An Independent Analysis of a Retrospective Cohort of 30,423 Covid-19 Patients Treated at IHU-Mediterranean in Marseille, France: Part 1, Efficacy of early Treatment with Hydroxychloroquine and Azithromycin
Valere Lounnas*, 1, Eleftherios Gkioulekas2, Marc Rendell3, Alexis Lacout4, Xavier Azalbert5, Christian Perronne6
1EMBL Heidelberg alumni, Meyerhofstraße 1, 69117 Heidelberg, Germany.
2School of Mathematical and Statistical Sciences,
University of Texas Rio Grande Valley, Edinburg, Texas, USA.
3Newport Coast, CA 92657.
4Centre de diagnostic ELSAN, 15000 Aurillac, France.
5Former student at Toulouse Schools of Economics and Econometrics - TSE, France.
6Infectious and Tropical Diseases, Paris, France
*Corresponding author:Valere LOUNNAS. EMBL Heidelberg alumni, Meyerhofstraße 1, 69117 Heidelberg, Germany.
Received: February 02, 2024; Accepted: February 09, 2024; Published: February 29, 2024
Article Information
Citation:
Valere Lounnas, Eleftherios Gkioulekas, Marc Rendell Alexis Lacout, Xavier Azalbert, Christian Perronne. An Independent Analysis of a Retrospective Cohort of 30,423 Covid-19 Patients Treated at IHU- Mediterranean in Marseille, France: Part 1, Efficacy of early Treatment with Hydroxychloroquine and Azithromycin. Archives of Microbiology and Immunology. 8 (2024): 51-66.
View / Download Pdf Share at FacebookAbstract
A cohort of 30,423 Covid-19 patients treated between March 2020 and December 2021 at the IHU-Méditerranée Infection in Marseille (France) was retrospectively analyzed in terms of treatment attempted and disease worsening factors to quantify efficacy with respect to the composite endpoint of transfer to intensive care unit or death, within a couple of months (56 days) from admission. Within limitations of the data and of the models, after adjustment for sampling biases, multivariate logistic regression analyses were performed to determine unadjusted and adjusted odds ratios (ORs) for the subset of patients having received the combined treatment hydroxychloroquine plus azithromycin (HCQ-AZ) or no specific treatment (i.e. no HCQ, no AZ and no ivermectin (IVM)) (24,943 patients). An efficacy of 58% in reducing the risk of ICU transfer and death was measured (HCQ-AZ unadjusted OR = 0.499; 95%CI = [0.343; 0.727], p < 0.001) (HCQ-AZ adjusted OR = 0.419; 95%CI = [0.327; 0.539], p < 0.001). AZ without HCQ but associated with ivermectin in 31.3% of the cases was significantly active as well with respect to no specific treatment, with a measured efficacy of 27% (unadjusted OR = 0.720, 95% CI = [0.574; 0.905] p = 0.005 and adjusted OR = 0.727, 95%CI = [0.608; 0.870] p < 0.001). Interactions between HCQ-AZ and the model covariates were systematically explored. No interaction between HCQ-AZ treatment and vaccination was detected. Statistically significant favorable interactions were detected between HCQ-AZ treatment and male sex, age categories ≥ 50 years, the UK variant and when the variant was not determined, obesity, chronic obstructive pulmonary disease (COPD), cancer, immunodeficiency, confirming the high efficacy of this early treatment. No statistically significant unfavorable interaction of HCQ-AZ with any covariate was detected. Limitations of the models and their implications for the results are discussed extensively.
Keywords
<p>Covid-19, observational cohort, hydroxychloroquine, azithromycin, multivariate analysis, logistic regression, propensity score matching, monocentric study, university medical institute, IHU-Méditerranée Infection</p>
Article Details
Introduction
About a year and a half after the end of the acute phase of the COVID-19 pandemic, reassessing the effectiveness of COVID-19 vaccination against severe outcomes using real-world data remains of keen interest for the scientific community and the broad public. Indeed, it turned out at the end of 2021 that vaccination did not hampered the virus inter-human transmission, as it was initially claimed by the manufacturers and their television affiliates [1-4]. The only potential benefit expected from mass vaccination was thus to reduce the number of aggravated disease conditions with the necessity of repeated injections at few months interval due to rapid waning of immunologic markers that were expected to reflect vaccine efficacy [5]. A retrospective cohort study conducted in the UK between December 8, 2020, and February 24, 2021 showed that at 28 days post vaccination with Oxford/AstraZeneca and Pfizer/BioNTech vaccines: “A very low proportion of hospital admissions were seen in vaccinated individuals who tested positive for SARS-CoV-2 (288/389,587, 0.07% of all patients vaccinated) providing evidence for vaccination effectiveness after a single dose” [6]. A preprint article in MedRxiv of October 2021 that has not yet been reviewed by a journal claimed that “among individuals infected with COVID-19, vaccination significantly reduced the risk of death (adj. HR: 0.20 [0.08 - 0.49])” [7].
However, the “announced” efficacy of COVID-19 vaccines proved to wane rapidly with an effectiveness on severe case of COVID-19 dropping to about 50 to 60% after 4 months [8]. Moreover, there was “some evidence for lower vaccine effectiveness in men than in women and in older individuals than in younger individuals” [8]. A critical appraisal of the marketed effectiveness of COVID-19 vaccines in reducing severe form of COVID-19 and death is all the more urgently needed since severe unwanted side effects have been associated with them. For instance, a nationwide, population-based, retrospective cohort study was conducted in Korea showing that the ChAdOx1, BNT162b2, mRNA-1273 vaccines significantly increased by a factor comprised between 1,4 and 2.6 the risk of ptosis both in the early (within 60 days) and late phase (61 - 180 days) post- vaccination [9]. The benefit/risk analysis of the vaccination appears to have moved towards increasing real life risks that would outweight the “marketed benefits”. At the present time, it is difficult to appraise the proportion of severe adverse effects resulting from the mass vaccination in Europe and in other westernized countries due to the reluctance of general practioners to declare to the pharmacovigilance units unexpected and expected suspected severe side effects following vaccine injection. The tip of the iceberg is revealed in the Eudravigilance database of the European Medical Agency (EMA), covering 32 countries, where 11 448 cases of Covid-19 vaccination with fatal outcomes, along with about 1 million adverse side effects, were reported by December 2022 [10]. In an observational study, from Dec 14, 2020, to June 14, 2021, the US vaccine adverse event report system (VAERS) processed 340 522 reports for 298 792 852 doses of mRNA vaccines administered in the USA: 313 499 (92·1%) were non-serious, 22 527 (6·6%) were serious (non-death), and 4496 (1·3%) were deaths [11]. A review of peer-reviewed published autopsy reports of vaccine-induced myocarditis has identified 28 deaths that have a clear causal link with COVID-19 vaccination [12]. What might very well be the tip of an iceberg of severe adverse events [13], is an unacceptable safety record for a vaccination that is intended to be administered, even mandated, to healthy individuals, especially when there was a safe and effective treatment option at hand, as shown in Part 1 (see Part 1 [14]).
In Part 1 of our study [14], we provided an overview of the research profile of IHU-Méditerranée Infection in Marseilles (France), and explained the circumstances and need for an independent analysis of the published data set[15] of 30,423 COVID-19 patients treated between March 2020 and December 2021 to ascertain the treatment efficacy of the innovative protocols used by the IHU-Méditerranée medical teams. Part 1 has confirmed [14] the recently published results by the IHU Méditerranée group [16] in support of demonstrating treatment efficacy, using a more comprehensive statistical approach, combining propensity score matching with logistic regression analysis. Because the vaccination status of a significant proportion of the patients in the IHU Méditerranée dataset is known, in Part 2 we shall analyze the same database using the same statistical methodology to investigate whether vaccination has any efficacy towards reducing deaths or admissions to the ICU, while controlling for demographic variables and choice of treatment.
Methods
The monocentric retrospective cohort of 30,423 COVID-19 patients of IHU-Méditerranée Infection was downloaded from a public depository site DRYAD [17]. A subset consisting of a very large number of patients (N=16063) with known vaccination status (vaccinated and unvaccinated) in the time period from 23 November 2020 until 21 December 2021 was extracted. Although this is not specified in the raw data supplied to the public, we assume that all patients for whom the last dose of vaccine was injected more than 4 months previously were considered as unvaccinated. Baseline characteristics, including all the variables (also indistinctly called covariates) at disposal, were established for the two subsets and Chi-square tests were calculated to evaluate the proportion imbalance in the percentage of occurrence between vaccinated and unvaccinated patients of every baseline demographic variable, treatment received: hydroxychloroquine (HCQ), ivermectin (IVM) and azithromycin (AZ)), disease severity and disease aggravating cofactors including risk factors (diabetes, high blood pressure and obesity) and other aggravating factors (asthma, chronic obstructive pulmonary disease - COPD -, immunodeficiency, auto-immune diseases and chronic cardiac diseases). A p-value < 0.05 means there are less than 5% chances to be wrong when asserting that the proportions differ between the vaccinated and unvaccinated subgroup for a given baseline characteristic. The “period” categorical variable was excluded from the covariates list (see result section of Part 1 of our study for detailed analysis). Of note, the treatment variables HCQ, IVM and AZ overlap (meaning they are not independent) to some extent since HCQ was administered with or without AZ and sometimes interrupted to be followed by ivermectin. In our study we have created the independent variable “AZ_only” to specify when only AZ was administered to the patient.
Variables assessing event-free survival were constructed at 42, 56, 90 and 640 days following admission at the IHU- Méditerranée either as outpatient (ambulatory care) or as inpatient (hospitalized patient), the first day was the day of admission. These variables were set either to 0 by default or to 1 when death or transfer to the intensive care unit (ICU) occurred in the period of time considered. Multivariate analysis using logistic models regressed on the baseline covariates was performed with the software package R version 4.3.1 [18] to assess the effect of vaccination on event- free survival. Detail of the statistical calculations performed is described in Part 1 of our study [14].
Results
Baseline characteristics:
Baseline characteristics of the subset “Vaccination” are collected in Table 1 showing imbalances (p < 0.001) for most of the variables and risk factor covariates between the treatment and control groups.
Analysis of the Variables (covariates) Independence
Tables 2 presents the detailed analysis of the categorical variable “Variant” in the subset “Vaccination”. It shows that the number of events (death or transfer to ICU within 56 days from admission) is homogeneously distributed (ca. 1.4%) in the unvaccinated patients across the different variants that have appeared between Nov. 23, 2020 and Dec. 21, 2021. Except for the “null” category (data missing or not determined) which was multicorrelated to other categories (see Part 1 of our study, [14]), the variant categories were independent from each other and thus the categorical variable “Variant” is well suited for logistic regression.
In Part 1 of our study [14] we noted that there was a large chunk of missing data for the covariates describing disease aggravating factors in the dataset of IHU-Méditerranée. In fact, for the “Period” category ≤ 3, all these covariates had null values. We had used a statistical procedure to deal with the issue, possibly introducing bias in the results. However, in the present case, the Vaccination subset corresponds to the “Period” ≥ 4 where all risk factors and disease aggravating factors covariates are informed. Table 3 shows that these covariates were actually highly correlated among themselves. Patients at risk who experienced one event (ICU transfer or death within 56 days) presented on average a ratio of 1.92 (317/165) disease aggravating factors. 77.8% (168/216) of the events were experienced by inpatients, 73.9% (216/287) of which presented at least one disease aggravating factors (comorbidities). 57.4% (74/129) of the inpatients at risk, who experienced an event, presented at least 2 disease aggravating factors.
Analysis of the events distribution across risk factors and aggravating factors
Tables 4a and 4b show the distribution of events across all covariates in the women and men subgroups, respectively. Covariates with zero event (highlighted in orange color) either in the vaccinated group or in the control group may have some impact on the accuracy of the logistic regression results because a null propensity (zero score) does not reflect reality, although calculation can still be performed.
Propensity score matching
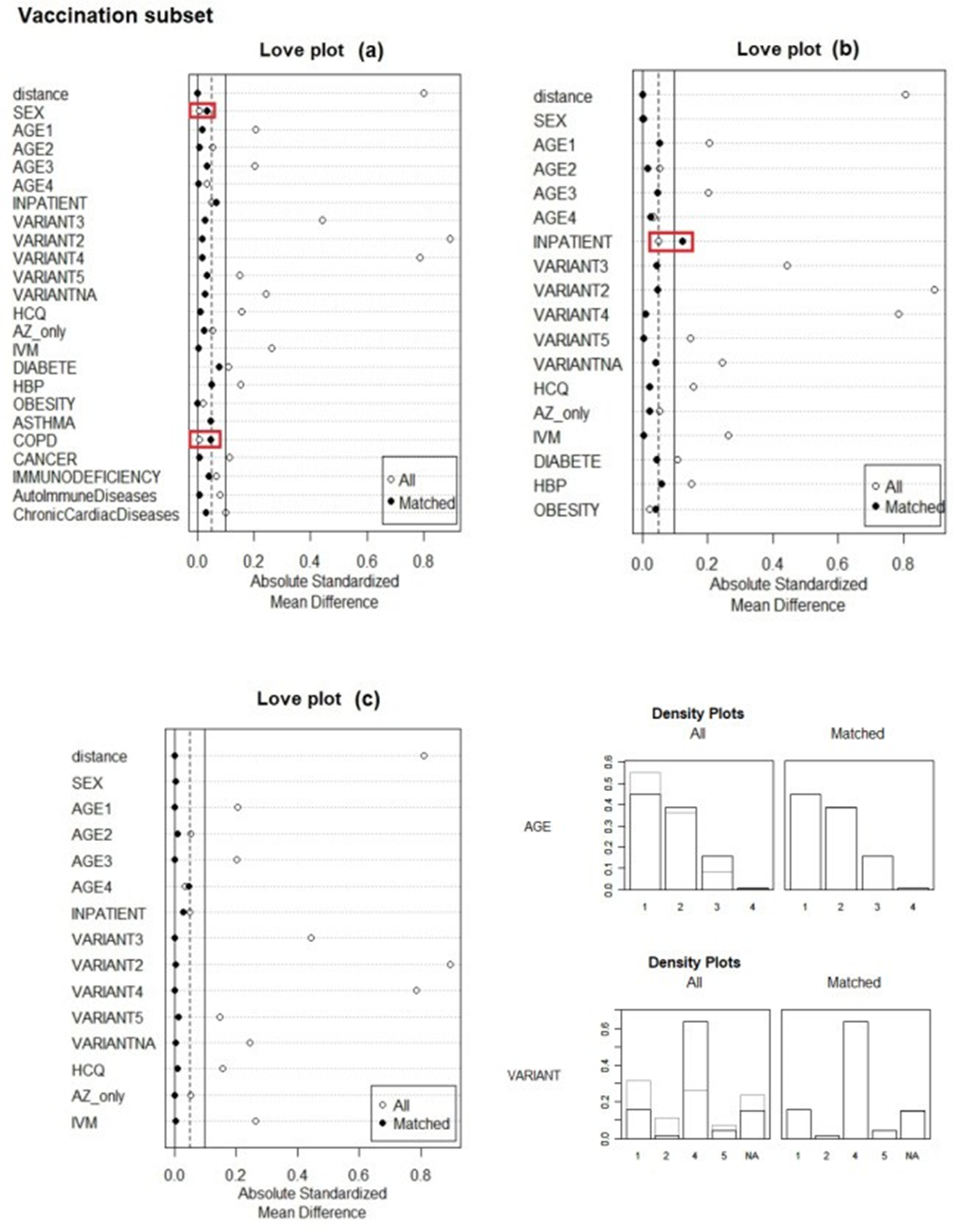
The results of the propensity score matching (PSM) procedure using Optimal Full Matching (“full” option) to correct for imbalances between treatment and control subgroups in the number of patients in every subgroups of the Vaccination subset are assessed in Figure 1. Calculations were performed for the 3 different models (a), (b) and (c). In model (a) all covariates were included. In model (b) disease aggravating factors were removed except risk factors (diabetes, high blood pressure and obesity) and in model (c) both risk factors covariates and disease aggravating factor covariates were removed. Figures of merit of the PSM calculations are presented in the supplementary material section (appendices 1a, 1b and 1c). Each subset required several minutes of central processing unit time (ca. 5 minutes). The Love plots calculated for the 3 different models (a) (b) and (c) showed the absolute standardized mean differences (ASMD) between treatment and control subgroups, before and after matching for each covariate (Figure 1).
Ideally, ASMDs should approach 0.0 and be lower than the ASMDs for unmatched subgroups. For instance, when all covariates were included (model (a)) we noted that the procedure could not converge to improve balance for the covariates SEX and COPD that were slightly deteriorated. In model (b) it is the covariate “Inpatient” that is deteriorated instead of being improved. In fact, PSM allowed improvement in all covariate subgroups only when risk factors and disease aggravating were removed in model (c). The reason is the high level of correlation between all these covariates (see previous section). Overall, all ASMDs ≤ 0.1 are usually considered as acceptable matching [19,20]. Here we have this condition fulfilled, especially for model with most values close to 0.05 insuring high reliability for this particular model which includes all confounding Density plots are shown to illustrate the efficacy of the PSM balancing procedure for the categorical covariates “Age” and “Variant” in model (c).
Table 1: Baseline characteristics for the “Vaccination” subset covering the pandemic periods from Nov. 23, 2020 until Dec. 21, 2021
|
All |
Vaccinated |
Not vaccinated |
p-value* |
||||
|
N |
% |
N |
% |
N |
% |
||
|
N |
16063 |
2124 |
13939 |
||||
|
Women |
7532 |
46.9 |
991 |
46,7 |
6541 |
46.9 |
0.906 |
|
Men |
8531 |
53.1 |
1133 |
53.3 |
7398 |
53.1 |
0.914 |
|
Age (yrs) |
|||||||
|
< 50 |
8627 |
53.7 |
953 |
44.9 |
7674 |
55.1 |
< 0.001 |
|
50-69 |
5898 |
36.7 |
827 |
38.9 |
5071 |
36.4 |
0.13 |
|
70-89 |
1483 |
9.2 |
332 |
15.6 |
1151 |
8.3 |
< 0.001 |
|
> 89 |
55 |
3.4 |
12 |
0.6 |
43 |
0.3 |
0.093 |
|
SARS-CoV-2 variants |
|||||||
|
Wuhan |
0 |
0 |
0 |
||||
|
B.1.160 (Marseille 4) |
1601 |
10 |
27 |
1.3 |
1574 |
11.3 |
< 0.001 |
|
B.1.7.7 (UK) |
4764 |
29.7 |
333 |
15.7 |
4431 |
31.8 |
< 0.001 |
|
B.1.617.2 (Delta) |
4973 |
31 |
1355 |
63.8 |
3618 |
26 |
< 0.001 |
|
Outpatients only |
14868 |
92.6 |
1989 |
93.6 |
12879 |
92.4 |
0.701 |
|
Inpatients only |
728 |
4.5 |
89 |
4.2 |
639 |
4.6 |
0.47 |
|
Out- and In-patients |
467 |
2.9 |
46 |
2.2 |
421 |
3 |
0.04 |
|
Diabetes |
1035 |
6.4 |
195 |
9.2 |
840 |
6 |
< 0.001 |
|
High blood pressure |
2214 |
13.8 |
402 |
18.9 |
1812 |
13 |
< 0.001 |
|
Obesity |
3272 |
20.4 |
417 |
19.6 |
2855 |
20.5 |
0.478 |
|
Patient with ≥ 2 of the 3 factors above |
1284 |
8 |
228 |
10.7 |
1056 |
7.6 |
< 0.001 |
|
COPD |
222 |
1.3 |
31 |
1.5 |
191 |
1.4 |
0.823 |
|
Cancer |
698 |
4.3 |
145 |
6.8 |
553 |
4 |
< 0.001 |
|
Auto-immune diseases |
870 |
5.41 |
154 |
7.3 |
716 |
5.1 |
< 0.001 |
|
Chronic cardiac diseases |
428 |
2.7 |
95 |
4.5 |
333 |
2.4 |
< 0.001 |
|
ICU transfer |
156 |
1 |
13 |
0.6 |
143 |
1 |
0.093 |
|
Death days |
|||||||
|
42 |
73 |
0.5 |
6 |
0.3 |
67 |
0.5 |
0.277 |
|
56 |
77 |
0.5 |
7 |
0.3 |
70 |
0.5 |
0.368 |
|
90 |
80 |
0.5 |
8 |
0.3 |
72 |
0.5 |
0.494 |
|
640 |
110 |
0.7 |
12 |
0.6 |
98 |
0.7 |
0.566 |
|
Received HCQ |
13258 |
82.5 |
1631 |
76.8 |
11627 |
83.4 |
0.003 |
|
Received |
12948 |
80.6 |
1599 |
75.3 |
11349 |
81.4 |
0.028 |
|
HCQ-AZ |
|||||||
|
Received HCQ only |
296 |
1.8 |
31 |
1.5 |
265 |
1.9 |
0.194 |
|
Received AZ only |
1025 |
6.4 |
114 |
5.4 |
911 |
6.5 |
0.036 |
|
Received ivermectin |
1577 |
9.8 |
398 |
18.7 |
1179 |
8.5 |
< 0.001 |
|
Received HCQ and ivermectin |
292 |
1.8 |
66 |
3.11 |
226 |
1.6 |
< 0.001 |
*Chi-square test for the equality of proportion: p < 0.05 indicates that the proportion statistically differ between the two groups for the considered baseline characteristic.
Table 2: “Variant” variable analysis: Vaccination subset at 56 days
|
Variant |
Patients |
Vaccinated |
Events in vaccinated |
Events in control group |
|||
|
N (%) |
N |
(%) |
N |
(%) |
N |
(%) |
|
|
Wuhan |
0 |
0 |
(0.0) |
0 |
(0.0) |
0 |
(0.0) |
|
Marseille 4 |
1601 |
27 |
(1.7) |
0 |
(0.0) |
24 |
(1.5) |
|
UK |
4764 |
333 |
(7.0) |
8 |
(2.4) |
71 |
(1.6) |
|
Delta |
4973 |
1355 |
(27.2) |
11 |
(0.8) |
51 |
(1.4) |
|
others |
1079 |
88 |
(8,2) |
0 |
(0.0) |
19 |
(1.9) |
|
null |
3646 |
321 |
(8.8) |
0 |
(0.0) |
30 |
(0.9) |
|
total |
16063 |
2124 |
(13.2) |
19 |
(0.9) |
195 |
(1.4) |
Table 3: Events distribution at 56 days for Covid-19 disease Aggravating Covariates
|
Vaccination subset reduced to “Period” ≥ 4 |
||||||||
|
Vaccinated (N=2124) |
Unvaccinated (N=13939) |
|||||||
|
N |
% |
Events |
% |
N |
% |
Events |
% |
|
|
Patients total |
2124 |
- |
19 |
0.9 |
13939 |
- |
195 |
1.4 |
|
Inpatient |
135 |
6.4 |
16 |
11.9 |
1060 |
7.6 |
151 |
14.3 |
|
Risk factors covariates |
||||||||
|
Diabetes |
195 |
9.2 |
5 |
2.6 |
840 |
6 |
45 |
5.4 |
|
Obesity |
417 |
19.6 |
5 |
1.2 |
2855 |
20.5 |
91 |
3.2 |
|
High blood pressure |
402 |
18.9 |
8 |
2 |
1812 |
13 |
79 |
4.4 |
|
Asthma |
184 |
8.7 |
1 |
0.5 |
1022 |
7.3 |
12 |
1.2 |
|
COPD |
31 |
1.5 |
2 |
6.5 |
191 |
1.4 |
11 |
5.8 |
|
Cancer |
145 |
6.8 |
2 |
1.4 |
553 |
4 |
17 |
3.1 |
|
Immunodeficiency |
62 |
2.9 |
4 |
6.5 |
249 |
17.9 |
6 |
2.4 |
|
Auto-immune diseases |
154 |
7.3 |
1 |
0.6 |
716 |
5.1 |
6 |
0.8 |
|
Chronic cardiac diseases |
95 |
4.5 |
1 |
1.1 |
333 |
2.4 |
19 |
5.7 |
|
Total redundant number of events1 |
29 |
286 |
||||||
|
Patient at risk2 |
1026 |
48.3 |
13 |
1.3 |
5858 |
42 |
153 |
2.6 |
|
Inpatient at risk3 |
95 |
4.5 |
10 |
10.5 |
729 |
5.2 |
121 |
16.6 |
|
Inpatient not at risk4 |
40 |
2.4 |
6 |
15 |
331 |
2.4 |
30 |
9.1 |
|
Inpatient with one aggravating cofactor |
40 |
1.9 |
3 |
7.5 |
355 |
2.5 |
52 |
14.6 |
|
Inpatient with ≥ 2 aggravating cofactors |
52 |
2.4 |
6 |
11.5 |
354 |
2.5 |
68 |
19.2 |
1a number of patients that experienced a defined event (ICU transfer or death with 56 days) had more than 1 baseline disease aggravating factors.
2patients presenting at least one disease aggravating cofactor.
3inpatients with at least one disease aggravating cofactor.
4inpatients with no disease aggravating cofactor.
Table 4a: Analysis of events at 56 days in women subgroup (N=8531)
|
Vaccination Status |
||||||
|
Vaccinated |
Unvaccinated |
|||||
|
N |
Event |
% |
N |
Event |
% |
|
|
Women |
1133 |
7 |
0.6 |
7398 |
61 |
0.8 |
|
Age (yrs) |
||||||
|
< 50 |
548 |
1 |
0.2 |
4110 |
4 |
0.1 |
|
50-69 |
427 |
3 |
0.7 |
2637 |
32 |
1.2 |
|
70-89 |
151 |
1 |
0.7 |
629 |
22 |
3.5 |
|
> 89 |
7 |
2 |
28.6 |
22 |
3 |
13.6 |
|
SARS-CoV-2 variants |
||||||
|
Wuhan |
0 |
0 |
0 |
0 |
0 |
|
|
B.1.160 (Marseille 4) |
22 |
0 |
836 |
8 |
||
|
B.1.7.7 (UK) |
165 |
2 |
2295 |
27 |
||
|
B.1.617.2 (Delta) |
716 |
5 |
1908 |
19 |
||
|
Others |
44 |
0 |
504 |
3 |
||
|
Null |
186 |
0 |
1855 |
4 |
||
|
Inpatients |
53 |
6 |
11.3 |
452 |
47 |
10.4 |
|
Treatments |
||||||
|
HCQ |
883 |
2 |
0.2 |
6098 |
28 |
0.5 |
|
AZ only |
55 |
2 |
3.6 |
519 |
14 |
2.7 |
|
Ivermectin |
213 |
3 |
1.4 |
664 |
19 |
2.9 |
|
Risk factors |
||||||
|
Diabetes |
74 |
1 |
1.4 |
404 |
16 |
4 |
|
High blood pressure |
186 |
3 |
0.5 |
881 |
27 |
3.1 |
|
Obesity |
202 |
2 |
1 |
1401 |
28 |
2 |
|
Comorbidities |
||||||
|
COPD |
16 |
0 |
NA |
95 |
2 |
2.1 |
|
Cancer |
82 |
1 |
1.2 |
373 |
5 |
1.3 |
|
Asthma |
114 |
1 |
0.9 |
633 |
5 |
0.8 |
|
Immunodeficiency |
30 |
1 |
3.3 |
143 |
2 |
1.4 |
|
Auto-immune diseases |
119 |
1 |
0.8 |
546 |
3 |
5.5 |
|
Chronic cardiac diseases |
17 |
0 |
NA |
84 |
2 |
2.4 |
|
Events summary |
7 |
0.6 |
61 |
0.8 |
||
|
ICU transfer |
4 |
0.4 |
41 |
0.6 |
||
|
Death days |
||||||
|
42 |
3 |
0.3 |
25 |
0.3 |
||
|
56 |
3 |
0.3 |
25 |
0.3 |
||
|
90 |
4 |
0.4 |
27 |
0.4 |
||
|
640 |
5 |
0.5 |
38 |
0.5 |
||
Table 4b: Analysis of events at 56 days in men subgroup (N=7532)
|
Vaccinated |
Unvaccinated |
|||||
|
N |
Event |
% |
N |
Event |
% |
|
|
Men |
991 |
12 |
1.2 |
6541 |
136 |
2.1 |
|
Age (yrs) |
||||||
|
< 50 |
405 |
1 |
0.2 |
3564 |
23 |
0.6 |
|
50-69 |
400 |
5 |
1.3 |
2434 |
66 |
2.7 |
|
70-89 |
181 |
6 |
3.3 |
522 |
40 |
7.7 |
|
> 89 |
5 |
0 |
NA |
21 |
5 |
23.8 |
|
SARS-CoV-2 variants |
||||||
|
Wuhan |
0 |
0 |
||||
|
B.1.160 (Marseille 4) |
2 |
0 |
738 |
16 |
2.2 |
|
|
B.1.7.7 (UK) |
168 |
6 |
2136 |
44 |
2.1 |
|
|
B.1.617.2 (Delta) |
639 |
6 |
3.6 |
1710 |
32 |
1.9 |
|
Others |
44 |
0 |
0.9 |
487 |
16 |
3.3 |
|
Null |
135 |
0 |
1470 |
26 |
1.8 |
|
|
Inpatients |
82 |
10 |
12.2 |
608 |
104 |
17.1 |
|
Treatments |
||||||
|
HCQ with or without AZ |
748 |
6 |
0.8 |
5529 |
89 |
1.6 |
|
AZ only |
59 |
2 |
3.4 |
392 |
22 |
5.6 |
|
Ivermectin |
185 |
5 |
2.7 |
515 |
29 |
5.6 |
|
Risk factors |
||||||
|
Diabetes |
121 |
4 |
3.3 |
436 |
29 |
6.7 |
|
High blood pressure |
216 |
5 |
2.3 |
931 |
52 |
5.6 |
|
Obesity |
215 |
3 |
1.4 |
1454 |
63 |
4.3 |
|
Comorbidities |
||||||
|
COPD |
5 |
2 |
40 |
96 |
9 |
9.4 |
|
Cancer |
63 |
1 |
1.6 |
180 |
12 |
6.7 |
|
Asthma |
70 |
0 |
NA |
389 |
7 |
1.8 |
|
Immunodeficiency |
106 |
3 |
2.83 |
32 |
4 |
12.5 |
|
Auto-immune diseases |
35 |
0 |
NA |
170 |
3 |
NA |
|
Chronic cardiac diseases |
78 |
1 |
1.2 |
249 |
17 |
6.8 |
|
Events summary |
12 |
1.2 |
134 |
2 |
||
|
ICU transfer |
9 |
0.9 |
100 |
1.5 |
||
|
Death days |
||||||
|
42 |
3 |
0.3 |
42 |
0.6 |
||
|
56 |
4 |
0.4 |
45 |
0.7 |
||
|
90 |
4 |
0.4 |
45 |
0.7 |
||
|
640 |
7 |
0.7 |
60 |
0.9 |
||
Logistic regression model - multivariate analysis
The logistic models regressed on all the IHU-Méditerranée baseline predefined covariates (except for the “Period” covariate that was excluded) converged. Results at 56 days cutoffs are presented for models (a), (b) and (c) in Table 5. Results for model (a) with PSM at 42, 56, 90 and 640 days cutoffs using full optimal matching are presented in Table 5b. One can notice a regular decrease of vaccination efficacy with the cutoff time increasing. There is an absolute 6 7% decrease in the reduction of the risk of events between 42 days and 640 days cutoffs indicating that after Covid-19 remission, vaccinated patients survive less well over the next two years than unvaccinated patients.
Multivariate logistic regressions with adjusted ORs at 56 days cutoff using Optimal Full Matching are presented with all covariates (model (a)), with only risk factors covariates (model (b)) and without risk factors and comorbidities covariates (model (c)) for the “Vaccination” subset (Table 6a, Table 6b and Table 6c). The reference for the “Variant” categorical variable was set to B.1.7.7 (UK) instead of B.1.160 (Marseille 4) because of too few vaccinated individuals and an absence of event in the vaccinated group for the variant Marseille 4 category (see Table 2) leading to strong model bias.
In all models the calculated OR evaluating the average treatment effect in the treated patients (ATT) was statistically significant indicating the overall efficacy of vaccination. Adjusted treatment ORs were comprised between 0.28 and 0.46 with respect to no vaccination, that is to say a reduction of the risk of being transferred to ICU or dying reduced by 54% (model (a)) to 72% (model (c)). We see the calculated treatment OR is quite sensitive to the inclusion of risk factors and comorbidities in the model because many covariates are partial confounders. Thus, the effect of vaccination must be carefully appraised taking into account all covariates. A too simplistic approach (model(c)) may actually lead to an overestimate of the vaccination benefit. The inclusion of risk factors and comorbidities covariates in the models largely suppressed this apparent global effect of vaccination (model (c)).In this respect, according to model (a) with risk factors and comorbidities covariates included the general trend of the vaccination effect is around an absolute risk reduction of 54% only. The problem with vaccination is that it interacts negatively with certain categories of patients as we show in the next section (Interaction analysis).
Analysis of the covariates ORs (that assess their separate effects independently of other covariates by setting all their values to 0) showed that the variables “sex”, “age” and “inpatient”, describing the patient demographic characteristics and disease severity, all had a statistically significant impact (p < 0.001) with comparable OR values trends between model (a), (b) and (c). The odds ratio was very favorable for women whereas it became very unfavorable as age increases and with disease severity. The trends are consistently the same indicating model robustness in this respect. For age categories ≥ 50 years, a statistically significant increase in the risk of event (OR >>1) was observed for each age subgroup with respect to patients of age < 50 years.
OR = 13.9 for the covariate “inpatient” (model(a)) means that hospitalized patients had overall 14 times more chances to be transferred to ICU or to die than ambulatory (day hospital) patients.
Table 5a: Treatment ORs for the different logistic regression models calculated at 56 days cutoff using PSM with Optimal Full Matching
|
“Vaccination” subset |
||||||
|
Model |
OR |
95%CI |
p-value |
OR |
95%CI |
p-value |
|
unadjusted after PSM |
adjusted after PSM |
|||||
|
a |
0.498 |
[0.313 ; 0.794] |
0.003 |
0.462 |
[0.307 ; 0.670] |
< 0.001 |
|
b |
0.393 |
[0.222 ; 0.698] |
0.001 |
0.336 |
[0.209 ; 0.516] |
< 0.001 |
|
c |
0.353 |
[0.186 ; 0.669] |
0.001 |
0.278 |
[0.162 ; 0.450] |
< 0.001 |
Table 5b: Logistic regression models treatment adjusted ORs calculated at different time cutoffs for model (a) (PSM with Optimal Full
Matching)
|
Vaccination subset |
Vaccination subset |
|||||
|
Cutoff (days) |
unadjusted ORs after PSM |
adjusted ORs after PSM |
||||
|
OR |
95%CI |
p-value |
OR |
95%CI |
p-value |
|
|
42 |
0.475 |
[0.298 ; 0.757] |
0.002 |
0.438 |
[0.288 ; 0.640] |
< 0.001 |
|
56 |
0.498 |
[0.313 ; 0.794] |
0.003 |
0.462 |
[0.307 ; 0.670] |
< 0.001 |
|
90 |
0.521 |
[0.329 ; 0.826] |
0.006 |
0.486 |
[0.326 ; 0.700] |
< 0.001 |
|
640 |
0.542 |
[0.355; 0.827] |
0.005 |
0.508 |
[0.357 ; 0.704] |
< 0.001 |
As for the “Variant” categorical variable, results were very unstable across model (a), (b) and (c) depending strongly on whether or not risk factors and other comorbities covariates are included in the model, reflecting intrication between the “Variant” variable and comorbidities. There was no statistically significant impact (p > 0.05) for the Marseille 4, UK and Delta main variants with respect to the risk of event in the complete model (model (a)) nor for the categories “other” variant and “null” variant. Regarding the effect of other treatments, hydroxychloroquine (HCQ) (with or without azithromycin and sometimes with ivermectin subsequently) was significantly associated with a risk reduction in both models (a) and (b) whereas ivermectin (IVM) was significantly associated with an increase of the risk of event in the 3 models (a), (b) and model (c). The meaning of these ORs must not be mistaken with a measured average treatment effect. However, they indicate the relative risk of event in the overall vaccination subset with respect to the covariate HCQ, AZ and Ivermectin, respectively. OR < 1 for HCQ must be interpreted as patients having less risk of experiencing event when having received HCQ and OR > 1 for ivermectin as patients having a higher risk of event when having received ivermectin. Indeed ivermectin could have been prescribed to patients with a more severe illness. Azithromycin alone was not associated with any significant effect on the risk of event in any of the 3 models.
The percentage of events in the subgroups of patients having received HCQ and ivermectin is 5.82% (17/292) and ivermectin only was 3.11% (40/1285). However, in the “Vaccination” subset the overall percentage of events dropped to hardly 1.34%. Without additional information on the patients disease condition, these results do not necessarily mean a deleterious effect of ivermectin, but could be due to the fact that ivermectin was administered in first intention or second intention in patients with aggravated disease conditions with a markedly higher risk of event. Moreover, it is worth noting that the proportion of patients having received ivermectin in the vaccinated subgroup is 18.7% that is twice as much as in the unvaccinated subgroup (8.5%) (Table 1).
Interactions analysis
All the detected interactions for the models are listed in Table 7. The complete model (a) detected statistically significant interactions of some covariates with the treatment in the “Vaccination” subset : favorable with variant Delta (VARIANT4) and unfavorable with “Age > 89 years”, “Inpatient” and “Immunodeficiency”. We report also a possible interaction with COPD (this interaction has a p-value < 0.05 when the covariate “AZ_only” is excluded from the model (a)). In model (b) favorable interactions between vaccination and “69 < Age ≤ 89” and with the variant Delta
Table 6a: Multivariate logistic regression model (a) for the “Vaccination” subset at 56 days cutoff (PSM with Optimal Full Matching)
|
“Vaccination” subset (N= 16063) |
|||
|
Treatment actor |
OR* |
95%CI |
p-value |
|
Vaccination |
0.462 |
[0.307 ; 0.670] |
< 0.001 |
|
Demography |
|||
|
Sex (female/male) |
0.533 |
[0.431 ; 0.657] |
< 0.001 |
|
Age < 50 yrs (reference) |
|||
|
Age 50-69 (vs ref.) |
2.023 |
[1.457 ; 2.852] |
< 0.001 |
|
Age 70-89 (vs ref.) |
2.876 |
[2.021 ; 4.144] |
< 0.001 |
|
Age > 89 (vs ref.) |
3.865 |
[1.830 ; 7.751] |
< 0.001 |
|
Disease severity |
|||
|
Inpatient (vs ref.) |
13.96 |
[11.242 ; 17.384] |
< 0.001 |
|
Variant |
|||
|
B.1.7.7 (UK) (reference) |
|||
|
B.1.160 (Marseille 4) |
1.127 |
[0.517 ; 2.217] |
0.744 |
|
B.1.617.2 (Delta) |
1.006 |
[0.777 ; 1.304] |
0.966 |
|
Others |
0.902 |
[0.568 ; 1.398] |
0.653 |
|
Null |
0.8085 |
[0.562 ; 1.152] |
0.38 |
|
Other treatments |
|||
|
HCQ |
0.726 |
[0.530 ; 1.000] |
0.05 |
|
Ivermectin |
1.74 |
[1.270 ; 2.379] |
< 0.001 |
|
AZ only |
0.813 |
[0.510 ; 1.287] |
0.38 |
|
Comorbidities |
|||
|
Diabetes |
1.151 |
[0.904 ; 1.460] |
0.25 |
|
Obesity |
2.507 |
[2.023 ; 3.101] |
< 0.001 |
|
High blood pressure |
1.041 |
[0.830 ; 1.304] |
0.728 |
|
Asthma |
0.325 |
[0.189 ; 0.524] |
< 0.001 |
|
COPD |
4.554 |
[3.245 ; 6.316] |
< 0.001 |
|
Cancer |
1.003 |
[0.722 ; 1.369] |
0.985 |
|
Immunodeficiency |
1.71 |
[1.131 ; 2.516] |
0.008 |
|
Auto-immune diseases |
0.592 |
[0.345 ; 0.954] |
0.04 |
|
Chronic cardiac diseases |
1.05 |
[0.778 ; 1.403] |
0.745 |
*if not indicated otherwise ORs are with respect to covariates values
set to zeros
Table 6b: Multivariate logistic regression model (b) for the “Vaccination” subset at 56 days cutoff (PSM with Optimal Full Matching)
|
“Vaccination” subset (N= 16063) |
|||
|
Treatment actor |
OR* |
95%CI |
p-value |
|
Vaccination |
0.336 |
[0.209 ; 0.516] |
< 0.001 |
|
Demography |
|||
|
Sex (female/male) |
0.416 |
[0.331 ; 0.519] |
< 0.001 |
|
Age < 50 yrs (reference) |
|||
|
Age 50-69 (vs ref.) |
2.919 |
[1.976 ; 4.406] |
< 0.001 |
|
Age 70-89 (vs ref.) |
7.13 |
[4.817 ; 10.850] |
< 0.001 |
|
Age > 89 (vs ref.) |
8.436 |
[4.255 ; 16.483] |
< 0.001 |
|
Disease severity |
|||
|
Inpatient (vs ref.) |
14.119 |
[11.331 ; 17.650] |
< 0.001 |
|
Variant |
|||
|
B.1.7.7 (UK) (reference) |
|||
|
B.1.160 (Marseille 4) |
0.347 |
[0.111 ; 0.842] |
0.036 |
|
B.1.617.2 (Delta) |
0.813 |
[0.634 ; 1.046] |
0.104 |
|
Others |
0.54 |
[0.298 ; 0.917] |
0.03 |
|
Null |
0.31 |
[0.206 ; 0.451] |
< 0.001 |
|
Other treatments |
|||
|
HCQ |
0.454 |
[0.325 ; 0.634] |
< 0.001 |
|
AZ only |
0.655 |
[0.407 ; 1.047] |
0.079 |
|
Ivermectin |
1.587 |
[1.135 ; 2.213] |
0.007 |
|
Risk factors |
|||
|
Diabetes |
0.946 |
[0.721 ; 1.234] |
0.685 |
|
Obesity |
1.732 |
[1.378 ; 2.171] |
< 0.001 |
|
High blood pressure |
0.8 |
[0.629 ; 1.014] |
0.066 |
*if not indicated otherwise ORs are with respect to covariates values
set to zeros
Table 6c: Multivariate logistic regression model (c) for Vaccination subset at 56 days cutoff (PSM with Optimal Full Matching) without risk factors and aggravating factors (comorbidities)
|
“Vaccination” subset |
|||
|
Treatment factor |
OR* |
95%CI |
p-value |
|
Vaccination |
0.278 |
[0.162 ; 0.450] |
< 0.001 |
|
Demography |
|||
|
Sex (female/male) |
0.248 |
[0.186 ; 0.326] |
< 0.001 |
|
Age < 50 yrs (reference) |
|||
|
Age 50-69 (vs ref.) |
2.878 |
[1.866 ; 4.576] |
< 0.001 |
|
Age 70-89 (vs ref.) |
12.41 |
[8.218 ; 19.430] |
< 0.001 |
|
Age > 89 (vs ref.) |
82.807 |
[45.843 ; 151.433] |
< 0.001 |
|
Disease severity |
|||
|
Inpatient |
5.839 |
[4.513 ; 7.548] |
< 0.001 |
|
Variant |
|||
|
B.1.7.7 (UK) (reference) |
|||
|
B.1.160 (Marseille 4) |
1.905 |
[0.718 ; 4.526] |
0.168 |
|
B.1.617.2 (Delta) |
2.451 |
[1.760 ; 3.474] |
< 0.001 |
|
Others |
1.544 |
[0.790 ; 2.876] |
0.186 |
|
Null |
0.514 |
[0.281 ; 0.902] |
0.025 |
|
Other treatments |
|||
|
HCQ |
0.808 |
[0.531 ; 1.226] |
0.319 |
|
AZ only |
1.238 |
[0.677 ; 2.224] |
0.485 |
|
Ivermectin |
5.1 |
[3.327 ; 7.814] |
< 0.001 |
*if not indicated otherwise ORs are with respect to covariates values
set to zeros are also found. The favorable interaction with the variant Delta and the unfavorable interaction between vaccination and “Inpatient” was consistently found in the 3 models indicating the robustness of these two findings, especially of the latter.
It must be pointed out that the OR of an interaction is the risk of event calculated for a product covariate (eg. Vaccination*Inpatient) taking the value 1 relative to the situation of the three other possibilities where this product is 0 after propensity score matching to correct for imbalances between covariate distribution between vaccinated and unvaccinated patients. For instance, unfavorable interaction of vaccination with the inpatient status means that the risk of event is higher in the group of vaccinated inpatients than in the group constituted of unvaccinated inpatients, vaccinated outpatients and unvaccinated outpatients. In fact, one can see easily that there were 65% more events in the group of vaccinated inpatients not at risk compared with the unvaccinated inpatients not at risk (see Table 3).
Table 7: Treatment: covariate interactions detected in the logistic regression models (PSM with Optimal Full Matching) at 56 days cutoff.
|
Interaction |
OR |
95%CI |
p-value |
|
“Vaccination” subset |
|||
|
Model (a) |
|||
|
VACCINATION:AGE4 |
15.149 |
[1.460 ; 164.864] |
0.022 |
|
VACCINATION:INPATIENT |
8.071 |
[2.479 ; 34.612] |
0.002 |
|
VACCINATION:VARIANT4 |
0.201 |
[0.075 ; 0.531] |
0.001 |
|
VACCINATION:OBESITY |
0.409 |
[0.142 ; 1.098] |
0.084 |
|
VACCINATION:COPD |
4.773 |
[0.781 ; 25.577] |
0.072 |
|
VACCINATION:IMMUNODEFICIENCY |
10.958 |
[2.702 ; 44.150] |
< 0.001 |
|
VACCINATION:ChronicCardiacDiseases |
0.168 |
[0.014 ; 1.028] |
0.09 |
|
Model (b) |
|||
|
VACCINATION:AGE3 |
0.124 |
[0.020 ; 0.991] |
0.031 |
|
VACCINATION:INPATIENT |
5.017 |
[1.468 ; 22.664] |
0.018 |
|
VACCINATION:VARIANT4 |
0.324 |
[0.108 ; 0.973] |
0.042 |
|
Model (c) |
|||
|
VACCINATION:AGE3 |
0.078 |
[0.011 ; 0.805] |
0.015 |
|
VACCINATION:INPATIENT |
12.536 |
[3.181 ; 70.686] |
< 0.001 |
|
VACCINATION:VARIANT4 |
0.091 |
[0.026 ; 0.319] |
< 0.001 |
Sensitivity analysis
To assess the relative efficacy of vaccination with respect to age and sex we performed additional sensitivity calculations. Due to a reduced number of patients compared with the complete “Vaccination” subset the models could not converge (spurious ORs and confidence intervals) when all covariates were included in the logistic regression. However, removing the “AZ only” covariate from the models fairly allowed their convergence (see Appendix 2 for complete calculation results).
Vaccination subset with age < 50 years (N=8627): when we reduced the Vaccination subset to age ≤ 50 years 50 years (“Age” = 1) vaccination had no efficacy: unadjusted OR = 1.32; 95%CI = [0.48; 3.66], p = 0.588 and adjusted OR = 1.35; 95%CI = [0.09 ; 8.84], p = 0.781.
Vaccination subset with age ≥ 50 years (N=7436): when we reduced the Vaccination subset to age ≥ 50 years (“Age” ≥ 2) vaccination efficacy was: unadjusted OR = 0.406 ; 95%CI= [0.251 ; 0.657], p <0.001 and adjusted OR = 0.335; 95%CI= [0.183 ; 0.572], p < 0.001 (see complete result in Appendix 2a in supplementary material).
Vaccination subset reduced to women (N = 8531): vaccination unadjusted OR = 0.518, 95%CI = [0.259 ; 1.040] p = 0.0629 and adjusted OR = 0.472; 95%CI = [0.238; 0.855] p = 0.0203 (see Appendix 2b for complete data). A weakly significant unfavorable interaction was found with immunodeficiency OR = 2.447, 95%CI = [1.319 ; 1.855] p = 0.0636 (see complete data in Appendix 2b).
Vaccination subset reduced to men (N = 7532): vaccination unadjusted OR = 0.311, 95%CI = [0.164 ; 0.588] p < 0.001 and adjusted OR = 0.222, 95%CI = [0.107 ; 0.418] p < 0.001. Statistically significant unfavorable interactions were found between vaccination and immunodeficiency: OR = 6.027, 95%CI = [1.958 ; 3.078] p = 0.0021; and vaccination and COPD: OR = 4.1000 95%CI = [1.660 ; 2.470] p = 0.0135 (see complete data in Appendix 2c).
Discussion
The main concern in Part 2 of our study was the evaluation of the Covid-19 vaccination efficacy based on a large corpus of real life data provided by a very large single institution cohort treated for Covid-19 on both an outpatient and inpatient basis. Our aim was to establish a result on vaccination efficacy that allows side by side comparison with Part 1 of our study where we evaluated the efficacy of the hydoxychloroquine-azithromycin (HCQ-AZ) combination treatment deployed at the IHU Méditerranée [14]. Since the same single institution cohort was rigorously analyzed and the same statistical methods were used to assess the HCQ-AZ and the vaccination average effects on the treated patients, it is expected that the results convey meaningful information on the respective merit of the two approaches, within the limitations of the statistical method used (see article Part 1 of our study for the detailed description of the limitations) [14].
Importantly, no statistical significant interaction was detected between vaccination and the variable HCQ in all models constructed for the “Vaccination” subset. The 3 models constructed consistently assess an efficacy of vaccination with an absolute risk reduction of events (ICU transfer or death) comprised between 54% and 72% in the vaccinated subgroup with respect to the unvaccinated subgroup. It must be pointed out that model (a) with 54% absolute risk reduction is the most accurate model because it includes all covariates that are partial confounders (muti-correlated among themselves). Despite the number of covariates and their intrication, the PSM procedure converged for this model insuring its reliability. Similarly, in Part 1 of our study, the efficacy of the HCQ-AZ combination treatment was consistently established around 54-58% with no statistically significant interaction detected with the covariate vaccination.
Although tempting, a like for like comparaison of these two numbers would be misleading as a vaccination intervention entails several issues, that are preventing it from being considered as a treatment. Firstly, the approval of vaccination presumed the absence of efficacy of treatments, which is not the case. Secondly, vaccination posed an ethical problem since it presents an irreversible modification of the immune system of healthy individuals, with the risk of inducing potentially severe unwanted or unknown side effects due to the experimental nature of the genetic therapies. Third, it was previously known that vaccination against SARS coronavirus could induce an aggravated disease state in some healthy individuals (see further sections in the discussion). And fourth, peaks of contamination were observed within 2-3 weeks subsequent to mass vaccination episodes. Therefore, vaccination efficacy ought to be measured in a different manner, as a contingent efficacy, via more advanced probabilistic methods including a context wider than the IHU dataset.
Additionally, the respective merits of these approaches for combined HCQ-AZ therapy or vaccination must be assessed also taking into account their economical costs, their easiness to administrate and rapidity of access, as well as their short term and long term side effects (benefit/risk ratio). Sensitivity assessment conducted in the “Vaccination” subset reduced to age < 50 years shows that no statistical efficacy of vaccination could be detected in terms of risk of ICU transfer or death for this category of age. Indeed, in this category the percentage of events was very low, ≤ 0.2% in women and ≤ 0.6% in men, with in fact proportionally more events in vaccinated women than in unvaccinated women. This clearly raises the question of the relevance of vaccination for age < 50 years, especially if we consider that vaccination failed to diminish transmission and contamination [1-5].
Another point of potential concern was the difference in vaccination efficacy with respect to sex. We find that vaccination is more effective in reducing the risk of event in men than in women with adjusted OR of 0.222 and 0.472, respectively. In Part 1 of our study [14], we found the same trend for the efficacy of the combined treatment HCQ-AZ between men and women. We ascribed the difference to the propensity of women to respond to an infection and look for treatment faster than men, thus reducing the relative effectiveness of vaccination. The non-negligible absolute 7% decay over 640 days (21 months) of the measured vaccination efficacy, calculated at various event-free survival cutoffs (Table 5b), should be regarded cautiously. It could be ascribed to either a deleterious long-term effect of vaccination on event-free survival (EFS), regardless of the fact that patients have maintained or not their vaccination status after Covid-19 remission, or that there was a bias between vaccinated and unvaccinated patients, with a weaker general health condition in vaccinated patients, or conversely that healthier patients were less prone to accept vaccination, a possible bias that could not be reflected by the disease aggravating covariates included in the model. However, a long-term deleterious effect of vaccination cannot be excluded as well.
Finally, although results of interactions detected between treatment and covariates are often considered with caution because they are very sensitive to the inclusion confounder covariates, they are perhaps the most important considerations when debating the benefit of vaccination. Indeed the situation is complex since vaccination was officially initially supposed to stop the chain of transmission by preventing the development of the Covid-19 disease in individuals, and shortly after, only supposed to prevent severe forms in infected patients. However, a statistically significant unfavorable interaction was detected, consistently in the 3 different models, between vaccination and the “inpatient” status that reflects the severity of the disease. Despite the claim of the vaccine makers and proponents that Covid-19 vaccine reduced the occurrence of severe forms of Covid-19, this negative interaction detected by the 3 models indicates that, statistically, it was not always the case in the IHU Méditerranée cohort. Otherwise the interaction with the inpatient status would have been favorable with a decreased risk of ICU transfer or death. In fact, Table 3 shows clearly that for inpatients not at risk, the number of events was 65% higher in the vaccinated patients compared with the unvaccinated patients. Moreover, in complete contradiction with the official narrative, this finding is in line with what was demonstrated on laboratory animal tests showing that vaccines against SARS coronavirus may induce a worsening of the pneumonia and increase the risk of death [21-23].
Statistically significant favorable interactions of vaccination were detected with variant Delta (in the three models) and with age categories ≥ 50 years and ≤ 89 years (model (b) and model (c) less accurate) but not for age > 89 (model (a) more accurate) for which the interaction is unfavorable, coherently with a number of events elevated but roughly the same in vaccinated (16.3%) and unvaccinated (18.6) patients for this latter category. Other statistically significant interactions were detected in model (a) ; unfavorable between vaccination and immunodeficiency and between vaccination and chronic obstructive pulmonary disease (COPD), confirming the suspected capacity of vaccination to worsen the underlying illness, particularly severe chronic illnesses. A reference to this problem is made by an author heavily sponsored by Pfizer (institutional funding, see the conflict of interest statement [5]) and affiliates in an admirable semantic squirming published in Nature Reviews Immunology : “Estimating the protective effect of vaccination against severe illness from descriptive population-level statistics is non-trivial. For instance, in a population with lower average vaccine uptake but very high uptake among the key risk groups — namely the elderly and chronically ill — severe cases might still be expected to occur disproportionately among those vaccinated even if vaccine effectiveness is very high.” [5]
The negative interactions can be explained by combining the following insights: (a) the spike glycoprotein from both the SARS-CoV-2 virus and from COVID-19 vaccines has many known mechanisms of toxicity [25,26] and it is responsible for the formation of microscopic blood clots in the lung capillaries and alveoli [27-29] that result in the oxygen desaturation responsible for most hospitalizations and deaths; the spike protein is known to persist in the human body for at least 15 months, if not longer [30], and experimental treatment protocols for removing it from the body have only just started being explored [31,32]. Because the spike protein has also been used as the antigen for the genetic COVID-19 vaccines, vaccination followed with a breakthrough infection, within close temporal proximity of a few months, could result in excess bio-accumulation of spike protein. Thus, although the vaccine appears have some positive efficacy towards the reduction of ICU admissions or deaths, via some non- sterilizing antiviral effect of vaccine induced antibodies averaged over the entire cohort, for those patients where this antiviral response fails to mitigate an infection, the greater cumulative load of spike protein from both infection and prior vaccination, can explain the unfavorable interaction between vaccination and severity of the disease. It can also explain why the vaccine has similar negative interactions for fragile patients with age > 89 years and patients with immunodeficiency, where the elicited antibody response may be insufficient, resulting in no antiviral protection but some harm due to excess accumulation of the spike
Appendix 3, in supplementary material, includes the complete set of instructions needed to reproduce the calculations for model (a) using R. The design of our statistical analysis is retrospective and based on the same methodological approach used in Part 1, thus the same remarks apply regarding the retrospective observational design vs prospective randomized controlled trial designs [14]. All things being considered, the ORs we have calculated are reliably evidencing of a true treatment effect in both cases of HCQ-AZ treatment and vaccination (within the limitations evoked at the second paragraph of the Discussion section). Many will argue that the level of confidence of our analysis cannot be considered at the same value of proof as for results from randomized clinical trials. But randomized trials have many pitfalls and cannot be conducted to completion in times of urgency for the reasons presented in the Introduction section of the Part 1 of our study [14]. All things being considered, the ORs we have calculated are reliably evidencing of a true treatment effect in both cases HCQ-AZ and vaccination.
It is indisputable that both HCQ-AZ and vaccination exhibit efficacy independently in multivariate analysis with a sufficiently improved survival benefit for the category of age ≥ 50 years to preclude doubts on the reality of the measurement, especially for HCQ-AZ that has been severely criticized and denied. Somehow ironically, HCQ-AZ and vaccination shared a similar fate in the analysis of the IHU- Méditerranée cohort. Because their ORs were close and the calculation data suffered from the same imperfection for both of them (see results for HCQ-AZ in article Part 1 of our study, [14]), if the analysis was deemed not valid for HCQ-AZ it should be deemed not valid as well for vaccination and vice versa.
This is quite embarrassing for the proponents of vaccination as being the only valid response to the pandemic. The approval conditions for a vaccine candidate under FDA rule is that no alternative treatment exists for emergency use authorisation (EUA). However the potential efficacy of HCQ-AZ in Covid-19 was known to Glaxo and Pfizer as early as March-May 2020 [33-35]. Furthermore, the Indian Council of Medical Research (ICMR) had proposed, as early as March 23, 2020, the prophylactic use of weekly low-dose hydroxychloroquine as pre-exposure prophylaxis for health- care workers [36], based on the biological plausibility of its antiviral action against COVID-19. A meta-analysis [37] of 11 Indian studies that followed the ICMR protocol found an infection ratio risk reduction with RR=0.56 (p=0.004), and for the five studies that included only patients that followed the ICMR protocol for at least 6 weeks, allowing for an optimal buildup of hydroxychloroquine in the lungs, the infection ratio risk reduction was RR=0.25 (p<0.001), which is comparable with the infection ratio risk reduction efficacy claimed by several COVID-19 vaccines on the market. The toxicity of this experimental vaccination should be emphasized as well as its association with a very large number of severe side- effects and even deaths, worldwide, at least in countries with a high vaccination coverage. Irreversible or long lasting debilitations of all sorts are reported in a non-negligible fraction of the population, still to be evaluated thoroughly [38-40]. People with chronic symptoms may include patients who did not receive an early efficient treatment of their acute episode of Covid-19, such as HCQ-AZ, and developed a long Covid, but also persons suffering for long term vaccine side-effects. Moreover, the tens of billions US dollars benefit for the manufacturers and the too short delay to produce an effective and safe vaccine raise serious questions. Usually, it take 10 years to develop a new vaccine. It should also be emphasized that the vaccination mandates, enforced in many countries on a large proportion of the population, were in total disagreement with the international conventions. Indeed, it is not allowed to administrate an experimental product without a free informed consent.
An additional disturbing reality with vaccination was the dramatic peak of Covid-19 infections observed about 2 to 3 weeks after the massive campaign of injections had started [41]. Antibody-dependent enhancement (ADE) may explain this phenomenon (due to facilitating antibody, initially neutralizing at low concentration and immune complexes). It is known that infection facilitating antibodies can appear two to three weeks after one dose of vaccine [41-43]. For instance, the vaccine against the dengue fever sensitized some of the dengue-naïve recipients leading to severe dengue fever [43]. Indeed, the IHU Méditerranée cohort does not show any improvement with vaccination for the risk of aggravated disease state in fragile patients (age > 89 years). In addition there is an significant unfavorable interaction of vaccination with the severity of the disease at patient admission. In view of such data, we must remember the outrageous cost for countries of this global human experiment.
Conclusion
External and independent state-of-the-art statistical analysis of the IHU-Mediterranée data demonstrated that the potential efficacy of vaccination was not substantially better than the empirical treatment using a combination of hydroxychloroquine and azithromycin, given as an early treatment. Vaccination had no measured efficacy for age < 50 years and, to the contrary of what was officially claimed, a negative (unfavorable) interaction was evidenced between vaccination and Covid-19 disease severity. These results indicated a disease worsening effect of previous vaccination in many cases, raising the question of the relevance and legitimacy of mass vaccination. The very large size of the observational single-institution cohort of patients coherently treated, together with the quality of the statistical approach we used, made these results very challenging for those who have continuously denied the potential efficacy of hydroxychloroquine-based treatment of Covid-19 patients during the pandemic and advocated systematic vaccination for all age categories.
Funding
The authors declare that this study received funding from Association Bon Sens to cover the publication fees. Valère Lounnas declares having received a grant of 829 euros from association Bon Sens to purchase a laptop computer and perform calculations. All other authors were benevolent. The persons voting to provide funds were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgements
We thank all the medical teams and researchers of the IHU-Méditerranée Infection for fulfilling the Hippocratic Oath by providing the best cares to Covid-19 patients during the pandemic. Very wisely, they have first renounced to publish their analysis of the raw data and made them publicly accessible to allow unbiased assessments, before final publication [16]. Their ethical conduct has ultimately allowed this in-depth analysis of the treatment they deployed.
Supplementary Files
Note: Please download the Supplementary Files below link:
https://www.fortunejournals.com/suppli/AMI_10126.pdf
References
- Patterson BK, Francisco EB, Yogendra R et "Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post- Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection." Frontiers in Immunology 12 (2022): 746021.
- McCullough PA, Procter BC, Wynn "Clinical Rationale for SARS-Co-V-2 Base Spike Protein Detoxification in Post COVID-19 and Vaccine Injury Syndromes." Journal of the American Physicians and Surgeons 28 (2023): 90-93.
- Hulscher N, Procter BC, WynnC, McCullough "Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination." Cureus 15 (2023).
- Al-Kofahi M and Austin D. “Hydroxychloroquine and azithromycin as potential treatments for COVID-19; clinical status impacts the ” Journal of Pharmacokinetics and Pharmacodynamics 47 (2020): 187-188.
- France “Guerre du Vaccin - Etats-Unis, Russie, Chine, UE - il faut éliminer le soldat hydroxychloroquine” Publié le 12 août 19 (2020): 56.
- Pfizer Media Relation release note “Pfizer Shares Safety Data on Azithromycin-Hydroxychloroquine Combination Company Follows Up on Recently Made Commitment as Part of Five-Point Plan”.
- Indian Council of Medical Research "Revised advisory on the use of Hydroxychloroquine (HCQ) as prophylaxis for SARS-CoV-2 infection (in supersession of previous advisory dated 23rd March, 2020)", (2020).
- Stricker RB and Fesler MC. "Hydroxychloroquine Pre- Exposure Prophylaxis for COVID-19 in Healthcare Workers from India: A Meta-Analysis." Journal of Infection and Public Health 14 (2021): 1161-1163.
- Perez JC, Moret-Chalmin C, Montagnier L. “Emergence of a New Creutzfeldt-Jakob Disease: 26 Cases of the Human Version of Mad-Cow Disease, Days After a COVID-19 Injection.” International Journal of Vaccine Theory, Practice, and Research 3 (2023): 727.
- Korsia-Meffre “Vaccins contre la COVID-19 : doit- on s'inquiéter du risque de maladie aggravée chez les personnes vaccinées ?” Vidal Santé Publique 03 Novembre (2020)
- Barda N, Dagan N, Ben-Shlomo Y, et al. “Safety of the BNT162b2 mRNA Covid-19 Vaccine in a nationwide ” N. Engl. J. Med 385 (2021): 1078-1090.
- Raoult D “Vaccins et Omicron” (2022).
- Boldova AE, Korobkin JD, Nechipurenko YD, Sveshnikova “Theoretical Explanation for the Rarity of Antibody-Dependent Enhancement of Infection (ADE) in COVID-19.” Int J Mol Sci. 23 (2022): 11364.
- Lee WS, Wheatley AK, Kent SJ, DeKosky BJ “Antibody- dependent enhancement and SARS-CoV-2 vaccines and ” Nat Microbiol 5 (2020): 1185-1191.
- Shukla R, Ramasamy V, Shanmugam RK, et “Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine Front.” Cell Infect Microbiol 10 (2020): 572681.
- Brouqui P, Million M, Parola P, et “Outcomes after early treatment with hydroxychloroquine and azithromycin: An analysis of a database of 30,423 COVID-19 patients” New Microbes New Infect (2023).
- Matthieu “Monocentric retrospective cohort of 30,423 COVID-19 patients.” DRYAD 21 April (2023).
- Collaborative work The R Project for Statistical Computing R: The R Project for Statistical
- Neugebauer R, Laan Mvd. “Why prefer double robust estimators in causal inference?” J Stat Plan Inference 129 (2005): 405-26.
- HAS “Vaccins contre la Covid-19: protéger les plus fragiles via leur entourage.” PRESS RELEASE - Posted on Apr 30 129 (2021).
- Lee WS, Wheatley AK, Kent SJ, et “Antibody- dependent enhancement and SARS-CoV-2 vaccines and therapies.” Nat Microbiol 5 (2020): 1185-1191.
- Menachery VD, Yount BL Jr, Debbink K, et “A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence.” Nat Med 21 (2015): 1508-13.
- Tseng CT, Sbrana E, Iwata-Yoshikawa N, et “Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus” PLoS One 7 (2012): e35421.
- Parry PI, Lefringhausen A, Turni C, et "'Spikeopathy': COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA", Biomedicines 11 (2023).
- Lesgards JF, Cerdan D, Perronne C, et al. "Toxicity of SARS-CoV-2 Spike Protein from the Virus and Produced from COVID-19 mRNA or Adenoviral DNA Vaccines", Archives of Microbiology and Immunology 7 (2023): 121-138.
- McGonagle D, Bridgewood C, Meaney "A tricompartmental model of lung oxygenation disruption to explain pulmonary and systemic pathology in severe COVID-19. "The Lancet Respiratory Medicine 9 (2021): 665-672.
- Boschi C, Scheim DE, Bancod A et al. "SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects." International Journal of Molecular Sciences 23 (2022): 15480.
- Scheim DE "A Deadly Embrace: Hemagglutination Mediated by SARS-CoV-2 Spike Protein at Its 22 N-Glycosylation Sites, Red Blood Cell Surface Sialoglycoproteins, and Antibody." J. Mol. Sci. 23 (2022): 2558.
- Scheim DE, Vottero P, Santin AD, Hirsh, "Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19." Int. J. Mol. Sci. 24 (2023): 17039.
- Patterson BK, Francisco EB, Yogendra R et "Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post- Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection." Frontiers in Immunology 12 (2022): 746021.
- McCullough PA, Procter BC, Wynn "Clinical Rationale for SARS-Co-V-2 Base Spike Protein Detoxification in Post COVID-19 and Vaccine Injury Syndromes." Journal of the American Physicians and Surgeons 28 (2023): 90-93.
- Hulscher N, Procter BC, WynnC, McCullough "Clinical Approach to Post-acute Sequelae After COVID-19 Infection and Vaccination." Cureus 15 (2023).
- Al-Kofahi M and Austin “Hydroxychloroquine and azithromycin as potential treatments for COVID-19; clinical status impacts the outcome.” Journal of Pharmacokinetics and Pharmacodynamics 47 (2020): 187-188.
- France “Guerre du Vaccin - Etats-Unis, Russie, Chine, UE - il faut éliminer le soldat hydroxychloroquine” Publié le 12 août 19 (2020): 56.
- Pfizer Media Relation release note “Pfizer Shares Safety Data on Azithromycin-Hydroxychloroquine Combination Company Follows Up on Recently Made Commitment as Part of Five-Point Plan”.
- Indian Council of Medical Research "Revised advisory on the use of Hydroxychloroquine (HCQ) as prophylaxis for SARS-CoV-2 infection (in supersession of previous advisory dated 23rd March, 2020)", (2020).
- Stricker RB and Fesler MC. "Hydroxychloroquine Pre- Exposure Prophylaxis for COVID-19 in Healthcare Workers from India: A Meta-Analysis." Journal of Infection and Public Health 14 (2021): 1161-1163.
- Perez JC, Moret-Chalmin C, Montagnier L. “Emergence of a New Creutzfeldt-Jakob Disease: 26 Cases of the Human Version of Mad-Cow Disease, Days After a COVID-19 Injection.” International Journal of Vaccine Theory, Practice, and Research 3 (2023): 727.
- Korsia-Meffre “Vaccins contre la COVID-19 : doit- on s'inquiéter du risque de maladie aggravée chez les personnes vaccinées ?” Vidal Santé Publique 03 Novembre (2020)
- Barda N, Dagan N, Ben-Shlomo Y, et al. “Safety of the BNT162b2 mRNA Covid-19 Vaccine in a nationwide ” N. Engl. J. Med 385 (2021): 1078-1090.
- Raoult D “Vaccins et Omicron” (2022).
- Boldova AE, Korobkin JD, Nechipurenko YD, Sveshnikova “Theoretical Explanation for the Rarity of Antibody-Dependent Enhancement of Infection (ADE) in COVID-19.” Int J Mol Sci. 23 (2022): 11364.
- Lee WS, Wheatley AK, Kent SJ, DeKosky BJ “Antibody- dependent enhancement and SARS-CoV-2 vaccines and ” Nat Microbiol 5 (2020): 1185-1191.
- Shukla R, Ramasamy V, Shanmugam RK, et “Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine Front.” Cell Infect Microbiol 10 (2020): 572681.


 Impact Factor: * 3.5
Impact Factor: * 3.5 Acceptance Rate: 71.36%
Acceptance Rate: 71.36%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
