: Standard deviation; GSO: Gulf Standardization Organization; WHO: World Health Organization; SASO: Saudi Standards; Metrology and Quality Organization
aSD, Standard Deviation
Table 3: Detection limits and summary of the analysis of the certified standard reference material SRM1643d.
3. Results and Discussion
3.1 Physical properties
The physicochemical parameters values (TDS, pH, and EC) of all investigated water samples, together with the drinking water specifications stated by national and international organizations, are presented in Table 1. TDS represent the amount of minerals dissolved in water samples (Ritter [43]). The TDS values measured in the collected water varied from 68 to 793 mg L-1, with an average value of 499 mg L-1. It is clear from Table 1 that TDS values for all investigated samples were lower than recommended upper limits sets by WHO [1], SASO [13], and GSO [12]. It should be mentioned here that high TDS values of water samples could harm human health, particularly to the central nervous system, resulting in paralysis of the tongue, lips, and face, irritability, and dizziness reported by Chang [44].
The EC represents the level of ions in water; a higher EC level indicates a high mineral level in the water. The distribution of EC values in the collected waters were varied from 107 to 1124 µS cm-1, and more than 90% of the samples had EC values higher than 700 µS cm-1, with a mean value of 728 µS cm-1. In all samples, the EC values were found to be below the maximum limits of 1500 µS cm-1 proposed by (WHO [45]).
The pH indicates the degree of acidity or alkalinity in water; it significantly influences chemical, biological processes, and oxygen availability in the water (Kannel et al., [46]). Based on the pH measurements, water samples collected from the Riyadh network tended to be more alkaline and varied with a narrow range between 7.38 to 8.89. It should be mentioned here that if pH is less than 6.5, the human body stops creating vitamins and minerals. pH values higher than 8.5 induce a saltier taste of water, while pH more than 11 can cause skin disorder and eye irritation. The obtained pH values showed that 87.5% of the samples were within the recommended range for drinking water set up by SASO [13], WHO [1], and GSO [12] standards.
Although the water treatment plants used in this study employed the same treatment processes, there is a variation in TDS and EC in the water samples. This might be due to the variations in the removal efficiency of the treatment plants process. Furthermore, the water treatment plants' mixing with the desalinated seawater at different percentages may also contribute to TDS and EC variations. It should be mentioned here that during the major maintenance process for the water treatment plants, in particular, the Reverse osmosis (RO) process might cause variations in the quality of water, such as TDS and EC (Al-Jaseem et al., [47]).
3.2 Concentration of trace metals
The concentration of the measured trace metals and their comparison with drinking water specifications stated by GSO [12], WHO [1], SASO [13], and EU [3] guidelines are presented in Table 4. The average concentrations of the detected elements are presented according to the following order: Fe > Zn > Cu > Cr > Ni > Al > Pb > Cd.
The obtained concentration values for the investigated water samples were varied over a wide range: Al (0.61 – 4.16 μg L-1), Cr (1.06 –7.09 μg L-1), Fe (234.2 –1215.1 μg L-1), Ni (1.10 – 4.03 μg L-1), Cu (4.29 –11.56 μg L-1), Zn (8.68 – 52.22 μg L-1), Cd (0.004 – 0.289 μg L-1) and Pb (0.266 – 0.733 μg L-1). The results showed that the concentrations of the detected elements in water samples collected directly from the water treatment plant stations lied within the range of the tap water values collected from the houses, except for Al in both Manfuha and Salbokh, which shows relatively lower values inside the stations. It should be stated here that all water treatment plants in Riyadh provided the water after mixing the desalinated seawater with the treated groundwater. These water plants treated the water using the same physical, chemical, and desalination processes like RO and electro Dialysis (ED). The product water usually incorporates low levels of metals with various concentrations. This variation relies upon the removal efficiencies of different treatment plants and the amount of the added chemical used at some stage in the different treatment processes. Several studies have shown that Al concentration in product water enhances after the usage of alum as a coagulant in the softening process (Letterman and Driscoll [48]; Reiber et al., [49]).
Comparison of the obtained results with the national and international guidelines (GSO, WHO, SASO, and EU) showed that the average concentrations of heavy metals in the investigated samples were less than the maximum admitted limit for the corresponding elements except for iron concentration. The average concentration of iron was 833 μg L-1, which is four times higher than the recommended level stated by the EU standard (200 μg L-1) and almost 2.8 times higher than the recommended level set up by SASO (2000) [13] standard level for drinking water (300 µg L-1). This may be attributed to the water treatment plants' purification technique, which does not remove the iron properly from the water. Other potential resources that may affect heavy metals in water are the leaching and corrosion inside the transfer pipes and the intermittent pumping to the network and tanks (Fuge and Perkins [50]). The water's contact time with the pipes might also affect metals' concentrations, especially in old pipelines, which have poor maintenance.
Although iron is an essential element in the human body, the presence of a higher iron level in drinking water might cause a severe liver disease called haemosiderosis (Bhaskar et al., [14]). Also, excessive iron in water affects skin cells, which can lead to skin infections and wrinkles. In addition, ingestion of a high level of iron in water can lead to stomach problems such as nausea, vomiting, and other issues (Huang [51]).
STD: standard deviation; GSO: Gulf Standardization and Organizations; WHO: World Health Organization; EU: European Commission; SASO: Saudi Standards, Metrology and Quality Organization; HPI: Heavy Pollution Index; NM: Not Mentioned
Table 4: Comparison of trace metals concentrations (µg L-1 ± SD) in Riyadh water network with the standard guidelines, in addition to HPI of water at each sampling site.
The heavy metal concentrations of water samples in the present study were compared with similar studies conducted elsewhere in Saudi Arabia (Table 5). It was observed from Table 5 that bottled drinking water has the highest water quality, where none of the detected heavy metals exceeded the maximum recommended limits of drinking water (Alfadul and Khan [27]). For the groundwater samples collected from different wells in the Riyadh region, the results showed that Fe concentrations exceeded the drinking water standards in 45.6% of the samples (Alabdula’aly et al., [25]).
For cooler water samples collected from the same region, the results showed that the heavy metal concentrations of Fe, Pb, and Ni were higher than the maximum recommended limit in only 4.5% of the collected samples (Alabdula’aly and Khan [26]). However, the cooler water samples collected from Riyadh schools showed relatively high Fe, Ni, and Cd concentrations in some cases (Al-Saleh [24]). For the water samples collected from Wadi Hanifa, Riyadh, the results showed that all metal concentrations are within the recommended limits, except for Fe, Pb, and some concentrations of Cd (Al-Hammad and El-Salam [23]). With the exception of bottled drinking water, it should be noted here that Fe is the only common heavy metal in drinking water samples with concentration levels that exceeded the maximum recommended limit in all previous studies conducted in Saudi Arabia. Therefore, it seems the high concentration of iron in water is a chronic general problem.
Thus, further investigations and monitoring agencies are needed to improve the water body and to elucidate the causes of these high concentrations of iron in water samples. These investigations should also be extended to other environmental aspects to include microbiological contaminants and other chemical compounds that could result from the corrosion of transfer lines.
BDL: Below detection limits; ND: not detected
Table 5: Comparison (range and mean) values (µg L-1) of heavy metals concentrations in water samples of this study with those reported in Saudi Arabia.
3.3 Heavy Pollution Index (HPI)
The HPI was calculated for each water sample based on the investigated heavy elements' concentration values using equ. 1. Table 6 presented the standard parameter values (Si, Ii, and Wi) of the heavy elements which are used to calculate HPI for the investigated water samples based on the WHO standard for drinking water (WHO [1]). The calculated values of HPI for each sampling location was given in Table 4. The HPI of the investigated samples ranged from the lowest value of 51.7 in Al-Nasim1 region to the highest value of 62.7 in Al-Malaz 2 region with an average value of ( ± SD) 60.1 ± 2.1. The HPI values are less than the critical value of 100. Thus, the overall pollution due to heavy metals in the investigated water samples is insignificant.
Table 6: Standard parameter values used to calculate HPI for the investigated water samples based on WHO ( 2008 [1]).
4. Conclusion
In the present study, twenty-nine samples of tap water collected from houses in Riyadh city and three water samples collected from water treatment plants in Riyadh were analyzed for their concentration of Al, Cr, Fe, Ni Cu, Zn, Cd, and Pb and their physical parameters.
The detected metals concentration in each investigated sample was below the national and international acceptable drinking water standard limits for Al, Cr, Ni Cu, Zn, Cd, and Pb. However, Fe levels were higher than 95% of the investigated samples, with an overall average Fe concentration of 833 mg L-1. The high concentration of Fe present in both tap water and treatment plants water might be attributed to a failure in the water treatment plants' purification technique and the leaching and corrosion of water in the distribution pipes. The presence of a higher level of iron in drinking water might adversely affect both humans and the ecosystem. Thus, further investigations and monitoring agencies are needed to improve the water body in the public water distribution network and elucidate the causes of these high iron concentrations in water samples.
Furthermore, the overall water quality of the investigated samples was assessed based on the measured heavy elements using HPI. The average value of HPI was found to be less than the critical value indicating that the overall water quality is acceptable.
Author Contributions
Fahad Almasoud: Conceptualization, methodology, writing the original draft. Yousef Alanazi; data curation, formal analysis, writing the original draft. Zaid Ababneh: investigation, writing-reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank the chemistry department technical staff at King Saud University (KSU), Riyadh, Saudi Arabia. Also, extended thanks are conveyed to King Abdulaziz City for Science and Technology (KACST) in Riyadh, Saudi Arabia, for their financial support to complete this study.
References
- World Health Organization. Guidelines for drinking water quality. Incorporating the first and second addenda, Recommendations, 3rd ed 1 (2008).
- Apollaro C, Buccianti A, Vespasiano G, et al. Comparative geochemical study between the tap waters and the bottled mineral waters in Calabria (Southern Italy) by compositional data analysis (CoDA) developments. Applied Geochemistry 107 (2019): 19-33.
- Directive, Council: On the quality of water intended for human consumption. Official Journal of the European Communities 330 (1998): 32-54.
- Humans I.W.G.o.t.E.o.C.R.t. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Chlorinated Drinking Water; Chlorination By-products; Some Other Halogenated Compounds; Cobalt and Cobalt Compounds. IARC (1990).
- Sastre J, Sahuquillo A, Vidal M, et al. Determination of Cd, Cu, Pb and Zn in environmental samples: microwave-assisted total digestion versus aqua regia and nitric acid extraction. Analytica Chimica Acta 462 (2002): 59-72.
- Schroeder HA. The Trace Elements and Nutrition. Faber and Faber, London (1973).
- Wiesenberger A. The influence of minerals in water. Excerpted from, The Taste of Water (2001).
- Zhou C, Wong M, Koh L, et al. Soil lead and other metal levels in industrial, residential and nature reserve areas in Singapore. Environmental Monitoring and Assessment 44 (1997): 605-615.
- Lee CS, Li XD, Zhang G, et al. Heavy metals and Pb isotopic composition of aerosols in urban and suburban areas of Hong Kong and Guangzhou, South China—evidence of the long-range transport of air contaminants. Atmospheric Environment 41 (2007): 432-447.
- Radwan MA, Salama AK. Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food and Chemical Toxicology 44 (2006): 1273-1278.
- Wong C, Li X, Zhang G, et al. Atmospheric deposition of heavy metals in the Pearl River Delta, China. Atmospheric Environment 37 (2003): 767-776.
- Gulf Cooperation Council Standards. Unbottled drinking water standards, Standardization and Metrology Organization for the Gulf Cooperation Council Countries GSO. Riyadh, Saudi Arabia 149 (2004).
- Saudi Arabian Standards Organization. Unbottled Drinking Water (SASO 701). Riyadh, Saudi Arabia (2000).
- Bhaskar CV, Kumar K, Nagendrappa G. Assessment of heavy metals in water samples of certain locations situated around Tumkur, Karnataka, India. Journal of Chemistry 7 (2010): 349-352.
- Fallahzadeh RA, Ghaneian MT, Miri M, et al. Spatial analysis and health risk assessment of heavy metals concentration in drinking water resources. Environmental Science and Pollution Research 24 (2017): 24790-24802.
- Reimann C, Birke M, Filzmoser P. Bottled drinking water: water contamination from bottle materials (glass, hard PET, soft PET), the influence of colour and acidification. Applied Geochemistry 25 (2010): 1030-1046.
- Saleh HN, Panahande M, Yousefi M, et al. Carcinogenic and non-carcinogenic risk assessment of heavy metals in groundwater wells in Neyshabur Plain, Iran. Biological trace element research 190 (2019): 251-261.
- Vardè M, Servidio A, Vespasiano G, et al. Ultra-trace determination of total mercury in Italian bottled waters. Chemosphere 219 (2019): 896-913.
- Veschetti E, Achene L, Ferretti E, et al. Migration of trace metals in Italian drinking waters from distribution networks. Toxicological and Environmental Chemistry 92 (2010): 521-535.
- Wulan DR, Marganingrum D, Yoneda M. Distribution, source identification, and assessment of heavy metal pollution in the surface and pore waters of Cipeles River, West Java, Indonesia. Environmental Science and Pollution Research (2020): 1-12.
- Al-Bassam AM, Al-Rumikhani YA. Integrated hydrochemical method of water quality assessment for irrigation in arid areas: application to the Jilh aquifer, Saudi Arabia. Journal of African Earth Sciences 36 (2003): 345-356.
- (The Ministry of Economy and Planning):The Ninth Development Plan (2010-2014) (The Kingdom of Saudi Arabia) (2010).
- Al-Hammad BA, El-Salam MMA. Monitoring water pollution levels in Wadi Hanifa, Riyadh, Saudi Arabia and its public health implications. Bulletin of environmental contamination and toxicology 98 (2017): 525-533.
- Al-Saleh IA. Trace elements in drinking water coolers collected from primary schools, Riyadh, Saudi Arabia. Science of the total environment 181 (1996): 215-221.
- Alabdula’aly AI, Al Zarah AI, Khan MA. Assessment of trace metals in groundwater sources used for drinking purposes in Riyadh region. Int J Water Resour Arid Environ 1 (2001): 5-9.
- Alabdula’aly AI, Khan MA. Heavy metals in cooler waters in Riyadh, Saudi Arabia. Environmental monitoring and assessment 157 (2009): 23-28.
- Alfadul SM, Khan MA. Water quality of bottled water in the kingdom of Saudi Arabia: A comparative study with Riyadh municipal and Zamzam water. Journal of Environmental Science and Health, Part A 46 (2011): 1519-1528.
- Population. Statistical Yearbook 47 (2011). Central Department of Statistics and Information (Saudi Arabia). Retrieved 15 November 2013 (2011).
- Shepherd JM. Evidence of urban-induced precipitation variability in arid climate regimes. Journal of Arid Environments 67 (2006): 607-628.
- Sharaf MA, Hussein M. Groundwater quality in the Saq aquifer, Saudi Arabia. Hydrological sciences journal 41 (1996): 683-696.
- Powers R, Ramirez L, Redmond C, et al. Geology of the Arabian peninsula. Geological survey professional paper 560 (1966): 1-147.
- Sharief FA, Magara K, Abdulla HM. Depositional system and reservoir potential of the Middle Cretaceous Wasia Formation in central-eastern Arabia. Marine and Petroleum Geology 6 (1989): 303-315.
- Allen H, Henderson M, Haas C. What's in that bottle of water? Chemtech 21 (1991): 738-742.
- Allen HE, Halley-Henderson MA, Hass CN. Chemical composition of bottled mineral water. Archives of Environmental Health: An International Journal 44 (1989): 102-116.
- Banks D, Hall G, Reimann C, et al. Distribution of rare earth elements in crystalline bedrock groundwaters: Oslo and Bergen regions, Norway. Applied Geochemistry 14 (1999): 27-39.
- Gießmann U, Greb U. High resolution ICP-MS-a new concept for elemental mass spectrometry. Fresenius' journal of analytical chemistry 350 (1994): 186-193.
- Hall G, Vaive J, Pelchat J. Performance of ICP mass spectrometric methods used in the determination of trace elements in surface waters in hydrogeochemical surveys. Journal of Analytical Atomic Spectrometry 11 (1996): 779-786.
- Moens L, Jakubowski N. Double-focusing mass spectrometers in ICP-MS. Anal.Chem 70 (1998): 251A-256A.
- Reimann C, Hall G, Siewers U, et al. Radon, fluoride and 62 elements as determined by ICP-MS in 145 Norwegian hard rock groundwater samples. Science of the Total Environment 192 (1996): 1-19.
- Riondato J, Vanhaecke F, Moens L, et al. Fast and reliable determination of (ultra-) trace and/or spectrally interfered elements in water by sector field ICP-MS. Journal of Analytical Atomic Spectrometry 15 (2000): 341-345.
- Mohan SV, Nithila P, Reddy SJ. Estimation of heavy metals in drinking water and development of heavy metal pollution index. Journal of Environmental Science and Health Part A 31 (1996): 283-289.
- Horton RK. An index number system for rating water quality. Journal of Water Pollution Control Federation 37 (1965): 300-306.
- Ritter JA. Water quality. CO: American Water Works Association (2010).
- Chang H. Spatial and temporal variations of water quality in the Han River and its tributaries, Seoul, Korea, 1993-2002. Water, Air, and Soil Pollution 161 (2005): 267-284.
- Guidelines for Drinking-water Quality. Chapter 9 Radiological Aspects, fourth ed. WHO, Geneva (2011).
- Kannel PR, Lee S, Lee YS, et al. Application of water quality indices and dissolved oxygen as indicators for river water classification and urban impact assessment. Environmental monitoring and assessment 132 (2007): 93-110.
- Al-Jaseem QK, Almasoud FI, Ababneh AM, et al. Radiological assessment of water treatment processes in a water treatment plant in Saudi Arabia: Water and sludge radium content, radon air concentrations and dose rates. Science of the Total Environment 563 (2016): 1030-1036.
- Letterman RD, Driscoll CT. Survey of residual aluminum in filtered water. Journal?American Water Works Association 80 (1988): 154-158.
- Reiber S, Kukull W, Standish-Lee P. Drinking water aluminum and bioavailability. Journal of the American Water Works Association 87 (1995).
- Fuge R, Perkins W. Aluminium and heavy metals in potable waters of the north Ceredigion area, mid-Wales. Environmental geochemistry and health 13 (1991): 56-65.
- Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 533 (2003): 153-171.


 Impact Factor: * 3.6
Impact Factor: * 3.6 Acceptance Rate: 76.49%
Acceptance Rate: 76.49%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 